Abstract
Changes in the nutrient availability of mammalian cell cultures are reflected in the β-dispersion parameter characteristic frequency (fC) and the on-line dual frequency permittivity signal. Multi-frequency permittivity measurements were therefore evaluated in fed-batch cultivations of two different CHO cell lines. Similar responses to nutrient depletions and discontinuous feed additions were monitored in different cultivation phases and experimental setups. Sudden increases in permittivity and fC occurred when feed additions were conducted. A constant or declining permittivity value in combination with a decrease in fC indicated nutrient limitations. fC correlated well with changes in oxygen uptake rate when cell diameter remained constant, indicating that metabolic activity is reflected in the value of fC. When significant cell size changes occurred during the cultivations, the analysis of the β-dispersion parameters was rendered complex. For the application of our findings in other systems it will be hence required to conduct additional off-line measurements. Based on these results, it is hypothesized that multi-frequency permittivity measurements can give information on the intracellular or physiological state in fed-batch mode. Similar observations were made when using different cell lines and feeding strategies, indicating that the findings are transferable to other cell lines and systems. The results should lead to an improved understanding of routine fed-batch processes. Additional studies are, however, required to explore how these observations can be used for fed-batch process development and optimization.
Keywords: Permittivity, Physiological state, Fed-batch, CHO cell culture, Characteristic frequency
Introduction
Fed-batch processes continue to be the most relevant culture mode for the vast majority of high-yielding commercial bioprocesses due to their ease of operation and reliability (Wurm 2004; Chee Furng Wong et al. 2005). Improvement in process monitoring and subsequent optimization of CHO cell-based processes is consequently of particular interest for the production of biotherapeutics (Chee Furng Wong et al. 2005). It can be hypothesized that advances in the optimization of bioprocesses can in particular be achieved by an improved understanding and control of the metabolic activity and physiological state of a cell population. However, with current sensor technology, on-line monitoring of the physiological state remains a challenging task, rendering its use as a bioprocess control parameter complex (Konstantinov 1996; Henry et al. 2007).
Permittivity-based in situ probes are now an established tool for the measurement of biomass in real-time in a wide variety of cell culture processes (Kamen et al. 1996; Zeiser et al. 1999; Elias et al. 2000; Zeiser et al. 2000; Ducommun et al. 2001, 2002; Cannizzaro et al. 2003). Permittivity is a direct measure of the membrane enclosed volume fraction or biovolume of the cell suspension. These measurements also reflect changes in cell physiology which has been demonstrated for several cell types (Fehrenbach et al. 1992; Noll and Biselli 1998; Zeiser et al. 2000; Ducommun et al. 2002; Cannizzaro et al. 2003; Sarrafzadeh et al. 2005).
Multi-frequency permittivity measurements can be used to monitor nutrient limitations during batch cultivations of CHO cells (Ansorge et al. 2010). In this previous study, a decrease in the on-line dual frequency permittivity signal (ΔεFogale) was an indicator for a metabolic shift after glutamine depletion. This decrease was caused by a drop in the characteristic frequency (fC) which is a function of the intracellular conductivity (σi). It was possible to identify σi as the parameter that was responsible for the decrease in ΔεFogale and fC. Changes in the nutrient availability are consequently reflected in the β-dispersion parameter characteristic frequency (fC) and the permittivity signal (ΔεFogale).
We consequently evaluated the use of on-line permittivity measurements as monitoring tool in fed-batch cultivations of two different CHO cell lines (CHO I and II). It was hypothesized from previous results that the β-dispersion parameters are indicative of changes in metabolic activity or physiological state of cell cultures. We therefore investigated if nutrient additions, typically performed in discontinuous mode (bolus additions), would have an effect on fC and ΔεFogale. The results give first insights into how permittivity measurements might provide additional information on, and understanding of, the physiological state of mammalian cell cultures.
Materials and methods
Bioreactor setup
A benchtop Biostat MCD (B. Braun Biotech, Melsungen, Germany) (3 L total volume) as previously described was employed for cultivations in lab scale (Ansorge et al. 2007b).
For the cultivations performed in pilot scale, a 25 L airlift bioreactor CF 3000 (Alfa Laval Chemap, Volketswil, Switzerland) was used. This system was additionally equipped with a turbidity probe (Aquasant Messtechnik AG, Bubendorf, Switzerland). All process parameters were recorded on a pen recorder, manually transferred into txt format and filtered using Matlab software (Mathworks, Natick, MA).
Cell lines and media
Two different suspension-grown in-house CHO cell lines were employed in this study. First, a CHO K1/dhfr− (dehydrofolate reductase negative) host cell line was cultivated in fed-batch mode (Ansorge et al. 2010). Second, cultivations were conducted with a recombinant CHO cell line, expressing a human monoclonal antibody (CHO II). An in-house medium mixture (DHI) was used as basal medium for both CHO cell lines (Schlaeger 1996). Final nutrient concentrations, e.g. glutamine and glucose concentrations and other media supplements, differed for each medium composition and were cell line dependent. Discontinuous feeding (bolus addition) consisted of an addition of 4% (v/v) (CHO I) or 2% (v/v) (CHO II) of a concentrated nutrient mixture per day.
Metabolite and product analyses
Amino acid analysis was performed on a HP 1100 Series HPLC (Palo Alto, CA) as suggested by the manufacturer (Gratzfeld-Huesgen 1998). Glucose concentration was determined with a Beckman Coulter Glucose Analyzer 2 system (Beckman 1999).
The human monoclonal antibody produced by cell line CHO II was quantified by a standard sandwich ELISA assay and by ProSep A chromatography (Millipore, Billerica, MA) according to the manufacturer’s instructions.
Biovolume, cell number and cell size determination
A 0.05% (w/v) solution of Trypan Blue (Sigma–Aldrich, MO) was routinely used for staining and manual cell counting in a Neubauer hemacytometer. A CASY®1 device (Innovatis AG, Bielefeld, Germany) was employed to determine mean cell diameter and ‘total biovolume (CASY)’. Furthermore, the Vi-CELL™ XR system (VC) (Beckman Coulter, Fullerton, CA) was used for automated cell counting and for measurement of viability and cell size. The VC allows distinguishing between the diameters of non-stained (viable) and stained (non-viable) cells. Total biovolume (PCV) was measured using disposable PCV measurement tubes (Techno Plastics Products AG, Trasadingen, Switzerland) (Stettler et al. 2006).
Permittivity measurements with the Fogale Biomass System® (BMS)
The underlying theory on the dielectric properties of biological cells has been extensively described elsewhere (Kell and Harris 1985; Harris et al. 1987; Pethig and Kell 1987; Markx and Davey 1999).
In summary, the permittivity of a cell suspension can be easily measured because a characteristic fall in its value, the β-dispersion, occurs with increasing frequency. This decrease is caused by the polarization of cell membranes and characterized by several β-dispersion parameters. The first parameter, the dielectric increment (Δεmax) can be measured as the difference in permittivity at the low-frequency and the high-frequency plateau and is defined by the equation of Schwan (1957) (Eq. 1):
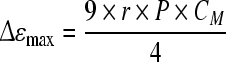 |
1 |
where Δεmax is the permittivity increment (difference in permittivity at very low and very high frequency relative to fC; F m−1), r the cell radius (m), N the cell density (m−3), CM the capacitance per membrane area (F m−2), and P the volume fraction of cells (biovolume) defined by:
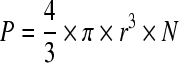 |
1.1 |
The second parameter, the characteristic frequency (fC) is the frequency at which a permittivity of 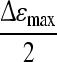 is reached. fC is a function of cell size, capacitance per membrane area (CM) and also intracellular (σi) and medium conductivity (σm) and defined by a simplified equation (Eq. 2) (Harris et al. 1987):
is reached. fC is a function of cell size, capacitance per membrane area (CM) and also intracellular (σi) and medium conductivity (σm) and defined by a simplified equation (Eq. 2) (Harris et al. 1987):
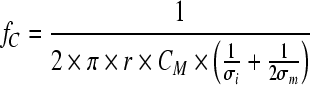 |
2 |
where σi is the conductivity of the cytoplasm (intracellular conductivity; mS cm−1) and σm the conductivity of the medium (mS cm−1).
A third parameter describing the β-dispersion is α (also: Cole–Cole α). It is an empirical parameter describing the fall in permittivity with increasing frequency in the Cole–Cole equation (Cole and Cole 1929). α is believed to increase when the distribution in cell electrical properties widens in the cell population (Davey 1993).
The BMS makes use of the β-dispersion to measure the biovolume. The system uses a dual-frequency mode with a high frequency f2 (10 MHz) and a working frequency f1 in the region of fC. Here, commercial in situ autoclavable DN 12 and DN 25 probes were employed for the lab and pilot scale bioreactor system, respectively. The system setup was performed according to manufacturer’s instructions. The permittivity signal given by the BMS, ΔεFogale, is the result of the difference in permittivity at f1 and f2. f1 is adjustable and it is hence possible to measure ΔεFogale for any given cell type in the region of fC (for mammalian cells: ~1 MHz) and not in the low-frequency range of Δεmax (Eq. 1) (Schwan 1957). A constant ΔεFogale response can consequently be observed when the cell radius is changing at a constant biovolume. ΔεFogale is henceforth linear correlating with the biovolume even in the case of cell size changes (FogaleNanotech 2004). The BIOMASS+ software automatically analyzes the permittivity over a total of 20 frequencies from 0.3 to 10 MHz. The software then determines the β-dispersion parameters fC, Δεmax and α. To minimize noise in the calculated parameters, it is optional to either set the Cole–Cole α to a constant (fixed) value or to calculate it depending on the results of the other β-dispersion parameters (value of α is variable). During our analyses, a calculated (variable) α did not affect the qualitative patterns of the other β-dispersion parameters. All analyses were therefore conducted with a calculated (variable) α as the setting of a fixed value did not result in a significant noise reduction. Matlab software (Mathworks, Natick, MA) was finally used to filter the generated datasets and further minimize noise in all calculated parameters.
Results and discussion
The two CHO cell lines (CHO I and CHO II) used in this work showed differences when correlating the permittivity signal (ΔεFogale) from preliminary batch and fed-batch experiments to off-line cell counts and biovolume measurements (Table 1). Linear regression resulted in a linear relationship (R2 = 0.86) of the viable cell count and ΔεFogale for cell line CHO I in batch experiments (during which only small cell size changes were observed). For the same cell line, a lower R2 value (R2 = 0.76), was observed for total cell count and ΔεFogale, caused by values from later stages of the cultivation when viability and ΔεFogale decreased. In contrast, the permittivity signal correlated better with the total cell count for cell line CHO II (R2 = 0.89 vs. 0.74 for the viable cell count). In later cultivation phases this effect seemed to be even more pronounced, but cell diameter and viability changes made it difficult to interpret the results (data not shown). The differences in slope (i.e. the permittivity signal per cell) were most likely related to larger cell diameters of cell line CHO II and other cell-specific properties. It was also observed for cell line CHO I that, at the end of batch cultivations in different scales, ΔεFogale returned to its baseline value when viability tended to zero. Yet, for cell line CHO II, a remaining permittivity signal was observed when viability had reached zero. This value was clearly caused by non-viable/stained cells as disintegration of the cell suspension by sequential ultrasonication decreased the signal close to the baseline value before inoculation (results not shown). As viability and cell size changes occurred simultaneously in fed-batch cultures, these data were not considered for the regression values in Table 1A.
Table 1.
Linear regression values for permittivity signal (ΔεFogale) (Y) and off-line cell counting/biovolume determination (X)
| A | |||
|---|---|---|---|
Slope 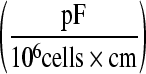
|
R2 | ||
| CHO I | vcc | 0.82 | 0.86 |
| tcc | 0.58 | 0.76 | |
| CHO II | vcc | 1.45 | 0.74 |
| tcc | 1.22 | 0.89 | |
| B | |||
|---|---|---|---|
Slope 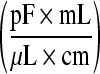
|
R2 | ||
| CHO I | CASY | 0.926 | 0.99 |
| PCV | 0.675 | 0.99 | |
| CHO II | CASY | 1.35 | 0.97 |
| PCV | 0.54 | 0.98 | |
Remarks: vcc: viable cell count (hema); tcc: total cell count (hema); data from a total of four cultivations were included in the table: (CHO I: 1 batch, 1 fed-batch cultivation (both in 25 L scale); CHO II: 1 batch, 1 fed-batch cultivation (both in 2 L scale)
(A): Values for regression of off-line cell counts (X) and ΔεFogale (Y); only data from batch cultivations are represented (see text for details); cell diameter variation for samples represented in the table: 13.78 ± 0.72 μm (CHO I), 16.97 ± 0.82 μm (CHO II)
(B): Values for regression of biovolume measurements (CASY and PCV) (X) and ΔεFogale (Y)
Only samples for which viability was >70% were considered for the regression
For both cell lines, a regression of ΔεFogale and off-line biovolume measurements (PCV and CASY) resulted in very high R2 values for batch and fed-batch cultures, being independent of cell size changes in fed-batch and decreasing viability until very late stages of the cultures (Table 1B).
These results appeared to be independent of the chosen bioreactor scale, with cultivations in different bioreactor systems resulting in slightly different but overall similar correlations for both cell lines (Ansorge et al. 2010 and unpublished data). This indicates that the permittivity signal is not in all cases reflecting off-line viable cell counts by dye exclusion. The degree to which non-viable cells contribute to the permittivity signal seems to rather depend on the level of disintegration of cells after inactivation or the conservation of membrane properties (Patel et al. 2008a, b).
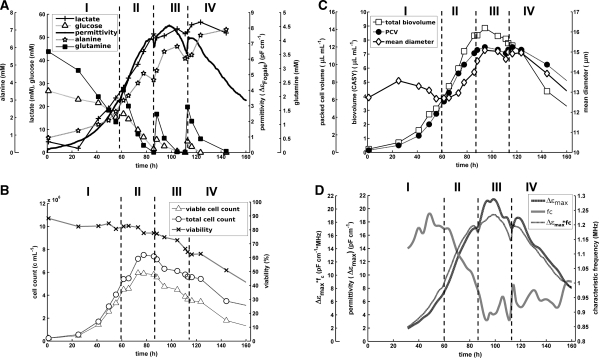
In a subsequent fed-batch cultivation with cell line CHO I, the goal was first to evaluate the impact of feed additions on ΔεFogale and fC. We hence performed feed additions at different physiological states (Fig. 1), expecting that both parameters would reflect the metabolic activity/nutrient availability of the cultures. The cultivation could be divided in four distinct phases (FB I–IV). During phase FB I (0–60 h), cells started growing exponentially with a specific growth rate of μ = 1.59 ± 0.14 d−1 (Fig. 1b). When the cell density reached 4 × 106 cells/mL, the first feed addition (~60 h) was performed at a time when none of the analyzed nutrients was depleted (amino acid data not completely shown). A small decrease of ΔεFogale was observed at the time of the feed addition representing the dilution effect of the bolus addition. Otherwise there was no change in ΔεFogale or its slope. Our hypothesis is that the physiological state of the culture was not significantly changed after this first feed addition.
Fig. 1.
Fed-batch cultivation of cell line CHO I in pilot scale. Feed additions are marked by vertical dashed lines and divide the cultivation in phases FB I–IV. a permittivity and metabolite concentrations. b viability and cell counts (hema). c off-line biovolume (CASY, PCV)/cell size (CASY) measurements. d β-dispersion parameters
The second addition took place when glutamine was already depleted and alanine started to get consumed. Under these conditions, ΔεFogale leveled off (~85 h) and its slope tended to zero. This time the addition resulted in a significant change of ΔεFogale. The third and last feed addition (~112 h) was deliberately conducted at a time when the culture had already entered death phase and the viability had decreased substantially. ΔεFogale had a negative slope and glucose as well as glutamine were depleted. At that time, both lactate and alanine were consumed. Adding extra nutrient resulted in a less prominent change of the on-line signal.
We observed significant changes in cell diameter during phase FB I, similar to those observed at the start of the cultivation for previously reported batch experiments (Ansorge et al. 2010) (Fig. 1c). A striking difference compared to the batch runs was a dramatic increase in cell diameter from ~12.5 to 15 μm starting at ~70 h (phase FB II) until late stages of the culture (phase FB IV). As in batch mode, we observed that changes in biovolume could not explain sudden changes in ΔεFogale at the time of nutrient limitations and additions. The data for PCV and total biovolume (CASY) did not follow the sudden changes in ΔεFogale after or before the feed additions in phases FB III–IV. The OUR, however, roughly matched the pattern of ΔεFogale, showed similar changes after, and more significant decreases before the three feed additions (not shown).
We analyzed the β-dispersion parameters (Δεmax, fC,Δεmax* fC) to further characterize the cultivation (Fig. 1d). fC increased at the beginning of the cultivation (~25–45 h), probably because of a decreasing cell diameter (Fig. 1c). At the end of phase FB I and immediately after the first feed addition (in phase FB II), fC remained constant at ~1.2 MHz. This supported our initial hypothesis after which there was no change in the physiological state of the culture (because of no change in ΔεFogale or its slope). fC decreased in phase FB II, most likely as a result of the increase in cell size after ~63 h (Eq. 1). This decrease continued until ~78 h when ΔεFogale leveled off and glutamine was depleted. Although the second feed addition should have resulted in a change in the nutrient availability there was no direct change in fC. Thus, fC was affected by changes in nutrient state as well as cell size which is in-line with the underlying theory (Schwan 1957; Harris et al. 1987; Ansorge et al. 2010). This became even more evident in phase FB III when cell size remained almost constant for the entire cultivation phase and the first part of phase FB IV. At that time (~90 h), fC increased sharply when none of the analyzed nutrients were limiting and dropped shortly after (~110 h) as soon as glutamine and glucose were depleted and alanine was again consumed. At that point, changes in fC seemed to indicate a metabolic shift as described for previous batch cultivations (Ansorge et al. 2010). The following third and last feed addition (~115 h) caused a sudden increase in fC, indicating higher metabolic activity and improved physiological state.
Δεmax followed the pattern of ΔεFogale very closely and only showed a slightly different profile in phase FB III, during which it gave a higher relative value compared to ΔεFogale. We could also observe that Δεmax*fC gave an identical pattern compared to ΔεFogale. ΔεFogale is only dependent on the biovolume and the intracellular conductivity σi when the pattern of Δεmax*fC and ΔεFogale are identical (Ansorge et al. 2010). Yet, it was impossible to draw conclusions based on the dataset of this cultivation because the biovolume (based on PCV and CASY) did not remain constant for an extended period (Fig. 1c).
Viability had already decreased significantly in phases FB III and IV. Yet, the underlying equations are only valid for cells at high viability. For this cell line, however, we observed that ΔεFogale follows the viable cell density/biovolume in all cultivation phases (Table 1; Ansorge et al. 2010). This means that, for cell line CHO I, dead cells did not significantly contribute to the permittivity signal and indicates that ΔεFogale only reflected changes in the dielectric properties of viable cells.
In summary, changes in the physiological state seemed to be reflected in ΔεFogale and fC in fed-batch mode with cell line CHO I (in particular after feed additions). However, cell size was significantly changing during the cultivation. As ΔεFogale is a function of the biovolume of the cell suspension it is directly affected by changes in cell size (Eq. 1). fC is also directly related to the cell diameter (Eq. 2) and it was therefore difficult to draw conclusions on the reasons for the changes in the different parameters.
After this first evaluation of the impact of feed additions on ΔεFogale and fC using the host cell line CHO I, we continued our efforts to evaluate permittivity measurements in fed-batch cultivations of a recombinant cell line (CHO II).
A lab scale fed-batch cultivation was conducted. Feed additions were performed independent of changes in the permittivity signal (ΔεFogale), following a strictly time-based feeding strategy. At each feed addition, 2% (v/v) of a concentrated nutrient mix was added (Fig. 2a). The first feed addition was performed at ~50 h cultivation time at a cell density of ~1.5 × 106 c/mL. One feed addition per day was then performed over a period of 9 days. The maximum in viable cell density of 3.5 × 106 c/ml was reached after ~100 h. The viable cell count then remained constant over a period of 3 days until viability decreased significantly after ~170 h. The total cell count, however, kept increasing and this was most likely the reason why ΔεFogale showed an increase until the beginning of death phase at ~170 h. Stained cells can, depending on cell line and bioreactor system, contribute significantly to the permittivity which seemed to have been the case for cell line CHO II (Table 1; Davey 1993; Guan et al. 1998; Ducommun et al. 2002; Cannizzaro et al. 2003; Ansorge et al. 2007a).
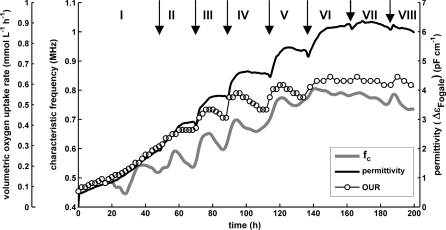
Fig. 2.
Fed-batch lab scale cultivation of CHO II. Feed additions are marked by vertical arrows and divide cultivation in phases FB I–X. a permittivity and cell counts (hema). b biovolume measurements and cell diameter. c β-dispersion parameters and product concentration; product concentration. increased linearly over time from FB II-FB X: conc (mAb(ProSep A)) = 0.31 × −8.7, R2 = 0.997 (regression not shown in figure)
Similar to previously described cultivations we divided this run in phases FB I-X (Fig. 2a). A sudden slope change and an increase in ΔεFogale was observed after each feed addition from phase FB II–VI (Figs. 2a, 3). Thereafter, ΔεFogale leveled off and only ~10 h after each feed addition its slope tended to zero again. When viability had significantly decreased (FB VII–X), ΔεFogale did not further increase but rather showed a continuous decrease with sudden short rises when feed was added.
Fig. 3.
On-line measurements until 200 h of fed-batch cultivation with cell line CHO II (graph presents data for same cultivation also shown in Fig. 2)
The cell diameter did not change significantly in the first part of the cultivation (until ~140 h) with average values of 16.57 ± 0.25 μm (CASY) and 16.61 ± 0.25 μm (VC) (Fig. 2b). This was a significant difference compared to the fed-batch cultivation of CHO I (see Fig. 1). Dramatic increases in cell size occurred in both cultivations when nutrient concentrations had significantly decreased, i.e. starting at around ~70 h for CHO I but not before ~150 h for CHO II. At that time, viability dropped in both cases and the biovolume had almost reached its maximum value.
For cell line CHO II, the mean diameter increased until death phase started in phase FB X (~250 h). At high viability, the viable mean diameter (VC) showed an almost identical pattern but increased much more starting in phase FB V and VI from a mean of ~16.5 to ~23 μm in phase FB X. In contrast, the mean diameter of stained cells (VC) was showing constant values (~14 μm) over the whole time course of the cultivation (not shown).
The off-line biovolume measurements (PCV and CASY) generally followed the pattern of ΔεFogale (Fig. 2b), explaining the overall increase in permittivity signal until ~150 h. In late phases of the process (FB VII–X), the PCV gave higher values relative to ΔεFogale.
The product concentration showed a linear increase over the whole time course of the cultivation (Fig. 2c). Hence, volumetric productivity (qprod = 0.31 RU/h (from phase FB II–X; see legend for Fig. 2c)) was constant although the number of viable cells was decreasing after ~170 h. This might have possibly been an indication for the fact that the remaining viable cells (with a larger (viable) cell diameter (Fig. 2b)), showed a higher specific productivity. This observation was also observed in a batch cultivation of CHO II (not shown) and is supported by literature findings according to which productivity is also a function of cell size (Lloyd et al. 2000; Berdichevsky et al. 2008). Another possibility is that stained cells were partly metabolically active and contributed to antibody production in late stages of the culture. It could also not be excluded that cells growing on the inner surface of the bioreactor were still productive. A final conclusion explaining the product kinetics could therefore not been drawn because only a limited dataset was available for this study. These results also make clear that more studies are needed to further shed light on a relationship of on-line permittivity signals and their evolution, the physiological state, product formation and overall process performance.
For the analysis of the β-dispersion parameters (Fig. 2c) it is important to note that the cell diameter was almost constant from 20 to 140 h. Viability was also high (>80%) during that time of the process. This was an advantage for the analysis of the calculated parameters compared to the previous CHO I fed-batch process.
We could observe a behavior of fC which was much more corresponding to our expectations than the first fed-batch cultivation with cell line CHO I concerning the relationship of fC and the physiological state/metabolic activity of the cell suspension. After each feed addition from phase FB II–IV, fC increased significantly. It then decreased shortly after when ΔεFogale leveled (see also Fig. 3). This finding was in-line with our previous results according to which fC indicated nutrient availability and limitations. A decrease in fC and hence σi was indicating a depletion in nutrients in batch cultivations (Ansorge et al. 2010). During these batch cultivations, OUR and fC correlated well at the time of glutamine depletion and the metabolic shift with regression coefficients of R2 > 0.82 when cell size was constant (data not shown). During fed-batch cultivations, we observed here increases in fC as responses to nutrient feed additions. The hypothesis that the changes in fC were related to the metabolic activity was substantiated in fed-batch where a linear correlation of fC and OUR was found from FB II–IX (Fig. 3, R2 = 0.92; regression not shown), when cell diameter was subject to only small changes. Compared to the on-line permittivity signal, the OUR also correlated with ΔεFogale after the feed additions (phase FB II–VI; Fig. 3), although resulting in a lower R2 value (R2 = 0.86 for FB I–VIII). This means that the responses of fC to the feed additions were also, in part, reflected in ΔεFogale. The responses of the oxygen consumption to nutrient additions were more pronounced than the respective changes in the permittivity signal (Fig. 3). Whenever ΔεFogale leveled and its slope tended to zero the OUR decreased, reaching almost the lower value it showed before the feed addition. Changes in the oxygen uptake rate after the addition of nutrients have been described for hybridoma cultures (Ramirez and Mutharasan 1990). These authors found typical peaks in OUR after additions of limiting nutrients.
The results show that OUR and fC better reflected changes in the metabolic activity of the culture whereas permittivity is rather an indicator of the biovolume (Fig 2b; Table 1). The changes in OUR found here were similar to those observed in fC and ΔεFogale. It was consequently hypothesized that decreases in OUR and fC before the addition of feed were an indicator for a nutrient limitation of the culture. Yet, our standard metabolite analyses (amino acids, glucose) did not reveal any clear limitations or depletions of major nutrients during this cultivation (not shown). For cell line CHO II, the observed changes in ΔεFogale and fC could hence not be attributed to one single nutrient component. They were possibly either a result of a complex interaction of changes in the concentration of several nutrients or caused by one or several unidentified medium components. In later stages of the culture (>150 h), an increase in cell size in combination with decreasing viability was probably responsible for the less pronounced responses to feed additions. During phases FB VI–IX, only small, temporary changes in fC and ΔεFogale were observed when feed was added. Similar to previous findings, a significant continuous increase in fC (from 0.7 to 0.8 MHz) marked the beginning of death phase in phase FB X.
Again, Δεmax followed the pattern of ΔεFogale very closely during the first part of the cultivation. It showed a slightly different profile in phases FB VII–X (until ~250 h) with higher values relative to ΔεFogale. This was probably related to the significantly increased cell diameter at that time and was in-line with the higher biovolume (Fig. 2b). As observed earlier, Δεmax*fC gave an identical pattern compared to ΔεFogale.
In summary, it was demonstrated how our previous findings from batch cultivations might be used when analyzing changes in β-dispersion parameters during fed-batch cultivations. When nutrient availability was high (CHO I, phase FB I), no responses to feed additions were observed in ΔεFogale and fC. In contrast, sudden increases in ΔεFogale and fC were observed in later culture stages at decreasing viability and viable cell count (CHO I, phases FB II/III and FB III/IV) but also at high viability and increasing viable cell count (CHO II, FB I–VI) in fed-batch cultivations of two different CHO cell lines. The OUR correlated well with changes in fC when cell size remained constant during the fed-batch cultivation of CHO II. For cell line CHO I, a direct correlation of OUR and fC in fed-batch was not possible because dramatic cell size changes occurred. However, batch cultivations resulted in comparable relationships at the time of glutamine depletion.
In addition to our previous findings (Ansorge et al. 2010), an increase in fC might therefore serve as an indicator of the nutrient availability/metabolic activity of mammalian cell cultures after feed additions. When the cell diameter remained constant in critical phases of the cultivation, the analysis was significantly simplified. In batch cultivations, this allowed identifying σi as underlying parameter indicating changes in the intracellular state of the culture after nutrient depletions (Ansorge et al. 2010). For the fed-batch cultivations presented here, it was, however, not possible to unambiguously identify σi as responsible for the sudden increases in ΔεFogale. This is due to the fact that σi is reflected in fC but not directly determined by multi-frequency permittivity measurements. Despite the off-line measurement of cell size and biovolume it can consequently remain difficult to separate simultaneously occurring changes in biovolume, viability, cell diameter, CM and σi which is consistent with other reports (Patel et al. 2008a, b). This complex analysis is certainly a challenge when interpreting datasets of β-dispersion parameters. However, the development of metabolic models for the determination of the physiological state with a full analysis of consumption rates for amino acids and other components is possibly an even greater challenge (Henry et al. 2007). Additionally, as we observed for cell line CHO II, analyses of concentrations of major nutrients do not necessarily lead to the identification of limitations in complex systems (which is also in agreement with other non-published observations in our lab).
In general, permittivity measurements appear to be advantageous for processes in which a scale-up is intended and/or different scales need to be compared. This also holds for turbidity measurements whilst the calculation of the OUR is often hampered by the complexity of differences in mass transfer and aeration setups in different bioreactors. The OUR mainly indicates changes in the metabolism of cultures (Kussow et al. 1995; Zeiser et al. 2000). In contrast, turbidity and infrared sensors appear to measure the viable cell count during growth phases but also dead cells and debris in later phases of cultivations (Merten et al. 1987; Konstantinov et al. 1992; Ansorge et al. 2007a). The majority of methods for biovolume measurements give identical patterns in situations where viability is high (growth phase) (Ansorge et al. 2007b). It is generally more difficult in late phases to identify which part of any on-line signal is caused by debris or dead cells and which by viable cells.
Possible applications of the findings would include the on-line control of feed additions based on the permittivity signal although this approach would likely be sensitive to noise and algorithms including several process variables such as the β-dispersion parameter fc are preferable. This could allow maintaining or driving mammalian cell cultures towards a more desirable productive state (Konstantinov 1996; Henry et al. 2007).
Conclusion
The on-line permittivity signal (ΔεFogale) and the characteristic frequency (fC) were indicators of nutrient limitations and depending on nutrient availability. Responses to feed additions could be monitored in real-time in the values of ΔεFogale and fC. Correlations of OUR and fC confirmed that the characteristic frequency reflects the metabolic activity. It is likely that changes in intracellular conductivity were the cause of the variations in the on-line signals, as was previously observed in batch cultivations. Permittivity measurements have thus the potential to give important information on the intracellular state of mammalian cell cultures.
Multi-frequency permittivity measurements provide more information on the culture than classical on-line monitoring methods but the resulting dataset seems to be more difficult to interpret. The most important information provided by the technology remains the real time in situ monitoring of the biomass content. Secondly, the metabolic activity and physiological state of mammalian cell cultures is reflected in the on-line signals. To obtain meaningful results and for the application of our findings in other systems it will be required to conduct additional off-line measurements for an ‘in-depth’ characterization of the respective production system.
We give here first insights into how permittivity measurements could be used for the development and optimization of fed-batch cultivations. This study is however only a first step towards an improved understanding of the metabolic/physiological and, in particular, productive state of cell cultures in fed-batch mode. Future work will have to investigate the relationship of nutrient limitations, fC, σi and other parameters such as the OUR to reveal possible underlying mechanisms and directly provide usable process control parameters from the measurements. It is possible that improvements in productivity can be achieved when these measurements are employed to identify and minimize nutrient limitations or control feed additions.
Acknowledgments
The authors would like to acknowledge C. Ghommidh from Université Montpellier II and M. Biselli from the Aachen University of Applied Sciences (Department Juelich) for the stimulating and fruitful discussions and remarks, D. Zacher for conducting preliminary experiments, M. Foggetta, M. Siegrist, J.-M. Vonach and J.-C. von Bueren for support with small scale experiments and setup of the bioreactor system, A. Weiss for product analyses and H. Remy for support with the CASY®1 system. S. Ansorge was supported during his internship by a Hoffmann-La Roche AG fellowship. The presented results form a part of his diploma thesis at the Aachen University of Applied Sciences.
Abbreviations
- BMS/Fogale BMS
Fogale Biomass System®
- CASY
CASY® 1 system
- CM
Capacitance per membrane area (F m−2)
- ΔεFogale
(online dual-frequency) permittivity signal; difference in permittivity measured at f1 and f2
- Δεmax
Permittivity increment (difference in permittivity at very low and very high frequency relative to fC)
- Δεmax*fC
Mathematical product of Δεmax and fC
- eq.
Equation
- fC
Characteristic frequency
- fig.
Figure
- hema
Hemacytometer
- N
Cell density
- P
Volume fraction of cells (biovolume)
- pHi
Intracellular pH
- r
Cell radius
- VC
Beckman Coulter Vi-CELL XR™ system
References
- Ansorge S, Esteban G, Ghommidh C, Schmid G (2007a) Monitoring nutrient limitations by online Capactitance measurements in batch and fed-batch CHO fermentations. Conference Proceedings to the 19th ESACT Meeting: Cell Technology for Cell Products, pp 723–726
- Ansorge S, Esteban G, Schmid G. On-line monitoring of infected Sf-9 insect cell cultures by scanning permittivity measurements and comparison with off-line biovolume measurements. Cytotechnology. 2007;55:115–124. doi: 10.1007/s10616-007-9093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge S, Esteban G, Schmid G. Multifrequency permittivity measurements enable on-line monitoring of changes in intracellular conductivity due to nutrient limitations during batch cultivations of CHO cells. Biotechnol Prog. 2010;26(1):272–283. doi: 10.1002/btpr.347. [DOI] [PubMed] [Google Scholar]
- Beckman (1999) Beckman Coulter: Glucose Reagent Kit Manual
- Berdichevsky M, Gentile MP, Hughes B, Meis P, Peltier J, Blumentals I, Auniņs J, Altaras NE. Establishment of higher passage PER.C6 cells for adenovirus manufacture. Biotechnol Prog. 2008;24(1):158–165. doi: 10.1021/bp070258u. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Gugerli R, Marison I, Stockar U. On-line biomass monitoring of CHO perfusion culture with scanning dielectric spectroscopy. Biotechnol Bioeng. 2003;84(5):597–610. doi: 10.1002/bit.10809. [DOI] [PubMed] [Google Scholar]
- Chee Furng Wong D, Tin Kam Wong K, Tang Goh L, Kiat Heng C, Gek Sim Yap M. Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO cell cultures. Biotechnol Bioeng. 2005;89(2):164–177. doi: 10.1002/bit.20317. [DOI] [PubMed] [Google Scholar]
- Cole KS, Cole RH. Dispersion and absorption in dielectrics. 1- Alternating current characteristics. J Chem Phys. 1929;9:341–351. doi: 10.1063/1.1750906. [DOI] [Google Scholar]
- Davey CL. The Biomass Monitor Source Book. Department of Biological Sciences, University of Wales: Aberystwyth; 1993. [Google Scholar]
- Ducommun P, Bolzonella I, Rhiel M, Pugeaud P, Stockar U, Marison IW. On-line determination of animal cell concentration. Biotechnol Bioeng. 2001;72(5):515–522. doi: 10.1002/1097-0290(20010305)72:5<515::AID-BIT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ducommun P, Kadouri A, Stockar U, Marison IW. On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng. 2002;77(3):316–323. doi: 10.1002/bit.1197. [DOI] [PubMed] [Google Scholar]
- Elias CB, Zeiser A, Bedard C, Kamen AA, Voyer R, Jardin B, Tom R. Enhanced growth of Sf-9 cells to a maximum density of 5.2 x 10(7) cells per mL and production of beta-galactosidase at high cell density by fed batch culture. Biotechnol Bioeng. 2000;68(4):381–388. doi: 10.1002/(SICI)1097-0290(20000520)68:4<381::AID-BIT3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Fehrenbach R, Comberbach M, Petre JO. On-line biomass monitoring by capacitance measurement. J Biotechnol. 1992;23(3):303–314. doi: 10.1016/0168-1656(92)90077-M. [DOI] [PubMed] [Google Scholar]
- FogaleNanotech (2004) Biomass System User Manual V 3.0
- Gratzfeld-Huesgen A (1998) Sensitive and reliable amino acid analysis in protein hydrolysates using the HP 1100 Series HPLC, Hewlett-Packard Co
- Guan Y, Evans PM, Kemp RB. Specific heat flow rate: an on-line monitor and potential control variable of specific metabolic rate in animal cell culture that combines microcalorimetry with dielectric spectroscopy. Biotechnol Bioeng. 1998;58(5):464–477. doi: 10.1002/(SICI)1097-0290(19980605)58:5<464::AID-BIT2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Harris CM, Todd RW, Bungard SJ, Lovitt JGM, Kell DB. Dielectric permittivity of microbial suspensions at radio frequencies: a novel method for the real-time estimation of microbial biomass. Enzyme Microb Technol. 1987;9:181–186. doi: 10.1016/0141-0229(87)90075-5. [DOI] [Google Scholar]
- Henry O, Kamen A, Perrier M. Monitoring the physiological state of mammalian cell perfusion processes by on-line estimation of intracellular fluxes. J Process Control. 2007;17(3):241–251. doi: 10.1016/j.jprocont.2006.10.006. [DOI] [Google Scholar]
- Kamen AA, Bédard C, Tom R, Perret S, Jardin B. On-line monitoring of respiration in recombinant-baculovirus infected and uninfected insect cell bioreactor cultures. Biotechnol Bioeng. 1996;50:36–48. doi: 10.1002/(SICI)1097-0290(19960405)50:1<36::AID-BIT5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kell DB, Harris CM. Dielectric spectroscopy and membrane organization. J Bioelectr. 1985;4:317–348. [Google Scholar]
- Konstantinov KB. Monitoring and control of the physiological state of cell cultures. Biotechnol Bioeng. 1996;52(2):271–289. doi: 10.1002/bit.260520203. [DOI] [PubMed] [Google Scholar]
- Konstantinov KB, Pambayun R, Matanguihan R, Yoshida T, Perusich CM, Hu WS. On-line monitoring of hybridoma cell growth using a laser turbidity sensor. Biotechnol Bioeng. 1992;40:1337–1342. doi: 10.1002/bit.260401107. [DOI] [PubMed] [Google Scholar]
- Kussow CM, Zhou W, Gryte DM, Hu WS. Monitoring of mammalian cell growth and virus production process using on-line oxygen uptake rate measurement. Enz Microb Tech. 1995;17(9):779. doi: 10.1016/0141-0229(94)00035-P. [DOI] [Google Scholar]
- Lloyd DR, Holmes P, Jackson LP, Emery AN, Al-Rubeai M. Relationship between cell size, cell cycle and specific recombinant protein productivity. Cytotechnology. 2000;34(1–2):59. doi: 10.1023/A:1008103730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markx GH, Davey CL. The dielectric properties of biological cells at radiofrequencies: applications in biotechnology. Enz Microb Tech. 1999;25:161–171. doi: 10.1016/S0141-0229(99)00008-3. [DOI] [Google Scholar]
- Merten O-W, Palfi GE, Stäheli J, Steiner J. Invasive infrared sensor for the determination of the cell number in a continuous fermentation of hybridomas. Dev Biol Standard. 1987;66:357–360. [PubMed] [Google Scholar]
- Noll T, Biselli M. Dielectric spectroscopy in the cultivation of suspended and immobilized hybridoma cells. J Biotechnol. 1998;63(3):187–198. doi: 10.1016/S0168-1656(98)00080-7. [DOI] [PubMed] [Google Scholar]
- Patel PM, Markx GH. Dielectric measurement of cell death. Enz Microb Tech. 2008;43(7):463–470. doi: 10.1016/j.enzmictec.2008.09.005. [DOI] [Google Scholar]
- Patel PM, Bhat A, Markx GH. A comparative study of cell death using electrical capacitance measurements and dielectrophoresis. Enz Microb Tech. 2008;43(7):523–530. doi: 10.1016/j.enzmictec.2008.09.006. [DOI] [Google Scholar]
- Pethig R, Kell DB. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol. 1987;32:933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- Ramirez OT, Mutharasan R. Cell cycle- and growth phase-dependent variations in size distribution, antibody productivity, and oxygen demand in hybridoma cultures. Biotechnol Bioeng. 1990;36(8):839–848. doi: 10.1002/bit.260360814. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh MH, Belloy L, Esteban G, Navarro JM, Ghommidh C. Dielectric monitoring of growth and sporulation of Bacillus thuringiensis. Biotechnol Lett. 2005;27(7):511–517. doi: 10.1007/s10529-005-2543-x. [DOI] [PubMed] [Google Scholar]
- Schlaeger EJ. The protein hydrolysate, Primatone RL, is a cost-effective multiple growth promoter of mammalian cell culture in serum-containing and serum-free media and displays anti-apoptosis properties. J Immunol Methods. 1996;194:191–199. doi: 10.1016/0022-1759(96)00080-4. [DOI] [PubMed] [Google Scholar]
- Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phy. 1957;5:147–208. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Stettler M, Jaccard N, Hacker D, Jesus MD, Wurm FM, Jordan M. New disposable tubes for rapid and precise biomass assessment for suspension cultures of mammalian cells. Biotechnol Bioeng. 2006;95(6):1228–1233. doi: 10.1002/bit.21071. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Zeiser A, Bedard C, Voyer R, Jardin B, Tom R, Kamen AA. On-line monitoring of the progress of infection in Sf-9 insect cell cultures using relative permittivity measurements. Biotechnol Bioeng. 1999;63(1):122–126. doi: 10.1002/(SICI)1097-0290(19990405)63:1<122::AID-BIT13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zeiser A, Elias CB, Voyer R, Jardin B, Kamen AA. On-line monitoring of physiological parameters of insect cell cultures during the growth and infection process. Biotechnol Prog. 2000;16(5):803–808. doi: 10.1021/bp000092w. [DOI] [PubMed] [Google Scholar]