Abstract
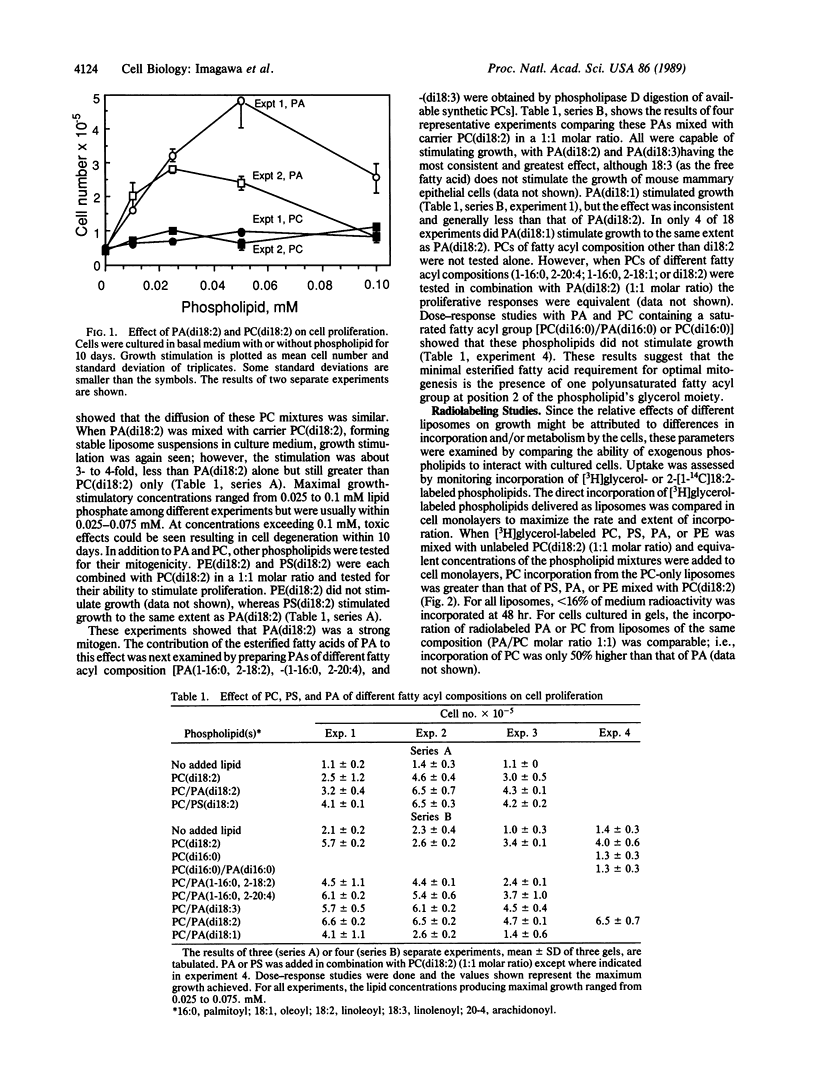
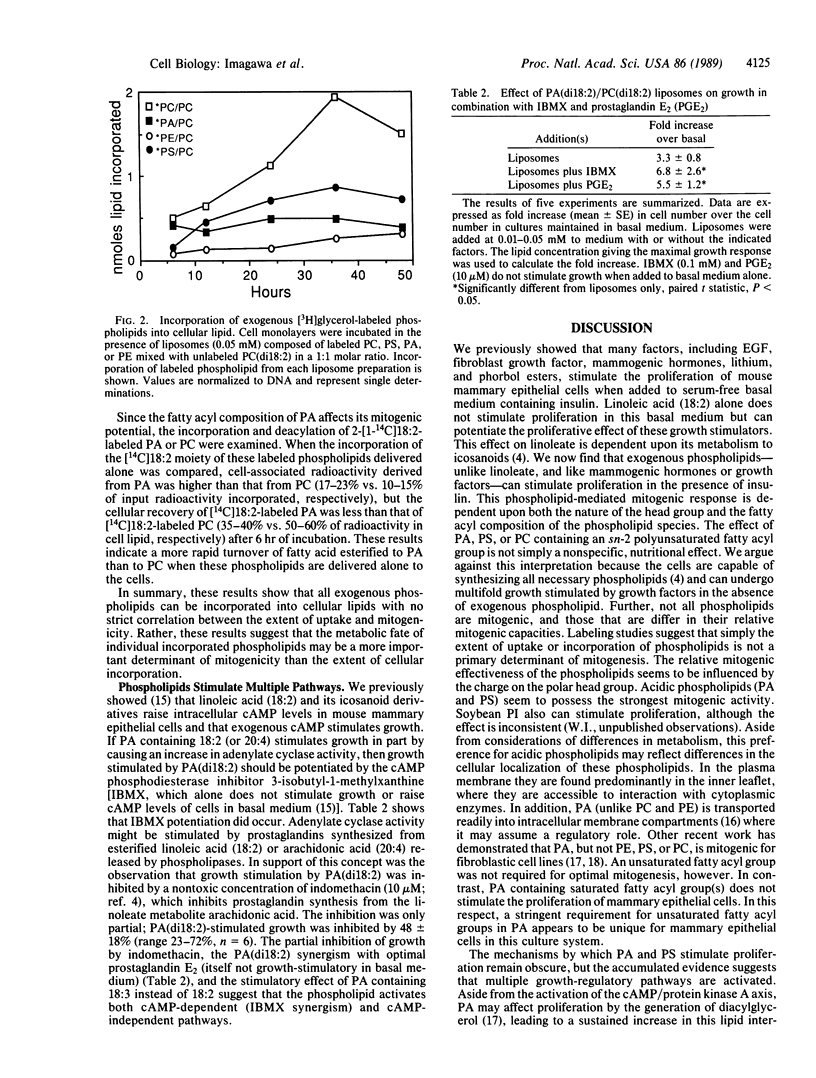
Epithelial cells obtained by collagenase digestion of mammary glands from virgin BALB/c mice were cultured in collagen gels in serum-free basal medium containing insulin (10 micrograms/ml), to which lipids or growth factors were added. Synthetic phospholipids were added as liposomes. Dilinoleoyl phosphatidic acid or phosphatidylserine or epidermal growth factor stimulated multifold growth. The optimum mitogenic effect of the phospholipids was dependent upon the presence of a polyunsaturated fatty acid esterified to the sn-2 position of the glycerol moiety. Dilinoleoyl phosphatidylcholine also stimulated growth but was generally less stimulatory than phosphatidylserine or phosphatidic acid, and phosphatidylethanolamine did not stimulate growth. Studies using phospholipids radiolabeled in either the sn-2 fatty acyl group or the glycerol backbone showed that the relative effect of phospholipids on growth did not correlate directly with the extent of their incorporation into cellular lipid, indicating that phospholipid turnover was the more important determinant for mitogenesis. Analysis of phosphatidic acid-stimulated growth suggested that both cAMP-dependent and cAMP-independent pathways were involved. Thus, mitogenic phospholipids stimulate proliferation by activating (directly or indirectly) multiple growth-regulatory pathways in mammary epithelial cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. V., Conn P. M. Measurement of inositol phospholipid metabolites by one-dimensional thin-layer chromatography. Methods Enzymol. 1987;141:156–168. doi: 10.1016/0076-6879(87)41064-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Dunbar L. M. Essential fatty acid requirements of cells in tissue culture: a review. Exp Mol Pathol. 1973 Apr;18(2):142–161. doi: 10.1016/0014-4800(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D. R., Nandi S. Proliferative effects of insulin and epidermal growth factor on mouse mammary epithelial cells in primary culture. Enhancement by hydroxyeicosatetraenoic acids and synergism with prostaglandin E2. J Biol Chem. 1988 Jun 5;263(16):7567–7573. [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D., Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987 Feb 25;262(6):2750–2756. [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl H., Kovatchev S. Preparation of phospholipids and their analogs by phospholipase D. Methods Enzymol. 1981;72:632–639. doi: 10.1016/s0076-6879(81)72055-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hamilton R. L., Jr, Goerke J., Guo L. S., Williams M. C., Havel R. J. Unilamellar liposomes made with the French pressure cell: a simple preparative and semiquantitative technique. J Lipid Res. 1980 Nov;21(8):981–992. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Wallace D., Nandi S. Growth stimulation by PGE2 and EGF activates cyclic AMP-dependent and -independent pathways in primary cultures of mouse mammary epithelial cells. J Cell Physiol. 1988 Jun;135(3):509–515. doi: 10.1002/jcp.1041350320. [DOI] [PubMed] [Google Scholar]
- Levay-Young B. K., Bandyopadhyay G. K., Nandi S. Linoleic acid, but not cortisol, stimulates accumulation of casein by mouse mammary epithelial cells in serum-free collagen gel culture. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8448–8452. doi: 10.1073/pnas.84.23.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. F., Stockdale F. E. 3T3-L1 adipocytes promote the growth of mammary epithelium. Exp Cell Res. 1984 Mar;151(1):112–122. doi: 10.1016/0014-4827(84)90361-6. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Sleight R. G. Defining lipid transport pathways in animal cells. Science. 1985 Sep 13;229(4718):1051–1057. doi: 10.1126/science.4035344. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]



