Abstract
Purpose
Renal cell carcinomas (RCC) frequently express the gastrin-releasing peptide receptor (GRP-R). Gastrin-releasing peptide (GRP) stimulates tumor cell proliferation and neoangiogenesis. Tumor-associated macrophages (TAM) comprise an important cellular component of these tumors. We analyzed the GRP/GRP-R network in clear cell RCC (ccRCC) and non-clear cell RCC (non-ccRCC) with special regard to its expression by macrophages, tumor cells and microvessels.
Methods
Gastrin-releasing peptide and GRP-R expression in 17 ccRCC and 9 non-ccRCC were analyzed by RT-PCR, immunohistochemistry and double immunofluorescence staining.
Results
Tumor-associated macrophages expressed GRP and GRP receptor in ccRCC. Tumor cells and microvessels showed low to intermediate GRP-R expression in nearly all cases. In 12 ccRCC tumor epithelia also expressed low levels of GRP. Microvascular GRP expression was found in nine cases of ccRCC. For non-RCC, the expression of GRP and GRP receptor expression pattern was similar.
Conclusions
Tumor-associated macrophages are the main source of GRP in RCC. GRP receptor on TAM, tumor epithelia and microvessels might be a molecular base of a GRP/GRP receptor network, potentially acting as a paracrine/autocrine modulator of TAM recruitment, tumor growth and neoangiogenesis.
Keywords: Tumor-associated macrophages, Renal cell carcinoma, Gastrin-releasing peptide, Growth factor
Introduction
Gastrin-releasing peptide (GRP) is the human analog of the amphibian tetradecapeptide bombesin. McDonald et al. [1] was the first to describe GRP in the gastrointestinal tract of mammals. GRP, originally named for its ability to stimulate the release of gastrin, modulates the secretion of a variety of gastrointestinal hormones and affects pancreatic secretion, intestinal transit, muscle contractility, metabolism and behavior [2]. The expression of GRP is not only restricted to the gastrointestinal tract and it is also widely distributed in the central nervous system.
GRP interacts with four different G-protein coupled receptors: the GRP receptor (subtype I), the neuromedin-B receptor, the Bombesin receptor (subtype 3), and a novel subtype 4 that has been identified in frogs. In humans, GRP binds to the GRP receptor with high affinity and to the subtype 3 receptor with low affinity.
GRP has been identified as an important growth factor not only for neuroendocrine malignancies, such as medullary thyroid carcinomas [3], small cell lung carcinomas [4] and neuroblastomas [5], but also for carcinomas of the breast [6], colon [7], prostate [8] and rat pancreatic cancer [9]. The growth of renal cell carcinoma (RCC) has been linked to the presence of GRP and its receptor by Pansky et al. [10] who stimulated the growth of various RCC cell lines with GRP. Reubi et al. [11] identified the GRP receptor on tumor epithelia in human RCC specimens using a radioactive ligand. We have shown recently that the GRP receptor is not only found on tumor epithelia, but also expressed in microvessels [12]. We likewise demonstrated that the microvascular GRP receptor is potentially relevant for neoangiogenesis in RCC, because microvessel formation is inhibited by the GRP receptor antagonist RC-3095 in an animal model of a dorsal skinfold chamber.
Malignant tumors comprise up to 50% stromal cells of which monocytes/macrophages are a quantitative and functional important subpopulation. The tumor microenvironment and hypoxia induce a differentiation of tumor-associated macrophages (TAM) into a phenotype with depressed cytotoxicity [13–15] which may enhance growth and spread of tumor cells [16–18].
Neuropeptides, e.g. GRP, significantly modulate the function of macrophages. The GRP receptor has been found on macrophages, and GRP has an effect on macrophage function such as displayed by an alteration of adhesion, migration and phagocytosis [19, 20]. Interestingly, GRP expression in macrophages has been reported only under inflammatory conditions, such as chronic bronchitis and lung inflammation and fibrosis [21, 22]. In inflammation, the findings of simultaneous peptide and receptor expression suggest an autocrine loop in the macrophage environment that may be important for the macrophage/epithelium interaction.
In the present study, we confirm the presence of GRP and GRP receptor mRNA transcripts in tumor tissue of human clear cell RCC (ccRCC) and non-clear cell RCC (non-ccRCC). Moreover, as novel finding, we co-localize an expression of GRP and GRP-R in TAM and also in microvessels. We conclude that the GRP/GRP receptor system in RCC and TAM may represent a link between growth regulation in the tumor and local immune response.
Materials and methods
Patients and tumors
Seventeen ccRCC and nine non-ccRCC were freshly obtained at the time of surgery from the urological department of our institution. Approval of the ethics committee had been obtained before initiation of the study. Histopathological data are summarized in Table 1.
Table 1.
Synopsis of clinicopathological data and expression analysis of ccRCC and non-ccRCC
| CcRCC | Grade | pTNM | Tumor cells | Stromal cells | RNA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophages | Vessels | ||||||||||
| GRP | GRP-R | GRP | GRP-R | GRP | GRP-R | GRP | GRP-R | PBGD | |||
| Case | |||||||||||
| 1 | G1 | pT1b, Nx, Mx | 1 | 1 | 1 | n.a. | 0 | 3 | 2 | 2 | 2 |
| 2 | G1 | pT1a, Nx, Mx | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 3 |
| 3 | G1 | pT1b, Nx, Mx | 0 | 2 | 3 | 2 | 1 | 2 | 2 | 3 | 2 |
| 4 | G2 | pT2, Nx, M1 | 0 | 2 | 3 | 3 | 1 | 2 | 3 | 3 | 3 |
| 5 | G2 | pT3a, N0, Mx | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 |
| 6 | G2 | pT2, Nx, M1 | 1 | 1 | 2 | 3 | 0 | 1 | 3 | 3 | 2 |
| 7 | G2 | pT1b, Nx, Mx | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 2 | 2 |
| 8 | G2 | pT2, Nx, Mx | 0 | 1 | 3 | 2 | 0 | 1 | 2 | 2 | 2 |
| 9 | G2 | pT3a, Nx, Mx | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 2 |
| 10 | G2 | pT3b, Nx, Mx | 1 | 1 | 1. | 2 | 1 | 1 | 2 | 2 | 2 |
| 11 | G2-3 | pT3b, Nx, Mx | 1 | 1 | 0 | 2 | 0 | 1 | Degraded RNA | ||
| 12 | G2-3 | pT3b, Nx, Mx | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| 13 | G2-3 | pT2, Nx, Mx | 0 | 2 | 2 | 2 | 0 | 1 | 3 | 3 | 2 |
| 14 | G3 | pT3b, Nx, M1 | 1 | 1 | 2 | 2 | 2 | 1 | 3 | 3 | 2 |
| 15 | G3 | pT3b, Nx, Mx | 1 | 1 | 3 | 3 | 1 | 2 | 2 | 3 | 2 |
| 16 | G2 | pT1b, Nx, Mx | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 2 |
| 17 | pT3b, Nx, M1 | 1 | 1 | 1 | 2 | 0 | 1 | 3 | 2 | 3 | |
| Non-ccRCC | Tumor type | pTNM | Tumor cells | Stromal cells | RNA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophages | Vessels | ||||||||||
| GRP | GRP GRP-R | GRP | GRP-R | GRP | GRP-R | GRP | GRP-R | PBGD | |||
| Case | |||||||||||
| 18 | pap | pT2, Nx, Mx | 2 | 1 | 0 | 1 | 0 | 1 | 3 | 2 | 3 |
| 19 | chr | pT3b, N2, M1 | 0 | 2 | 3 | 3 | 2 | 3 | 2 | 3 | 2 |
| 20 | sarc | pT4, Nx, Mx | 1 | 1 | 2 | 3 | 0 | 1 | 2 | 2 | 2 |
| 21 | sarc | pT3a, N2, Mx | 1 | 2 | 3 | 3 | 0 | 2 | 2 | 2 | 3 |
| 22 | sarc | pT2, N0, Mx | 0 | 3 | 2 | 2 | 0 | 1 | 3 | 3 | 2 |
| 23 | sarc | pT3a, N2, M1 | 0 | 1 | 3 | 3 | 0 | 1 | 3 | 2 | 3 |
| 24 | sarc | pT3a, Nx, M1 | 2 | 2 | 2 | 3 | 0 | 1 | 0 | 1 | 1 |
| 25 | db | pT3b, N2, Mx | 1 | 3 | 3 | 2 | 0 | 2 | 0 | 2 | 3 |
| 26 | db | pT3a, Nx, Mx | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 3 | 2 |
pap papillary carcinoma, sarc sarcomatoid carcinoma. db Duct Bellini carcinoma, n.a. not applicable
Immunohistochemistry and immunofluorescence
Tissues were fixed in HOPE™ (Hepes–glutamic acid buffer-mediated organic solvent protection effect) solution (DCS Diagnostic Systems, Hamburg, Germany) and embedded in paraffin according to the manufacturer’s instructions. Tissue sections (3 μm) were mounted on glass slides, de-paraffinized, rehydrated and subjected to immunohistochemistry. Sections were incubated with a polyclonal rabbit anti-gastrin-releasing peptide antibody (1:50 dilution, Dako, Hamburg Germany) or polyclonal rabbit anti-gastrin-releasing peptide receptor (GRP-R) antibody (1:200 dilution; kindly donated by Dr. Battey, NIDCD, Rockville, MD) at room temperature for 2 h. After several washing steps, the sections were subsequently incubated with Envision™ peroxidase (Dako) for 30 min. Signals were detected with diaminobenzidine as substrate (Dako). Finally, sections were counterstained with hematoxylin, dehydrated with ethanol and xylene and covered with cover slips.
Immunohistochemical staining for GRP and its receptor was semiquantitatively analyzed by means of an immunoreaction score: 0, no staining; 1 staining <10% of cells; 2 10–50% of cells stained; 3 >50% of cells stained.
For double immunofluorescence analysis, sections were incubated with a mixture of anti-GRP (1:25) and CD68 (1:3,000, Dako Clone KP-1) at 4°C overnight. Anti-CD 68 antibody was detected with an anti-mouse Cy3 conjugate (1:500 dilution for 1 h at RT). Subsequently, the sections were incubated with anti-rabbit Envision-Peroxidase (Dako) for 1 h at RT and incubated with tyramide-FITC to detect GRP (tyramid signal amplification, PerkinElmer/NEN, Rodgau, Germany). Finally, sections were mounted with fluoromount (Dako). Immunofluorescence stainings were visualized by means of an Axiophot fluorescence microscope (Zeiss, Göttingen, Germany) and a fluorescence camera (Fview, Olympus, Hamburg, Germany).
mRNA expression analysis
Paraffin sections of HOPE-fixed tissue were cut under RNAse-free conditions and transferred to reaction tubes. The sections were de-paraffinized with three changes of isopropanol at 60°C for at least 90 min. The sections were washed with absolute ethanol, air dried and lysed in lysis buffer applying the RNAeasy mini kit (Qiagen, Hilden, Germany), and RNA was extracted according to the manufacturer’s instructions. The quality and quantity of isolated RNA were tested in an automated capillary electrophoresis system (Agilent BioAnalyzer 2100; Agilent Technologies, Waldbronn, Germany). A maximum of 500 ng of total RNA was reverse transcribed using random hexamer primers (Invitrogen, Karlsruhe, Germany) and Omniscript™ reverse transcriptase (Qiagen) in a 20 μl volume (25 ng RNA/μl) at 37°C for 60 min according to the manufacturer′s instructions.
A 25 ng of cDNA was subjected to PCR analysis in a total volume of 20 μl using Hotstart Mastermix (Qiagen) and primer pairs with conditions as summarized in Table 2. Porphobilinogen deaminase was used as a house-keeping gene which is constantly expressed in renal carcinomas. PCR products were separated on a 1.5% agarose gel, stained with ethidium bromide and analyzed on a UV transilluminator. The used primers are given in Table 2.
Table 2.
Used primers in PCR
| Gene | Sequence (5′–3′) | T m (°C) | Product length (bp) |
|---|---|---|---|
| GRP | |||
| fw | tgggcggtggggcactta | 64 | 286 |
| rv | tcagctgggggttccttcct | ||
| GRP-R | |||
| fw | ctccccgtgaacgatgactgg | 61 | 389 |
| rv | atcttcatcagggcatgggag | ||
| PBGD | |||
| fw | tgtctggtaacggcaatgcggctgcaac | 72 | 127 |
| rv | tcaatgttgccaccacactgtccgtct | ||
Statistics
For the analysis of the immunohistological staining, the one-way ANOVA with Dunnett’s post test was performed using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego CA USA, http://www.graphpad.com.
Results
mRNA expression analysis of GRP and GRP receptor in ccRCC
mRNA expression analysis of GRP and GRP receptor was performed in serial sections of the same tissue blocks used for immunohistochemistry. Particularly for GRP, we could demonstrate that GRP is synthesised within the tumor environment of ccRCC and non-ccRCC (Fig. 1; Table 1).
Fig. 1.
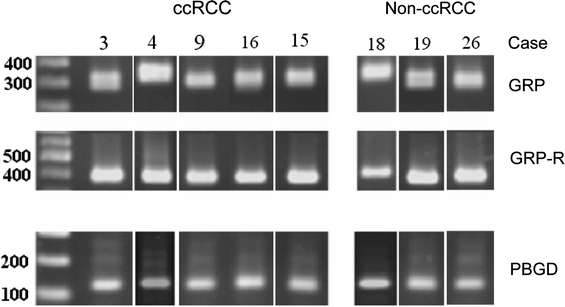
Gel electrophoresis of RT-PCR products of typical cases separated on a 1.5% agarose gel. Blots have been resorted into groups of ccRCC and non-ccRCC. A summary of the cases is shown in Table 1
Immunohistochemical localization of GRP and GRP receptor in ccRCC
In ccRCC tumor cells with a low GRP signal were observed in 12 cases. The mean GRP scores in tumor cells (scoremean 0.71) and tumor vessels (scoremean 0.59) were significantly lower than in TAM (scoremean 1.65) (P < 0.01). Therefore, TAM were the main source of GRP in ccRCC.
Addressing the question which cell types express GRP receptor, a low to high signal intensity was detected in ccRCC. The mean GRP receptor expression was significantly lower in tumor epithelia (scoremean 1.35) than in TAM (scoremean 1.94) (P < 0.0.5) and lower in association with tumor microvessels (scoremean 1.58).
In non-ccRCC, a low GRP signal of tumor cells was detected in three cases and an intermediate signal in two cases. The mean GRP scores in non-ccRCC tumor cells (scoremean 0.78) and tumor vessels (scoremean 0.44) were significantly lower than in TAM (scoremean 2.11) (P < 0.05). For GRP-R expression, non-ccRCC exhibited the same pattern as ccRCC, but no significant difference was detected (data not shown).
When comparing immunohistochemically stained serial sections, we also found GRP and GRP receptor-positive macrophages and GRP receptor-positive tumor cells located in close vicinity (Fig. 2).
Fig. 2.
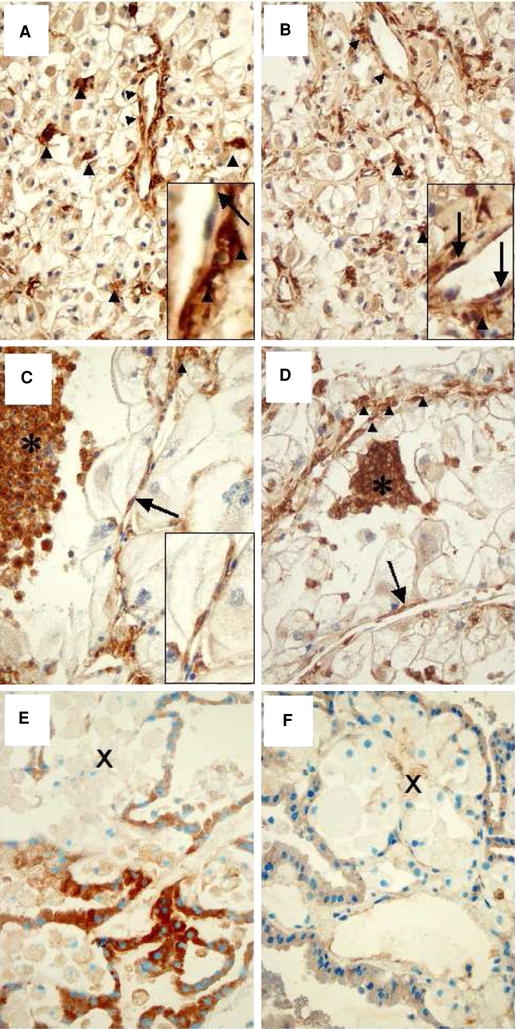
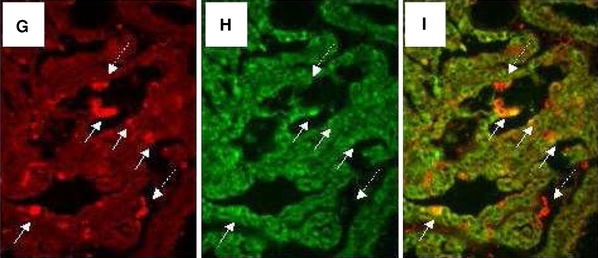
Immunohistochemistry of GRP and GRP receptor of case 15 (ccRCC a, b), 19 (chromophobe RCC c, d) and case 18 (papillary RCC e, f) (×400). GRP expression is shown in a, c, and e. GRP-R expression is demonstrated in b, d, and f. In case 15 (ccRCC), GRP and GRP-R are expressed by tumor cells, microvessels (right arrow), and with highest intensity by interstitial macrophages (filled triangle) which is highlighted in the figure inlets (a, b). In case 19 (chromophobe RCC) GRP is expressed by foamy (asterisk) and perivascular (filled triangle) macrophages, microvessels (right arrow), but not by tumor cells. Endothelial GRP expression is highlighted in the figure inlet (a). In case 19, GRP-R is expressed by foamy (asterisk) and perivascular (filled triangle) macrophages, microvessels (right arrow), and with faint signals by tumor cells (b). In case 18, GRP is expressed by tumor cells, but not by foamy macrophages (X) (c). GRP-R signals are only faintly seen in macrophages (X) and tumor cells (d). For detailed description see Table 1. g–i Double immunofluorescence staining of GRP and CD68 in a papillary renal carcinoma, case 18 (×400). CD68-positive interstitial macrophages are shown in g (red fluorescence). GRP signals are demonstrated in h (green fluorescence). Overlay of g and h confines that interstitial macrophages express GRP (i)
To confirm this, we performed double immunofluorescence staining with CD 68 and GRP antibodies macrophages within RCC expressed both proteins on their surface as demonstrated by the orange composite signaling in confocal microscopy (Fig. 2).
Comparative analyses of the degree of differentiation, and the tumor stage did not reveal any correlation. The data on all patients are summarized in Table 1.
Analysis of tumor-free tissues of nine cases revealed a strong GRP and GRP-R expression in tubules and collecting ducts in contrast to peritubular capillaries where no expression could be found. No GRP or GRP receptor-positive macrophages were found in tumor-free tissue.
Discussion
The present study provides evidence that GRP and its high-affinity receptor are simultaneously expressed in renal carcinomas. The staining pattern of tumor cells and microvascular endothelia confirmed our previous results on the GRP receptor expression in 18 ccRCC [12]. In the present study, we used HOPE fixation, which allowed the simultaneous detection of specific mRNA transcripts and of proteins by means of RT-PCR and immunohistochemistry in 17 ccRCC and 9 non-ccRCC.
In most cases, we found a co-expression of mRNA and protein. Only in cases with RNA degradation and hypoxic necrosis differences between mRNA and protein expression were observed. Protein expression was located nearby or within the necrotic rims. The absence of mRNA expression for GRP can be explained by an advanced degradation of mRNA, even in cases with detectable PBGD mRNA which is abundantly expressed in renal carcinomas.
Immunohistochemical analysis of the GRP receptor expression has been described previously. Detection of the ligand using a polyclonal GRP antiserum has also been reported [23, 24]. We found cytoplasmatic signals for GRP, particularly in TAM and in lower intensity in tumor epithelia. Endothelial expression was found at low levels in few cases, predominately in ccRCC. GRP receptor expression analysis was positive, particularly in TAM and microvessels and with lower intensity in tumor cells of all cases. The discontinuous and granular staining for the GRP receptor was in contrast to the study of Scott et al. [25] who found a more homogeneous cytoplasmatic staining for the GRP receptor in carcinoid tumor epithelia, whereas the staining pattern for GRP corresponded well to our data. GRP and GRP expression has been analyzed in a broad number of neoplasias.
Up til now, investigators did not pay attention on the expression of GRP and GRP-R on macrophages and microvessels. The expression of bombesin/GRP in immune cells has been identified in circulating peripheral monocytes and in alveolar macrophages [26]. Furthermore, GRP acts as chemoattractant for murine peritoneal macrophages [20] and potentially stimulates cytotoxic natural killer cells both in vivo and in vitro [27]. In contrast, Meloni et al. [26] showed that bombesin/GRP stimulates the release of interleukin-8 (IL-8) by peripheral monocytes and may thereby promote neoangiogenesis and tumor growth indirectly. In renal carcinomas, a high number of infiltrating monocytes and macrophages have already been identified [28]. The stimulation of the GRP-R induced the release of interleukin-8 and vascular endothelial growth factor in human prostate cancer cell lines [29].
The detection of the GRP-R on tumor microvessels underlines the importance of GRP for the progression of renal carcinomas and suggests the presence of an autocrine or paracrine loop within the tumor. We have shown previously that the density of perfused microvessels and tumor blood flow are reduced if a specific GRP receptor antagonist is administered into nude mice bearing subcutaneously implanted renal carcinomas [12]. However, our finding has been questioned by Busby et al. [30] who demonstrated that bombesin/GRP did not have any mitogenic effect on isolated human vascular endothelial cells (HUVEC). This discrepancy may be explained by the fact that HUVEC originate from the umbilical cord and the findings that we have also identified GRP receptors on tumor pericytes that may also be involved in the proliferation of endothelia.
In conclusion, our study confirms the presence of both GRP and GRP receptor in ccRCC. TAM are the main source of intratumoral GRP. The results suggest a paracrine/autocrine signaling within renal carcinomas between TAM, tumor epithelia and microvessels; whereas, the functional relevance of GRP receptors in microvessels and tumor epithelia has been investigated, the significance and the function of the GRP receptor expression in macrophages needs further elucidation.
Conflict of interest statement
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
J. Bedke and B. Hemmerlein contributed equally to this work.
References
- 1.McDonald TJ, et al. A gastrin releasing peptide from the porcine nonantral gastric tissue. Gut. 1978;19:767–774. doi: 10.1136/gut.19.9.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh J, Dockray GJ, Bunnet et al (1994) Gut peptides. In: Walsh J, Dockray GJ (eds) Gastrin releasing peptide. Raven Press, New York, pp 423−445
- 3.Modigliani E, et al. Immunoreactive gastrin-releasing peptide in medullary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:831–835. doi: 10.1210/jcem-71-4-831. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SM, et al. Gastrin-releasing peptide receptor mediates activation of the epidermal growth factor receptor in lung cancer cells. Neoplasia. 2005;7:426–431. doi: 10.1593/neo.04454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafson WC, et al. Role of gastrointestinal hormones in neuroblastoma. World J Surg. 2005;29:281–286. doi: 10.1007/s00268-004-7815-4. [DOI] [PubMed] [Google Scholar]
- 6.Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am J Pathol. 1999;155:2067–2076. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartsmann G, et al. MAPK pathway activation in colorectal cancer: a therapeutic opportunity for GRP receptor antagonists. Lancet Oncol. 2005;6:444–445. doi: 10.1016/S1470-2045(05)70226-6. [DOI] [PubMed] [Google Scholar]
- 8.Lacoste J, Aprikian AG, Chevalier S. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol Cell Endocrinol. 2005;235:51–61. doi: 10.1016/j.mce.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Schuhmacher J, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68 Ga-labeled bombesin(6–14) analog. J Nucl Med. 2005;46:691–699. [PubMed] [Google Scholar]
- 10.Pansky A, et al. Gastrin releasing peptide-preferring bombesin receptors mediate growth of human renal cell carcinoma. J Am Soc Nephrol. 2000;11:1409–1418. doi: 10.1681/ASN.V1181409. [DOI] [PubMed] [Google Scholar]
- 11.Reubi JC, et al. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 12.Heuser M, et al. Expression of gastrin releasing peptide receptor in renal cell carcinomas: a potential function for the regulation of neoangiogenesis and microvascular perfusion. J Urol. 2005;173:2154–2159. doi: 10.1097/01.ju.0000158135.26893.bc. [DOI] [PubMed] [Google Scholar]
- 13.Daniliuc S, et al. Hypoxia inactivates inducible nitric oxide synthase in mouse macrophages by disrupting its interaction with alpha-actinin 4. J Immunol. 2003;171:3225–3232. doi: 10.4049/jimmunol.171.6.3225. [DOI] [PubMed] [Google Scholar]
- 14.Leek RD, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 15.Siegert A, et al. Suppression of the reactive oxygen intermediates production of human macrophages by colorectal adenocarcinoma cell lines. Immunology. 1999;98:551–556. doi: 10.1046/j.1365-2567.1999.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Crowther M, et al. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 18.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 19.De la Fuente M, et al. Modulation of phagocytic function in murine peritoneal macrophages by bombesin, gastrin-releasing peptide and neuromedin C. Immunology. 1991;73:205–211. [PMC free article] [PubMed] [Google Scholar]
- 20.Del Rio M, De la Fuente M. Chemoattractant capacity of bombesin, gastrin-releasing peptide and neuromedin C is mediated through PKC activation in murine peritoneal leukocytes. Regul Pept. 1994;49:185–193. doi: 10.1016/0167-0115(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire I. Bombesin-related peptides modulate interleukin-1 production by alveolar macrophages. Neuropeptides. 1991;20:217–223. doi: 10.1016/0143-4179(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire I, Jones S, Khan MF. Bombesin-like peptides in alveolar macrophage: increased release in pulmonary inflammation and fibrosis. Neuropeptides. 1991;20:63–72. doi: 10.1016/0143-4179(91)90041-G. [DOI] [PubMed] [Google Scholar]
- 23.Glover S, et al. Transient upregulation of GRP and its receptor critically regulate colon cancer cell motility during remodeling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1274–G1282. doi: 10.1152/ajpgi.00108.2004. [DOI] [PubMed] [Google Scholar]
- 24.Matkowskyj KA, et al. Expression of GRP and its receptor in well-differentiated colon cancer cells correlates with the presence of focal adhesion kinase phosphorylated at tyrosines 397 and 407. J Histochem Cytochem. 2003;51:1041–1048. doi: 10.1177/002215540305100807. [DOI] [PubMed] [Google Scholar]
- 25.Scott N, et al. Gastrin releasing peptide and gastrin releasing peptide receptor expression in gastrointestinal carcinoid tumours. J Clin Pathol. 2004;57:189–192. doi: 10.1136/jcp.2003.10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meloni F, et al. Bombesin enhances monocyte and macrophage activities: possible role in the modulation of local pulmonary defenses in chronic bronchitis. Respiration. 1996;63:28–34. doi: 10.1159/000196512. [DOI] [PubMed] [Google Scholar]
- 27.van Tol EA, et al. Intravenous administration of bombesin in man stimulates natural killer cell activity against tumour cells. Neuropeptides. 1991;18:15–21. doi: 10.1016/0143-4179(91)90158-F. [DOI] [PubMed] [Google Scholar]
- 28.Hemmerlein B, et al. Expression of acute and late-stage inflammatory antigens, c-fms, CSF-1, and human monocytic serine esterase 1, in tumor-associated macrophages of renal cell carcinomas. Cancer Immunol Immunother. 2000;49:485–492. doi: 10.1007/s002620000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine L, et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63:3495–3502. [PubMed] [Google Scholar]
- 30.Busby JE, et al. Angiogenesis is not mediated by prostate cancer neuropeptides. Angiogenesis. 2003;6:289–293. doi: 10.1023/B:AGEN.0000029409.94626.64. [DOI] [PubMed] [Google Scholar]


