Abstract
Hyponatremia is the most common electrolyte abnormality encountered in children. In the past decade, new advances have been made in understanding the pathogenesis of hyponatremic encephalopathy and in its prevention and treatment. Recent data have determined that hyponatremia is a more serious condition than previously believed. It is a major comorbidity factor for a variety of illnesses, and subtle neurological findings are common. It has now become apparent that the majority of hospital-acquired hyponatremia in children is iatrogenic and due in large part to the administration of hypotonic fluids to patients with elevated arginine vasopressin levels. Recent prospective studies have demonstrated that administration of 0.9% sodium chloride in maintenance fluids can prevent the development of hyponatremia. Risk factors, such as hypoxia and central nervous system (CNS) involvement, have been identified for the development of hyponatremic encephalopathy, which can lead to neurologic injury at mildly hyponatremic values. It has also become apparent that both children and adult patients are dying from symptomatic hyponatremia due to inadequate therapy. We have proposed the use of intermittent intravenous bolus therapy with 3% sodium chloride, 2 cc/kg with a maximum of 100 cc, to rapidly reverse CNS symptoms and at the same time avoid the possibility of overcorrection of hyponatremia. In this review, we discuss how to recognize patients at risk for inadvertent overcorrection of hyponatremia and what measures should taken to prevent this, including the judicious use of 1-desamino-8d-arginine vasopressin (dDAVP).
Keywords: Hyponatremia, Encephalopathy, Cerebral edema, Pulmonary edema, Fluid therapy, Saline, Sodium chloride, Myelinolysis, Arginine vasopressin
Introduction
Hyponatremia is one of the most common electrolyte abnormalities encountered in children, with mild hyponatremia, serum sodium (SNa) <135 mEq/L, occurring in ∼25% of hospitalized children and moderate hyponatremia, SNa <130 mEq/L, in ∼1% (Table 1). Recent data in both children and adults have demonstrated that hyponatremia is a far more serious condition than previously believed. The most serious complication of hyponatremia is hyponatremic encephalopathy. Hyponatremic encephalopathy is a topic that has been mired in controversy. The main disputes have centered on (1) the most appropriate fluid management strategies to prevent hyponatremic encephalopathy, (2) the optimal therapy for symptomatic hyponatremia, and (3) the risks of developing cerebral demyelination from the correction of hyponatremia. In this review, we discuss new aspects in the pathogenesis of hyponatremic encephalopathy with an emphasis on strategies for prevention and treatment.
Table 1.
Incidence of hyponatremia in hospitalized children
| Author | Inclusion criteria serum sodium (mEq/L) | Incidence (%) |
|---|---|---|
| Hasegawa et al. 2009 [121] | <135 on admission | 17 |
| Don et al. 2008 [122] | <135 on admission with community-acquired pneumonia | 45 |
| Hoorn et al. et al. 2004 [123] | <135 in emergency department patients with serum sodium checked | 22 |
| Armon et al. 2008 [124] | Hospitalized patients on intravenous fluids | |
| <135 | 24 | |
| <130 | 5 | |
| Wattad et al. 1992 [125] | <130 in hospitalized patients | 1.4 |
Why does hyponatremia develop?
Under normal circumstances, the human body can maintain plasma sodium levels within the normal range (135–145 mEq/L), even with wide fluctuations in fluid intake. The body’s primary defense against developing hyponatremia is the kidney’s ability to generate a dilute urine and excrete free water. Rarely is excess ingestion of free water alone the cause of hyponatremia, as an adult with normal renal function can typically excrete >15 L of free water per day [1]. It is also rare to develop hyponatremia from excess urinary sodium loss in the absence of free-water ingestion. In order for hyponatremia to develop, there must typically be a relative excess of free water in conjunction with an underlying condition that impairs the kidney’s ability to excrete free water (see Table 2). Excretion of free water will be impaired when there is either (1) a marked reduction in glomerular filtration rate, (2) renal hypoperfusion, or (3) arginine vasopressin (AVP) excess. Most cases of hyponatremia are the result of increased AVP production.
Table 2.
Disorders in impaired renal water excretion
| 1. Effective circulating volume depletion |
| a) Gastrointestinal losses: vomiting, diarrhea |
| b) Skin losses: cystic fibrosis |
| c) Renal losses: salt-wasting nephropathy, diuretics, cerebral salt wasting, hypoaldosteronism |
| d) Edematous states: heart failure, cirrhosis, nephrosis, hypoalbuminemia |
| e) Decreased peripheral vascular resistance: sepsis, hypothyroidism |
| 2. Thiazide diuretics |
| 3. Renal failure |
| a) Acute |
| b) Chronic |
| 4. Non-hypovolemic states of antidiuretic hormone (ADH) excess |
| a) Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) |
| b) Nausea, emesis, pain, stress |
| c) Post-operative state |
| d) Cortisol deficiency |
| 5. Nephrogenic syndrome of inappropriate antidiuresis (NSIAD) |
Renal water handling is primarily under the control of AVP, which is produced in the hypothalamus and released from the posterior pituitary. The action of AVP is mediated via the vasopressin V2 receptor. AVP binding of V2 receptors results in the insertion of aquaporin 2 (AQP2) water channels on the apical surface of the principal cells of the cortical collecting duct, which markedly increases water permeability and impairs water excretion [2]. There are osmotic, hemodynamic, and nonhemodynamic stimuli for AVP release. The body will attempt to preserve extracellular volume at the expense of SNa. Therefore, a hemodynamic stimulus for AVP production will override any inhibitory hypo-osmolar effect of hyponatremia [3]. There are numerous hemodynamic and nonhemodynamic stimuli for AVP production (Table 1) that occur in hospitalized patients and that can put virtually any hospitalized patient at risk for hyponatremia. In order for hyponatremia to develop in the presence of AVP excess, there must be an additional source of free-water intake, either intravenous or oral. Even isotonic fluid administration could in theory result in hyponatremia in the presence of AVP excess, as the kidney can generate free water by excreting a hypertonic urine, i.e. a urine sodium plus potassium concentration greater than that of the plasma [4].
Hyponatremia typically results from the combination of AVP excess plus free water intake.
Is asymptomatic hyponatremia a benign condition?
Increased attention has been focused on the possible deleterious consequences of asymptomatic hyponatremia. Most of this data comes from adult studies, but pediatric data are starting to appear. Recent data have revealed that hyponatremia is an independent predictor of mortality in adult patients with a variety of diseases, in particular, patients with pneumonia, congestive heart failure, and end-stage liver disease [5–7]. It has recently been demonstrated that mild chronic hyponatremia (mean SNa 128 mEq/L) in adults can result in subtle neurological impairment affecting both gait and attention, similar to that of moderate alcohol intake [8]. This appears to explain why hyponatremia has come to be associated with falls and bone fractures in the elderly [8–10].
There are data to suggest that hyponatremia has deleterious consequences in the preterm neonate, though data are lacking in older children. Preterm neonates with hyponatremia show impaired growth and development compared with those who have been salt supplemented [11, 12] and have increased sodium intake as adolescents [13]. Hyponatremia is also a significant risk factor for sensorineural hearing loss [14], cerebral palsy [15], and intracranial hemorrhage [16]. Hyponatremia has been shown to be a risk factor for increased mortality in neonates who suffered perinatal birth asphyxia [17].
Asymptomatic hyponatremia in adults is associated with attention and gait abnormalities, falls and fractures, and increased mortality in patients with pneumonia, heart failure and liver disease.
Asymptomatic hyponatremia in preterm neonates is associated with poor growth and development, sensorineural hearing loss, and increased sodium intake in later life.
What are the clinical features of hyponatremic encephalopathy?
Hyponatremic encephalopathy is a medical emergency that can be lethal. The pathogenesis and epidemiology of hyponatremic encephalopathy have been reviewed in detail by us elsewhere [18–20]. The primary symptoms of hyponatremia are those of cerebral edema (see Table 3). Hypoosmolality results in intracellular or cytotoxic cerebral edema caused by the influx of water into the intracellular space down a concentration gradient, resulting in parenchymal brain swelling. Cerebral edema results in increased intracranial pressure that can lead to brain ischemia, herniation, and death. The brain’s primary mechanism in adapting to hyponatremia is the intracellular extrusion of electrolytes and organic osmolytes. Some of these organic osmolytes are excitatory amino acids, such as glutamate and aspartate, that can produce seizures in the absence of detectable cerebral edema [21].
Table 3.
Clinical symptoms of hyponatremic encephalopathy
| 1. Early |
| a. Headache |
| b. Nausea and vomiting |
| c. Lethargy |
| d. Weakness |
| e. Confusion |
| f. Altered consciousness |
| g. Agitation |
| h. Gait disturbances |
| 2. Advanced |
| a. Seizures |
| b. Coma |
| c. Apnea |
| d. Pulmonary edema |
| e. Decorticate posturing |
| f. Dilated pupils |
| g. Anisocoria |
| h. Papilledema |
| i. Cardiac arrhythmias |
| j. Myocardial ischemia |
| k. Central diabetes insipidus |
Hyponatremic encephalopathy can be difficult to recognize, as the presenting symptoms are variable and can be nonspecific (see Table 3). The only universal presenting features of hyponatremic encephalopathy are headache, nausea, vomiting, and lethargy. These symptoms can easily be overlooked, as they occur in a variety of conditions. There must be a high index of suspicion for diagnosing hyponatremic encephalopathy, as the progression from mild to advanced symptoms can be abrupt and does not follow a consistent progression. A cranial computed tomography (CT) scan cannot consistently be used to rule out hyponatremic encephalopathy, as it is not sensitive enough to detect mild cerebral edema that could be detected by diffusion-weighted magnetic resonance imaging (MRI) [22, 23].
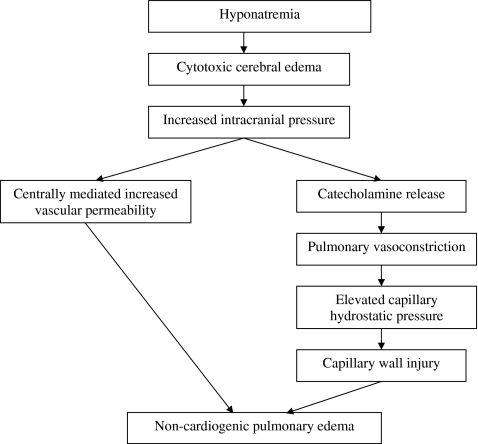
A common yet underrecognized feature of hyponatremic encephalopathy is noncardiogenic pulmonary edema, also referred to as Ayus-Arieff syndrome [24, 25]. Cerebral edema leads to increased intracranial pressure, which can result in pulmonary edema via two mechanisms: (1) centrally mediated increase in pulmonary vascular permeability to proteins, leading to increased alveolar and interstitial fluid [26], and (2) increased sympathetic neuronal activity with catecholamine release, resulting in pulmonary vasoconstriction with increased capillary hydrostatic pressure and capillary wall injury (Fig. 1) [27, 28]. This has primarily been reported in patients with postoperative hyponatremic encephalopathy and exercise-associated hyponatremia [29, 30]. It is important to recognize this syndrome, as it is rapidly reversible with hypertonic saline and is almost universally fatal if left untreated.
The most consistent clinical features of hyponatremic encephalopathy are headache, nausea, and vomiting.
Noncardiogenic pulmonary edema is an underrecognized feature of hyponatremic encephalopathy.
Hyponatremic encephalopathy can occur in the absence of CT evidence of cerebral edema.
Fig. 1.
Mechanism of noncardiogenic pulmonary edema in hyponatremic encephalopathy
What are the risks factors for developing hyponatremic encephalopathy?
Here we describe the major risk factors for hyponatremic encephalopathy development (Table 4).
Table 4.
Risk factors for developing hyponatremic encephalopathy
| 1) Impaired brain cell volume regulation and decreased cerebral perfusion |
| a) Elevated AVP levels |
| b) Female sex steroids |
| c) Hypoxia |
| 2) Decreased cranial capacity |
| a) Children <16 years |
| b) Space-occupying brain lesion |
| i) Tumor |
| ii) Hematoma/hemorrhage |
| c) Hydrocephalus |
| i) Chiari malformation |
| ii) Dandy Walker |
| 3) Central nervous system disorders (cytotoxic and vasogenic cerebral edema) |
| a) Infections |
| i) Meningitis/encephalitis |
| b) Encephalopathy |
| i) Metabolic |
| (1) Diabetic ketoacidosis |
| (2) Hyperammonemia |
| (3) Bilirubin |
| ii) Hepatic |
| iii) Ischemic |
| iv) Toxic |
| c) Cerebritis |
| d) Brain injury and neurosurgery |
| e) Seizure disorders |
Children
It is important to realize that children are at significantly higher risk than are adults for developing hyponatremic encephalopathy. The average SNa in children with hyponatremic encephalopathy is 120 mEq/L [31, 32], whereas that in adults is 111 mEq/L [9, 31–33]. More than 50% of children with an SNa <125 mEq/L will develop hyponatremic encephalopathy [20]. The reason for this is that children have a relatively larger brain to intracranial volume ratio compared with adults [34, 35]. A child’s brain reaches adult size by 6 years of age, whereas the skull does not reach adult size until 16 years of age [36, 37]. As a result, children have less room available in their rigid skulls for brain expansion and are likely to develop brain herniation from hyponatremia at higher SNa concentrations than adults. The fontanelles in infants appear to offer little protection, as the incidence of hyponatremic encephalopathy in infants is quite high [38].
Factors that impair brain-cell-volume regulation and decrease cerebral perfusion: female sex steroids, elevated AVP, and hypoxia
There are various factors that can impair the normal brain regulatory volume decrease and place patients at increased risk for the development of hyponatremic encephalopathy, independent of the degree of hyponatremia or the rate of fall in SNa. The primary factors are female sex steroids, elevated AVP levels, and hypoxia. Women in their reproductive years are at high risk for developing hyponatremic encephalopathy [33]. The reason for this appears to be that estrogens impair brain-cell-volume regulation by reducing the sodium/potassium/adenosine triphosphatase (Na+/K+/ATPase) pump activity, thereby inhibiting sodium extrusion from brain astrocytes. Androgens, on the other hand, appear to enhance Na+/K+/ATPase pump activity and confer a protective role in men [18]. Another reason that women are more susceptible to hyponatremic encephalopathy is that the vasoconstrictive effects of AVP are more pronounced in the female brain than in that of the male brain. AVP excess leads to cerebral vasoconstriction with corresponding decreased oxygen delivery [18]. AVP is known to increase brain-water content in the absence of hyponatremia and to impair brain regulatory volume mechanisms [39–41]. Postoperative patients are at particularly high risk for developing hyponatremic encephalopathy, and this may be explained in part by the high AVP levels associated with surgery [33]. Hypoxemia is a major risk factor for developing hyponatremic encephalopathy. The occurrence of a hypoxic event such as respiratory insufficiency is a major factor militating against survival without permanent brain damage in patients with hyponatremia [42]. The combination of systemic hypoxemia and hyponatremia is more deleterious than is either factor alone because hypoxemia impairs the ability of the brain to adapt to hyponatremia, leading to a vicious cycle of worsening hyponatremic encephalopathy [43]. Studies of hyponatremic animals have revealed that hypoxia impairs brain-cell-volume regulation, decreases cerebral perfusion, and increases the probability of developing neuronal lesions [44].
Underlying CNS disease
Hyponatremia is poorly tolerated in patients with central nervous system (CNS) disorders [45]. Even a small fall in SNa can aggravate cerebral edema and increase intracranial pressure (ICP) [46–48]. CNS pathology can lead to increased ICP from space-occupying brain lesions, hydrocephalus, or cerebral edema. Cerebra edema can occur via two mechanisms: vasogenic and cytotoxic. Vasogenic edema is the accumulation of fluid in the extracellular brain parenchyma from a disruption in the blood−brain barrier, such as is seen with a brain tumor, abscess, or meningitis. Cytotoxic cerebral edema is the accumulation of fluid in the intracellular space, which is seen with hypoxic brain injury, metabolic encephalopathy, and hyponatremia [49]. These mechanisms are not mutually exclusive. A patient with a CNS disorder will already be at risk for increased ICP and have impaired brain-cell-volume regulation. The additional water movement into the brain from even mild hyponatremia can be lethal.
It has been demonstrated that in children with a variety of neurologic diseases that hyponatremia is associated with prolonged hospital stay and poor neurologic outcome [50]. Similar findings have been reported in adults with traumatic brain injury [51]. In a study of children with Lacrosse encephalitis, mild hyponatremia was strongly associated with neurological deterioration [46]. SNa of patients with neurological deterioration was only 2 mmol/L less than in those without (131.9 vs. 133.8 mmol), and a fall in SNa of only 4 mmol/L resulted in neurological deterioration. In a study in children with pneumococcal meningitis, the mortality was 100% for those who presented with SNa <130 mEq/L [52]. Hyponatremia has been reported to lead to progressive cerebral edema in children with maple-syrup-urine disease during episodes of acute metabolic intoxication [53]. Mild hyponatremia, SNa <135 mEq/L, also appears to play a role in the development of cerebral edema in patients with diabetic ketoacidosis (DKA). It has been demonstrated on MRI that patients with DKA have evidence of vasogenic cerebral edema [54]. The development of worsening cerebral edema in DKA has been associated with a fall in serum osmolality during therapy and a slower rise in serum osmolality compared with controls [55, 56]. No degree of hyponatremia should be considered safe in a patient with CNS disease.
Major risk factors for developing hyponatremic encephalopathy are: (a) age <16 years, (b) hypoxemia, and (c) CNS disease.
Can hospital-acquired hyponatremia be prevented?
The majority of the morbidity and mortality from hyponatremic encephalopathy has occurred in hospitalized patients receiving hypotonic intravenous fluids, in particular, postoperative patients. In 2003 [57], we proposed that 0.9% sodium chloride (NaCl: Na 154 mEq/L) be administered to prevent hospital-acquired hyponatremia in patients at risk for AVP excess (see Table 5) and that the routine practice of administration of hypotonic and near-isotonic intravenous fluids (Na ≤ 130 mEq/L) be abandoned [45, 57, 58]. We recommended that hypotonic fluids be restricted in their use in patients with either hypernatremia (Na > 145 mEq/L) or ongoing urinary or extrarenal free-water losses. This concept, which challenged the traditional view of fluid therapy in children, was received with skepticism [59]. The main criticism of this approach was that 0.9% NaCl could result in either hypernatremia or fluid overload. Subsequent studies have confirmed that hypotonic fluids produce hyponatremia and that the administration of 0.9% NaCl does not result in either hypernatremia or fluid overload.
Table 5.
Primary indications for using 0.9% NaCl in parenteral fluids for the prevention of hospital-acquired hyponatremia
| 1. Central nervous system disorders |
| 2. Peri-operative state |
| a. Ear, nose, and throat (ENT) and orthopedic in particular |
| 3. Volume depletion |
| 4. Hypotension |
| 5. Pulmonary disease |
| a. Pneumonia and bronchiolitis in particular |
| 6. Hydration for chemotherapy |
| a. Cytoxan in particular |
In 2005, we presented data of >50 reports, from 1992–2004, of death or neurologic dysfunction from hyponatremic encephalopathy associated with the administration of hypotonic fluids in children. To the best of our knowledge, there have been nine additional reports of hyponatremic encephalopathy related to hypotonic fluids, with three deaths (Table 6) [58, 60–66]. It is estimated that there are >600 deaths per year from postoperative hyponatremic encephalopathy in children in the USA and one death per year in France [34, 65]. In Australia, it is estimated that about 10% of medical emergencies in the hospital involve hospital-acquired hyponatremia [67]. Despite the well-documented dangers of using hypotonic fluids, multiple recent studies in the USA and UK have revealed that between 80–100% of postoperative children are still being administered hypotonic fluids (0.18–0.45% NaCl) [58, 68–70]. In September 2007, the National Patient Safety Agency in the UK issued a warning about the use of hypotonic fluids in postoperative children and children with mild illness and recommended that 0.18% NaCl be removed from patient care areas [71].
Table 6.
Hospital-acquired hyponatremic encephalopathy in children receiving hypotonic fluids (2005–2009)
| Authors | Age (years) | Setting | Intravenous fluid | Sodium value (mEq/L) | Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Duke et al. 2005 [61] | 19 | ALL, dDAVP | 0.18% NaCl | 138 to 124 | Seizures | 3% NaCl | Survived |
| 2 | Medulloblastoma | 0.18% NaCl | 143 to 119 | Seizures & hypoxia | 3% NaCl | Survived | |
| 6 | ALL | 0.45% NaCl | 136 to 125 | Seizures & respiratory depression | 3% NaCl | Survived | |
| Ashraf and Albert 2006 [64] | 0.1 | Bronchiolitis | 0.22% NaCl | 142–107 | Lethargy | 3% NaCl | Survived |
| Osier et al. 2006 [62] | 8 | Burkitt’s lymphoma | 0.45% NaCl | 138 to 96 | Seizures | Fluid restriction | Survived |
| Agut Fuster et al. 2006 [60] | 3.5 | Adenoidectomy | D5 Water | 116 | Seizures, comatose | 3% NaCl | Survived |
| Donaldson et al. 2007 [63] | 5.5 | Adrenal suppression | 0.45% NaCl | 125 to 123 | Seizures, coma, respiratory arrest, cardiogenic shock | None | Death |
| Auroy et al. 2008 [65] | 4 | Dental extraction | D5 Water & 0.35% NaCl | 120 | Coma, respiratory distress, heart failure | None | Death |
| Cansick et al. 2009 [126] | 11 | Renal transplant | 0.45 NaCl | 140–121 | Seizures, cerebral herniation | Lorazepam | Death |
ALL acute lymphoblastic leukemia, dDAVP 1-desamino-8d-arginine vasopressin, NaCl sodium chloride, D5water 5% dextrose in water
Since our initial recommendations were made in 2003, there have been at least 11 studies in >1,000 children confirming our hypothesis that (a) hypotonic fluids, including Ringer’s Lactate (Na 130 mEq/L), produce hyponatremia and that (b) isotonic fluids prevent the development of hyponatremia (Table 7). The most convincing evidence that 0.9% NaCl is effective in preventing hospital-acquired hyponatremia has come from two recent prospective randomized studies comparing isotonic to hypotonic fluids. Yung and Keely conducted a prospective randomized controlled trial in 50 pediatric patients receiving either 0.9% NaCl or 0.18% saline at either standard maintenance or 2/3 maintenance rate [72]. Thirty-six (72%) were postsurgical. The 0.9% NaCl group had a fall in SNa of 0.2 mEq/L at 2/3 maintenance and 1.5 mEq/L at full maintenance, whereas the 0.18% NaCl group had a fall in SNa of 3 mEq/L at 2/3 maintenance and 4.9 mEq/L at full maintenance. They concluded that fluid type, not rate, was associated with a fall in SNa. Montanana et al. similarly conducted a prospective randomized controlled trial of 122 postoperative pediatric patients admitted to the intensive care unit who received either an isotonic fluid (Na + K = 155 mEq/L) or hypotonic fluids (Na < 100 mEq/L) [73]. The incidence of hyponatremia (Na < 135) at 24 h was 20.6% in the hypotonic fluid group compared with 5.1% in the isotonic group. Both studies failed to document any complications from isotonic fluids, such as hypertension or hypernatremia.
Table 7.
Relationship between intravenous fluid composition and development of hyponatremia (2003–2009)
| Authors | Study design | Number | Outcome |
|---|---|---|---|
| Hoorn et al. 2004 [123] | Retrospective. Incidence of acute (48 h) hospital-acquired hyponatremia (SNa < 136 mEq/L) in children presenting to the ED with a normal SNa | 432 | 40 patients (10%) developed acute hyponatremia with a fall in SNa from 139 ± 3 to 133 ± 2 mEq/L in 19 ± 10 h. All received hypotonic fluids |
| Neville et al. 2005 [127] | Prospective. Change in SNa at 4 h in normonatremic children with gastroenteritis receiving 0.45% NaCl | 25 | Fall in SNa from 138 ± 1.6 to 135 ± 2 mEq/L |
| Mehta et al. 2005 [128] | Prospective. Change in SNa at 24 h in jaundiced neonates receiving 0.18% NaCl | 37 | Fall in SNa from 141 ± 5 to 134 ± 4 mEq/L |
| Neville et al. 2006 [129] | Prospective randomized trial. Change in SNa at 4 h in normonatremic children with gastroenteritis receiving either 0.45% NaCl or 0.9% NaCl | 65 | Fall in SNa in the 0.45% NaCl group from 137 ± 7 to 135 ± 1.8 mEq/L |
| SNa unchanged in 0.9% NaCl group, 137 ± 2.2 to 138 ± 2.9 mEq/L | |||
| Dearlove et al. 2006 [130] | Retrospective. Incidence of hyponatremia in children following appendectomy; 87% received 0.45% NaCl | 51 | 32% incidence of hyponatremia (127–133 mEq/L) |
| Stewart and McGrath 2007 [131] | Prospective. Change in SNa in children following appendectomy treated with 0.45% NaCl or 0.9% NaCl | 30 | Fall in SNa in the 0.45% NaCl group by 1.2 mEq/L/day |
| Increase in SNa in the 0.9% NaCl group by 1.7 mEq/L/day | |||
| Coulthard et al. 2007 [132] | Retrospective. Change in SNa in children following spinal surgery treated with 0.3% NaCl at 2/3 maintenance or full maintenance with Hartmann’s solution (Na = 131 mEq/L) | 59 | Fall in SNa in 0.3% NaCl group from 140.7 ± 2.4 to 135.5 ± 2.5 |
| Fall in SNa in Hartmans’s group from 140.1 ± 2.5 to 137.6 ± 2.8 | |||
| Yung and Keely 2009 [72] | Prospective randomized trial. Change in SNa at 12–24 h in children admitted to the ICU randomized to either 0.18% NaCl or 0.9% NaCl at 2/3 or full maintenance rate | 50 | Fall in SNa in 0.18% NaCl group by 3 mEq/L and 4.9 mEq/L, respectively |
| Increase in SNa in the 0.9% NaCl group by 0.2 and 1.5 mEq/L, respectively | |||
| Armon et al. 2008 [124] | Cross-sectional survey. Incidence of hyponatremia (SNa < 135 mEq/L) in children receiving hypotonic fluids one day; 77% received hypotonic fluids | 86 | 24% incidence of hyponatremia |
| Au et al. 2008 [58] | Retrospective. Incidence of moderate hyponatremia (SNa < 130 mEq/L) within 24 h in postoperative children admitted to the ICU receiving hypotonic fluids (Na < 130 mEq/L) or near isotonic fluids (Na ≥ 130 mEq/L) | 145 | 12.9% incidence of moderate hyponatremia in the hypotonic group |
| 3.4% incidence in moderate hyponatremia in the near isotonic group | |||
| Montonana et al. 2008 [73] | Prospective randomized. Incidence of hyponatremia (SNa < 135 mEq/L) within 24 h in postoperative children admitted to the ICU receiving either hypotonic fluids (Na < 100 mEq/L) or isotonic fluid (Na + K = 155 mEq/L) | 122 | 20.6% incidence of hyponatremia in the hypotonic group |
| 5.1% incidence of hyponatremia in the isotonic group | |||
| Singhi and Jayashre 2009 [133] | Prospective observational. Incidence of hyponatremia (SNa <130) in children admitted to the ICU receiving 0.18% NaCl | 38 | 31% incidence of hyponatremia |
SNa serum sodium, ED emergency department, NaCl sodium chloride, Na sodium, ICU intensive care unit
Based on all the available data, 0.9% NaCl should be the fluid of choice in maintenance parenteral fluids, especially in the postoperative setting and in children with CNS or pulmonary disease. It must be emphasized that 0.9% NaCl is not appropriate for all clinical circumstances. Normal saline could result in hypernatremia if given to children with conditions causing ongoing urinary or extrarenal free-water losses, such as diabetes insipidus or profuse water diarrhea. Also, 0.9% NaCl may not always be able to prevent hyponatremia, in particular, in cases of CNS injury where there is cerebral salt wasting or Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) where the urine osmolality is >500 mOsm/kg. Patients receiving parenteral fluids should have close monitoring with daily weights, frequent vitals, strict intake and output measurement, and daily chemistries, especially during the first 72 h of therapy.
Randomized controlled trials have confirmed that 0.9% NaCl is effective in preventing the development of hospital-acquired hyponatremia.
What is the optimal therapy for treating hyponatremic encephalopathy?
Hyponatremic encephalopathy is a medical emergency that requires early recognition and treatment. The definitive therapy for treating hyponatremic encephalopathy is administration of hypertonic saline (3% NaCl, 513 mEq/L). The majority of morbidity associated with hyponatremic encephalopathy has resulted from insufficient therapy rather than overcorrection [9, 74–77]. As can be seen from Table 6, all recent deaths in children have resulted from failure to recognize and treat hyponatremic encephalopathy appropriately. Even in patients who had a good outcome, many had a significant delay in instituting therapy. This is consistent with recent data in adults with hyponatremic encephalopathy that reveal that there is an average delay of 11 h in instituting therapy with 3% NaCl because of either absence of severe neurological symptoms or failure to increase the SNa with other therapies [78].
Fluid restriction alone has no role in the management of symptomatic hyponatremia; 0.9% NaCl is also inappropriate for treating hyponatremic encephalopathy due to nonhemodynamic states of AVP excess, such as SIADH, postoperative hyponatremia, and exercise-associated hyponatremia, as it is not sufficiently hypertonic to induce the necessary reduction in cerebral edema central to the management of this condition [27]. In the presence of elevated AVP levels, there will be an impaired ability to excrete free water with the urine osmolality exceeding that of the plasma. This is a saline-resistant state in which the urinary electrolyte level can be hypertonic to that of the plasma. There is a new class of drug called V2-receptor antagonists (V2RA), or Vaptans, which cannot be recommended for the treatment of hyponatremic encephalopathy at this time [27]. V2RAs block the binding of AVP to its V2 receptor located in the renal collecting duct [79]. These drugs are primarily indicated for treating euvolemic hyponatremia from SIADH and hypervolemic hyponatremia in congestive heart failure. There are no data to suggest that V2-receptor antagonists will cause either a sufficiently rapid or sufficiently consistent increase in SNa for it to be used in the treatment of symptomatic hyponatremia. Current data indicate that V2-receptor antagonists do not exert an effect for 1−2 h, which would make it an inappropriate agent for symptomatic hyponatremia [79]. The only consistent way of acutely increasing plasma Na is to administer 3% NaCl, which has a sodium concentration that exceeds the kidney’s ability to generate free water. There may be a role for V2-receptor antagonists in conjunction with 3% NaCl for treating hyponatremic encephalopathy, but this needs to be further evaluated.
3% NaCl is the most effective therapy for treating hyponatremic encephalopathy.
V2-receptor antagonists should not be used as the sole treatment of hyponatremic encephalopathy.
Hypertonic saline (3% NaCl, 513 mEq/L) bolus therapy for treating hyponatremic encephalopathy
We introduced a new approach to using 3% NaCl for treating hyponatremic encephalopathy in order to (a) facilitate early and aggressive therapy and (b) prevent inadvertent overcorrection of hyponatremia (see Table 8). In 2005, we recommended using a 100-cc bolus of 3% NaCl to treat exercise-associated hyponatremic encephalopathy [80]. This approach has since been adopted by the Second International Exercise-Associated Hyponatremia Consensus Development Conference [81]. We have since recommended that any patient with suspected hyponatremic encephalopathy, with either mild or advanced symptoms, should receive a 2-cc/kg bolus of 3% NaCl with a maximum of 100 cc [27, 45]. Our approach has now been recommended by other experts to treat adults with hyponatremic encephalopathy [82, 83]. A single bolus would result in at most a 2-mEq/L acute rise in SNa, which would quickly reduce brain edema. The bolus could be repeated one or two times if symptoms persist. This approach can also serve as a diagnostic maneuver, as a patient who does not show some clinical improvement after two to three boluses of 3% NaCl most likely is not suffering from hyponatremic encephalopathy. The advantage of this approach over a continuous infusion of 3% NaCl is that there is a controlled and immediate rise in SNa and also little or no risk of inadvertent overcorrection from a 3% NaCl infusion running too long. No harm could come from using this approach in a patient with suspected hyponatremic encephalopathy, even if the patient proves not to have hyponatremic encephalopathy. It is our opinion that treatment of suspected symptomatic hyponatremic encephalopathy should begin with a 3% NaCl bolus. This should precede radiologic investigations because (a) neurologic deterioration could occur if there is a delay in therapy and (b) a CT scan cannot always rule out hyponatremic encephalopathy. This maneuver will stabilize the patient until further diagnostic studies can be done and serve as a bridge to instituting other therapies, such as V2-receptor antagonists.
Table 8.
Treatment of symptomatic hyponatremia
| 1. 2 cc/kg bolus of 3% NaCl over 10 min. Maximum 100 cc |
| 2. Repeat bolus 1–2 times as needed until symptoms improve. Goal: 5–6 mEql/L increase in serum sodium (SNa) in first 1–2 h |
| 3. Recheck SNa following second bolus or Q 2 h |
| 4. Hyponatremic encephalopathy is unlikely if no clinical improvement following an acute rise in serum sodium of 5–6 mEq/L |
| 5. Stop further therapy with 3% NaCl boluses when patient is either: |
| a. Symptom free: awake, alert, responding to commands, resolution of headache and nausea |
| b. Acute rise in sodium of 10 mEq/L in if first 5 h |
| 6. Correction in first 48 h should: |
| a. Not exceed 15–20 mEq/L |
| b. Avoid normo- or hypernatremia |
Recommended safe limits for the correction of hyponatremia vary among experts depending on the setting of hyponatremia, including from 6 to 8 mEq/L in 24 h [82], 10 mEq/L in 24 h [84], 15 mEq/24 h [85] or 20 mEq/L in 48 h [45], as do recommendations for using hypertonic saline. Our recommendation to use bolus therapy is a unifying approach that would stay well within all recommended limits of correction and can be used safely in any setting, from mildly symptomatic to severe encephalopathy and in acute or chronic hyponatremia. Our approach does not rely on formulas or complicated calculations, and it can be administered safely and quickly in the emergency department or at the bedside prior to transfer to a monitored setting.
A 2 cc/kg bolus of 3% NaCl, maximum 100 cc, should be administered promptly over 10 min if there are signs of hyponatremic encephalopathy.
A 3% NaCl bolus can be repeated one or two times as needed until symptoms improve.
The goal of correction should be 5–6 mEql/L in the first 1−2 h.
Who is at risk for developing cerebral demyelination?
A significant barrier to the use of hypertonic saline has been the perceived risk of developing cerebral demyelination from overcorrection of hyponatremia. Cerebral demyelination is a rare condition that has been reported in patients with chronic hyponatremia (>48 h) who have additional risk factors such as liver disease or alcoholism, severe malnutrition, hypoxia, or correction in SNa of >25 mEq/L in the first 24–48 h of therapy [74]. In these high-risk patients (see Table 9), it is not clear that cerebral demyelination is completely preventable, as there have been multiple reports of cerebral demyelination occurring with both careful correction or in the absence of hyponatremia [86–96]. Cerebral demyelination has not been reported in children with acute hospital-acquired hyponatremia, nor have neurological complications been associated with the use of 3% NaCl to treat children with acute hyponatremic encephalopathy [97–100].
Table 9.
Risk factors for developing cerebral demyelination in hyponatremic patients
| 1. Severe chronic hyponatremia: Na ≤115 mEq/L |
| 2. Development of hypernatremia |
| 3. Increase in serum sodium exceeding 25 mmol/L in 48 hours |
| 4. Hypoxemia |
| 5. Severe liver disease |
| 6. Thiazide diuretics |
| 7. Alcoholism |
| 8. Cancer |
| 9. Severe Burns |
| 10. Malnutrition |
| 11. Hypokalemia |
| 12. Diabetes |
| 13. Renal failure |
When cerebral demyelination does occur, it can be either symptomatic or asymptomatic [20]. The classical presentation typically follows a biphasic pattern, with initial clinical improvement of hyponatremic encephalopathy associated with correction of SNa, followed by a neurological deterioration 2–7 days following correction [42, 101]. Typical neurological features are mutism, dysarthria, spastic quadriplegia, pseudobulbar palsy, ataxia, and pseudobulbar palsy with “locked-in stare” [102]. Cerebral demyelination is best diagnosed by MRI 14 days following correction of hyponatremia, and the lesions can be both pontine and extrapontine [103].
Patients at highest risk for developing cerebral demyelination have chronic hyponatremia and either (a) liver disease, (b) malnutrition, (c) hypoxia, or (d) an increase in SNa of >25 mEq/l.
Patients with acute hyponatremia are not at significant risk for developing cerebral demyelination.
What are the dangers of overcorrection of hyponatremia?
There are limits to the brain’s ability to maintain cell volume and cellular integrity when faced with extreme elevations in osmolality. Animal studies have revealed that extreme elevations in SNa are injurious to the brain, producing cellular necrosis, myelinolysis, disruption of the blood−brain barrier, and increase in cerebral blood flow [104–110]. For chronically hyponatremic animals, the threshold for brain injury appears to be an acute elevation in SNa of 25 mEq/L within 24 h [106, 107, 111, 112]. Chronically hyponatremic rats can be corrected with hypertonic saline by as much as 20 mEq/L in 1 h without developing brain pathology [111]. Acutely hyponatremic and normonatremic animals have a significant mortality when SNa is increased by >25 mEq/L but a much lower incidence of brain injury when compared with chronically hyponatremic animals [104–106, 113, 114]. Clinical observations in chronically hyponatremic humans also suggest that increases in SNa of >25 mEq/L in 48 h can result in neurologic impairment [9, 74]. Based on these animal studies and clinical observations, increases in SNa of a magnitude >25 mEq/L in a 48-h period should be avoided.
Acute elevations in SNa exceeding 25 mEq/L in 48 h can produce brain injury.
Can inadvertent overcorrection of hyponatremia be prevented?
Preventing an extreme rise in SNa (>25 mEq/L in 48 h) can be difficult, particularly in the severely hyponatremic patient (SNa ≤ 115 mEq/L). The overall rate of correction of hyponatremia is primarily a determinant of the renal response to fluid therapy, more so than the composition of fluids administered. Under most circumstances, hyponatremia develops due to a state of high AVP production. Once the stimulus for AVP production abates, there will be brisk urinary excretion of free water and hyponatremia will correct rapidly. The main conditions in which correction by fluid therapy will induce a brisk free-water diuresis are (a) thiazide-induced hyponatremia, (b) water intoxication, (c) gastroenteritis, (d) adrenal insufficiency following replacement therapy, and (e) 1-desamino-8d-arginine vasopressin (dDAVP)-induced hyponatremia following dDAVP withdrawal. Even in patients who are not typically at high risk for overcorrection, such as those with SIADH and postoperative hyponatremia, when the stimuli for AVP production abates, a free-water diuresis will ensue. It is important to recognize that equations for predicting the correction of hyponatremia will not apply in these patients, as most of these equations are closed-system equations that due not take into account the renal response to fluid therapy [19, 78]. In general, if the SNa is >115 mEq/L, then even if there is a brisk free-water diuresis, the absolute rise in SNa will not likely exceed 25 mEq/L, and the risk of brain injury is small.
We recommend that the following measures be taken to prevent overcorrection of hyponatremia: (1) Patients with an SNa <115 mEq/L should be monitored to see whether a water diuresis ensues, as evidenced by an increase in SNa of >1 mEq/L per hour accompanied by a urine flow rate of >1 ml/kg per hour. In general, a urine tonicity (urine Na + K) <80 mEq/L or urine osmolality less than that of the plasma is consistent with a significant free-water diuresis during the correction phase of hyponatremia. (2) Hydration with either 3% NaCl or 0.9% NaCl should be limited to the minimal amount necessary to correct the SNa to a safe level or correct volume depletion. (3) Sodium-containing intravenous fluid should be restricted once a free-water diuresis commences, and oral intake should be encouraged. (4) Parenteral fluids, when needed, should be hypotonic, Na concentration <80 mEq/L, if there is a free-water diuresis. On the rare occasion that a pediatric patient with severe hyponatremia fails these above measures, the administration of dDAVP could be considered. DDAVP administration was first suggested to prevent the overcorrection of hyponatremia in 1993 [115] and has subsequently been used successfully in adult patients [116, 117]. DDAVP has also been used successfully to therapeutically re-lower the SNa in an adult patient with overcorrection of chronic hyponatremia. dDAVP should be used with caution and in consultation with someone familiar with this therapy, as it can result in inadvertent hyponatremia. If used, hypotonic fluid administration should be avoided following the administration of dDAVP, and isotonic saline should be administered at a restricted rate when needed. An inadvertent lowering of the SNa following dDAVP administration can be corrected with a bolus of 3% NaCl.
Management of dDAVP-induced hyponatremia
Hyponatremia caused by dDAVP is particularly difficult to manage. Hyponatremic encephalopathy from dDAVP-induced hyponatremia has been reported in children taking this medication for the treatment of enuresis, central diabetes insipidus (CDI), and as part of perioperative management of Von Willebrand’s disease [118, 119]. A common and dangerous way to manage dDAVP-induced hyponatremia is by stopping dDAVP and administering 0.9% NaCl. This can result in an overcorrection of hyponatremia, as withdrawal of dDAVP will result in a free-water diuresis, and in combination with 0.9% NaCl or 3% NaCl, hypernatremia could develop, especially in the case of CDI, where there will not be endogenous AVP production in response to hyperosmolality [120]. This is particularly likely to occur when the SNa is <115 mEq/L. We have previously reported on cases of brain injury from overcorrection of hyponatremia following dDAVP withdrawal [120]. The safer approach is to continue the dDAVP to allow controlled correction in SNa. Then, 3% NaCl boluses can be administered as needed to correct the SNa. Fluid restriction should then be instituted, with isotonic fluids used in parenteral fluids if needed. Once the SNa has been corrected to mildly hyponatremic values, dDAVP could be discontinued.
dDAVP should not be discontinued in the management of dDAVP-induced hyponatremia.
Questions
(Answers appear following the reference list)
- What is the main risk factor for developing hyponatremia?
- AVP excess
- Intravenous fluid therapy
- Prematurity
- Prolonged hospitalization
- Mechanical ventilation
- Which of the following is NOT a feature of hyponatremic encephalopathy?
- Headache
- Noncardiogenic pulmonary edema
- Hyperpyrexia
- Seizures
- Orthopedic injuries
- Why are children at increased risk for developing hyponatremic encephalopathy?
- Increased expression of aquaporin 4
- Increased sensitivity of AVP
- Increased basal metabolic rate
- Increased brain- to skull-size ratio
- Increased brain idiogenic osmole production
- What is the most effective therapy of hyponatremic encephalopathy?
- Vasopressin 2 antagonists
- Mannitol
- 0.9% NaCl plus Lasix
- Craniotomy
- 3% NaCl
- Which of the following is NOT a risk factor for developing cerebral demyelination?
- Liver disease
- Acute hyponatremic encephalopathy
- Hypoxia
- Correction in SNa of >25 mEq/L in 48 h
- Thiazide diuretics
Note added in proof
Following the submission of this manuscript, Neville et al. [134] reported on a prospective randomized trial of 124 postoperative children who received either 0.9% NaCl or 0.45% NaCl at either 100% or 50% of standard maintenance. The incidence of hyponatremia (Na < 135) within the first 24 hours of surgery was 30% in the 0.45% NaCl group compared to 10% in the 0.9% NaCl (p=0.02), with 15% of patients in the 0.45% NaCl group having a fall in SNa of ≥5 mEq/L compared to none in the 0.9% NaCl. Fluid restriction at 50% maintenance did not decrease the incidence of hyponatremia, but resulted in a 23% incidence of dehydration. Administration of 0.9% NaCl did not result in clinically significant hypernatremia.
Footnotes
Answers
1. a
2. c
3. d
4. e
5. b
References
- 1.Barlow ED, Wardener HE. Compulsive water drinking. Q J Med. 1959;28:235–258. [PubMed] [Google Scholar]
- 2.Oksche A, Rosenthal W. The molecular basis of nephrogenic diabetes insipidus. J Mol Med. 1998;76:326–337. doi: 10.1007/s001090050224. [DOI] [PubMed] [Google Scholar]
- 3.Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52:3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moritz ML. Urine sodium composition in ambulatory healthy children: hypotonic or isotonic? Pediatr Nephrol. 2008;23:955–957. doi: 10.1007/s00467-008-0757-6. [DOI] [PubMed] [Google Scholar]
- 5.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 7.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:e71–e78. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Ayus JC, Arieff AI. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281:2299–2304. doi: 10.1001/jama.281.24.2299. [DOI] [PubMed] [Google Scholar]
- 10.Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101:583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- 11.Al-Dahhan J, Haycock GB, Nichol B, Chantler C, Stimmler L. Sodium homeostasis in term and preterm neonates. III. Effect of salt supplementation. Arch Dis Child. 1984;59:945–950. doi: 10.1136/adc.59.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Dahhan J, Jannoun L, Haycock GB. Effect of salt supplementation of newborn premature infants on neurodevelopmental outcome at 10–13 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;86:F120–F123. doi: 10.1136/fn.86.2.F120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirazki A, Weintraub Z, Reich D, Gershon E, Leshem M. Lowest neonatal serum sodium predicts sodium intake in low birth weight children. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1683–R1689. doi: 10.1152/ajpregu.00453.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ertl T, Hadzsiev K, Vincze O, Pytel J, Szabo I, Sulyok E. Hyponatremia and sensorineural hearing loss in preterm infants. Biol Neonate. 2001;79:109–112. doi: 10.1159/000047076. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DJ, Hope PL, Johnson A. Neonatal risk factors for cerebral palsy in very preterm babies: case-control study. BMJ. 1997;314:404–408. doi: 10.1136/bmj.314.7078.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YF, Lu GJ, Han YK. Risk factors for intracranial hemorrhage in very low birth weight infants. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:297–300. [PubMed] [Google Scholar]
- 17.Mir NA, Faquih AM, Legnain M. Perinatal risk factors in birth asphyxia: relationship of obstetric and neonatal complications to neonatal mortality in 16, 365 consecutive live births. Asia Oceania J Obstet Gynaecol. 1989;15:351–357. doi: 10.1111/j.1447-0756.1989.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 19.Moritz ML, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant. 2003;18:2486–2491. doi: 10.1093/ndt/gfg394. [DOI] [PubMed] [Google Scholar]
- 20.Moritz ML, Ayus JC. Preventing neurological complications from dysnatremias in children. Pediatr Nephrol. 2005;20:1687–1700. doi: 10.1007/s00467-005-1933-6. [DOI] [PubMed] [Google Scholar]
- 21.Kimelberg HK. Increased release of excitatory amino acids by the actions of ATP and peroxynitrite on volume-regulated anion channels (VRACs) in astrocytes. Neurochem Int. 2004;45:511–519. doi: 10.1016/j.neuint.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Schoonman GG, Sandor PS, Nirkko AC, Lange T, Jaermann T, Dydak U, Kremer C, Ferrari MD, Boesiger P, Baumgartner RW. Hypoxia-induced acute mountain sickness is associated with intracellular cerebral edema: a 3 T magnetic resonance imaging study. J Cereb Blood Flow Metab. 2008;28:198–206. doi: 10.1038/sj.jcbfm.9600513. [DOI] [PubMed] [Google Scholar]
- 23.Sundgren PC, Reinstrup P, Romner B, Holtas S, Maly P. Value of conventional, and diffusion- and perfusion weighted MRI in the management of patients with unclear cerebral pathology, admitted to the intensive care unit. Neuroradiology. 2002;44:674–680. doi: 10.1007/s00234-002-0777-z. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Nguyen MK, Chang R, Kurtz I. Fatal hyponatremia in a young woman after ecstasy ingestion. Nat Clin Pract Nephrol. 2006;2:283–288. doi: 10.1038/ncpneph0167. [DOI] [PubMed] [Google Scholar]
- 25.Campbell GA, Rosner MH. The agony of ecstasy: MDMA (3, 4-methylenedioxymethamphetamine) and the kidney. Clin J Am Soc Nephrol. 2008;3:1852–1860. doi: 10.2215/CJN.02080508. [DOI] [PubMed] [Google Scholar]
- 26.McClellan MD, Dauber IM, Weil JV. Elevated intracranial pressure increases pulmonary vascular permeability to protein. J Appl Physiol. 1989;67:1185–1191. doi: 10.1152/jappl.1989.67.3.1185. [DOI] [PubMed] [Google Scholar]
- 27.Moritz ML, Ayus JC. Exercise-associated hyponatremia: why are athletes still dying? Clin J Sport Med. 2008;18:379–381. doi: 10.1097/JSM.0b013e31818809ce. [DOI] [PubMed] [Google Scholar]
- 28.Smith WS, Matthay MA. Evidence for a hydrostatic mechanism in human neurogenic pulmonary edema. Chest. 1997;111:1326–1333. doi: 10.1378/chest.111.5.1326. [DOI] [PubMed] [Google Scholar]
- 29.Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy. Noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest. 1995;107:517–521. doi: 10.1378/chest.107.2.517. [DOI] [PubMed] [Google Scholar]
- 30.Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132:711–714. doi: 10.7326/0003-4819-132-9-200005020-00005. [DOI] [PubMed] [Google Scholar]
- 31.Sarnaik AP, Meert K, Hackbarth R, Fleischmann L. Management of hyponatremic seizures in children with hypertonic saline: a safe and effective strategy. Crit Care Med. 1991;19:758–762. doi: 10.1097/00003246-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bruce RC, Kliegman RM. Hyponatremic seizures secondary to oral water intoxication in infancy: association with commercial bottled drinking water. Pediatrics. 1997;100:E4. doi: 10.1542/peds.100.6.e4. [DOI] [PubMed] [Google Scholar]
- 33.Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- 34.Arieff AI, Ayus JC, Fraser CL. Hyponatraemia and death or permanent brain damage in healthy children. BMJ. 1992;304:1218–1222. doi: 10.1136/bmj.304.6836.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arieff AI, Kozniewska E, Roberts TP, Vexler ZS, Ayus JC, Kucharczyk J. Age, gender, and vasopressin affect survival and brain adaptation in rats with metabolic encephalopathy. Am J Physiol. 1995;268:R1143–R1152. doi: 10.1152/ajpregu.1995.268.5.R1143. [DOI] [PubMed] [Google Scholar]
- 36.Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K. Intracranial volume change in childhood. J Neurosurg. 1999;91:610–616. doi: 10.3171/jns.1999.91.4.0610. [DOI] [PubMed] [Google Scholar]
- 37.Xenos C, Sgouros S, Natarajan K. Ventricular volume change in childhood. J Neurosurg. 2002;97:584–590. doi: 10.3171/jns.2002.97.3.0584. [DOI] [PubMed] [Google Scholar]
- 38.Farrar HC, Chande VT, Fitzpatrick DF, Shema SJ. Hyponatremia as the cause of seizures in infants: a retrospective analysis of incidence, severity, and clinical predictors. Ann Emerg Med. 1995;26:42–48. doi: 10.1016/s0196-0644(95)70236-9. [DOI] [PubMed] [Google Scholar]
- 39.Vajda Z, Pedersen M, Doczi T, Sulyok E, Stodkilde-Jorgensen H, Frokiaer J, Nielsen S. Effects of centrally administered arginine vasopressin and atrial natriuretic peptide on the development of brain edema in hyponatremic rats. Neurosurgery. 2001;49:697–704. doi: 10.1097/00006123-200109000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Doczi T, Laszlo FA, Szerdahelyi P, Joo F. Involvement of vasopressin in brain edema formation: further evidence obtained from the Brattleboro diabetes insipidus rat with experimental subarachnoid hemorrhage. Neurosurgery. 1984;14:436–441. doi: 10.1227/00006123-198404000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Kozniewska E, Gadamski R, Klapczynska K, Wojda R, Rafalowska J. Morphological changes in the brain during experimental hyponatraemia. Do vasopressin and gender matter? Folia Neuropathol. 2008;46:271–277. [PubMed] [Google Scholar]
- 42.Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314:1529–1535. doi: 10.1056/NEJM198606123142401. [DOI] [PubMed] [Google Scholar]
- 43.Vexler ZS, Ayus JC, Roberts TP, Fraser CL, Kucharczyk J, Arieff AI. Hypoxic and ischemic hypoxia exacerbate brain injury associated with metabolic encephalopathy in laboratory animals. J Clin Invest. 1994;93:256–264. doi: 10.1172/JCI116953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayus JC, Armstrong D, Arieff AI. Hyponatremia with hypoxia: effects on brain adaptation, perfusion, and histology in rodents. Kidney Int. 2006;69:1319–1325. doi: 10.1038/sj.ki.5000187. [DOI] [PubMed] [Google Scholar]
- 45.Moritz ML, Carlos Ayus J. Hospital-acquired hyponatremia–why are hypotonic parenteral fluids still being used? Nat Clin Pract Nephrol. 2007;3:374–382. doi: 10.1038/ncpneph0526. [DOI] [PubMed] [Google Scholar]
- 46.McJunkin JE, Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, Fu KD, Lovett GD, Tsai T, Thompson A. La Crosse encephalitis in children. N Engl J Med. 2001;344:801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- 47.Moritz ML, Ayus JC. La Crosse encephalitis in children. N Engl J Med. 2001;345:148–149. doi: 10.1056/NEJM200107123450216. [DOI] [PubMed] [Google Scholar]
- 48.Moritz ML, Ayus JC. Case 8–2006: a woman with Crohn's disease and altered mental status. N Engl J Med. 2006;354:2833–2834. doi: 10.1056/NEJMc061008. [DOI] [PubMed] [Google Scholar]
- 49.Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mt Sinai J Med. 2002;69:242–248. [PubMed] [Google Scholar]
- 50.Al-Zahraa Omar F, Al Bunyan M. Severe hyponatremia as poor prognostic factor in childhood neurologic diseases. J Neurol Sci. 1997;151:213–216. doi: 10.1016/s0022-510x(97)00133-0. [DOI] [PubMed] [Google Scholar]
- 51.Moro N, Katayama Y, Igarashi T, Mori T, Kawamata T, Kojima J. Hyponatremia in patients with traumatic brain injury: incidence, mechanism, and response to sodium supplementation or retention therapy with hydrocortisone. Surg Neurol. 2007;68:387–393. doi: 10.1016/j.surneu.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 52.Chao YN, Chiu NC, Huang FY. Clinical features and prognostic factors in childhood pneumococcal meningitis. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi. 2008;41:48–53. [PubMed] [Google Scholar]
- 53.Morton DH, Strauss KA, Robinson DL, Puffenberger EG, Kelley RI. Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics. 2002;109:999–1008. doi: 10.1542/peds.109.6.999. [DOI] [PubMed] [Google Scholar]
- 54.Glaser NS, Wootton-Gorges SL, Marcin JP, Buonocore MH, Dicarlo J, Neely EK, Barnes P, Bottomly J, Kuppermann N. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164–171. doi: 10.1016/j.jpeds.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 55.Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, Kaufman F, Quayle K, Roback M, Malley R, Kuppermann N. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 56.Hoorn EJ, Carlotti AP, Costa LA, MacMahon B, Bohn G, Zietse R, Halperin ML, Bohn D. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007;150:467–473. doi: 10.1016/j.jpeds.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 57.Moritz ML, Ayus JC. Prevention of hospital-acquired hyponatremia: a case for using isotonic saline. Pediatrics. 2003;111:227–230. doi: 10.1542/peds.111.2.227. [DOI] [PubMed] [Google Scholar]
- 58.Au AK, Ray PE, McBryde KD, Newman KD, Weinstein SL, Bell MJ. Incidence of postoperative hyponatremia and complications in critically-ill children treated with hypotonic and normotonic solutions. J Pediatr. 2008;152:33–38. doi: 10.1016/j.jpeds.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 59.Holliday MA, Segar WE, Friedman A. Reducing errors in fluid therapy. Pediatrics. 2003;111:424–425. [PubMed] [Google Scholar]
- 60.Agut Fuster MA, Campo Biosca J, Ferrer Rodriguez A, Ramos Martinez MJ, Viel Martinez JM, Agulles Fornes MJ. Post-tonsillectomy hyponatremia: a posible lethal complication. Acta Otorrinolaringol Esp. 2006;57:247–250. doi: 10.1016/s0001-6519(06)78701-5. [DOI] [PubMed] [Google Scholar]
- 61.Duke T, Kinney S, Waters K Hyponatraemia and seizures in oncology patients associated with hypotonic intravenous fluids. J Paediatr Child Health. 2005;41:685–686. doi: 10.1111/j.1440-1754.2005.00760.x. [DOI] [PubMed] [Google Scholar]
- 62.Osier FH, Berkley JA, Newton CR. Life-threatening hyponatraemia and neurotoxicity during chemotherapy for Burkitt's lymphoma. Trop Doct. 2006;36:177–178. doi: 10.1258/004947506777978055. [DOI] [PubMed] [Google Scholar]
- 63.Donaldson MD, Morrison C, Lees C, McNeill E, Howatson AG, Paton JY, McWilliam R. Fatal and near-fatal encephalopathy with hyponatraemia in two siblings with fluticasone-induced adrenal suppression. Acta Paediatr. 2007;96:769–772. doi: 10.1111/j.1651-2227.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 64.Ashraf A, Albert A. Bronchiolitis with hyponatremia. Clin Pediatr (Phila) 2006;45:101–102. doi: 10.1177/000992280604500119. [DOI] [PubMed] [Google Scholar]
- 65.Auroy Y, Benhamou D, Pequignot F, Jougla E, Lienhart A. Hyponatraemia-related death after paediatric surgery still exists in France. Br J Anaesth. 2008;101:741. doi: 10.1093/bja/aen282. [DOI] [PubMed] [Google Scholar]
- 66.Moritz ML, Ayus JC. 0.9% saline solution for the prevention of hospital-acquired hyponatremia: why is there still doubt? J Pediatr. 2008;153:444. doi: 10.1016/j.jpeds.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 67.Kinney S, Tibballs J, Johnston L, Duke T. Clinical profile of hospitalized children provided with urgent assistance from a medical emergency team. Pediatrics. 2008;121:e1577–e1584. doi: 10.1542/peds.2007-1584. [DOI] [PubMed] [Google Scholar]
- 68.Way C, Dhamrait R, Wade A, Walker I. Perioperative fluid therapy in children: a survey of current prescribing practice. Br J Anaesth. 2006;97:371–379. doi: 10.1093/bja/ael185. [DOI] [PubMed] [Google Scholar]
- 69.Snaith R, Peutrell J, Ellis D. An audit of intravenous fluid prescribing and plasma electrolyte monitoring; a comparison with guidelines from the National Patient Safety Agency. Paediatr Anaesth. 2008;18:940–946. doi: 10.1111/j.1460-9592.2008.02698.x. [DOI] [PubMed] [Google Scholar]
- 70.Davies P, Hall T, Ali T, Lakhoo K. Intravenous postoperative fluid prescriptions for children: a survey of practice. BMC Surg. 2008;8:10. doi: 10.1186/1471-2482-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Patient Safety Agency (2007) Reducing the risk of hyponatraemia when administering intravenous infusions to children. Available at http://www.nrls.npsa.nhs.uk/resources
- 72.Yung M, Keeley S. Randomised controlled trial of intravenous maintenance fluids. J Paediatr Child Health. 2009;45:9–14. doi: 10.1111/j.1440-1754.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 73.Montanana PA, Modesto i Alapont V, Ocon AP, Lopez PO, Lopez Prats JL, Toledo Parreno JD. The use of isotonic fluid as maintenance therapy prevents iatrogenic hyponatremia in pediatrics: a randomized, controlled open study. Pediatr Crit Care Med. 2008;9:589–597. doi: 10.1097/PCC.0b013e31818d3192. [DOI] [PubMed] [Google Scholar]
- 74.Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317:1190–1195. doi: 10.1056/NEJM198711053171905. [DOI] [PubMed] [Google Scholar]
- 75.Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21:70–76. doi: 10.1093/ndt/gfi082. [DOI] [PubMed] [Google Scholar]
- 76.Huda MS, Boyd A, Skagen K, Wile D, Heyningen C, Watson I, Wong S, Gill G. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J. 2006;82:216–219. doi: 10.1136/pmj.2005.036947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nzerue C, Baffoe-Bonnie H, Dail C. Predicters of mortality with severe hyponatremia. J Am Soc Nephrol. 2002;13:A0728. [Google Scholar]
- 78.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol. 2007;2:1110–1117. doi: 10.2215/CJN.00910207. [DOI] [PubMed] [Google Scholar]
- 79.Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624–1632. doi: 10.1016/S0140-6736(08)60695-9. [DOI] [PubMed] [Google Scholar]
- 80.Ayus JC, Arieff A, Moritz ML. Hyponatremia in marathon runners. N Engl J Med. 2005;353:427–428. [PubMed] [Google Scholar]
- 81.Hew-Butler T, Ayus JC, Kipps C, Maughan RJ, Mettler S, Meeuwisse WH, Page AJ, Reid SA, Rehrer NJ, Roberts WO, Rogers IR, Rosner MH, Siegel AJ, Speedy DB, Stuempfle KJ, Verbalis JG, Weschler LB, Wharam P. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18:111–121. doi: 10.1097/JSM.0b013e318168ff31. [DOI] [PubMed] [Google Scholar]
- 82.Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29:282–299. doi: 10.1016/j.semnephrol.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Palmer BF, Sterns RH. Fluid, electrolytes and acid-base disturbances. Nephrol Self Assess Program. 2009;8:136–142. [Google Scholar]
- 84.Decaux G, Soupart A. Treatment of symptomatic hyponatremia. Am J Med Sci. 2003;326:25–30. doi: 10.1097/00000441-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Lauriat SM, Berl T. The hyponatremic patient: practical focus on therapy. J Am Soc Nephrol. 1997;8:1599–1607. doi: 10.1681/ASN.V8101599. [DOI] [PubMed] [Google Scholar]
- 86.Tan H, Onbas O. Central pontine myelinolysis central pontine myelinolysis manifesting with massive myoclonus. Pediatr Neurol. 2004;31:64–66. doi: 10.1016/j.pediatrneurol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Hagiwara K, Okada Y, Shida N, Yamashita Y. Extensive central and extrapontine myelinolysis in a case of chronic alcoholism without hyponatremia: a case report with analysis of serial MR findings. Intern Med (Tokyo, Japan) 2008;47:431–435. doi: 10.2169/internalmedicine.47.0634. [DOI] [PubMed] [Google Scholar]
- 88.Schuster M, Diekmann S, Klingebiel R, Volk T. Central pontine myelinolysis despite slow sodium rise in a case of severe community-acquired hyponatraemia. Anaesth Intensive Care. 2009;37:117–120. doi: 10.1177/0310057X0903700120. [DOI] [PubMed] [Google Scholar]
- 89.Orakzai RH, Orakzai SH, Hasley PB. Treating hyponatremia: how slow is safe? Central pontine myelinolysis despite appropriate correction of hyponatremia. Eur J Intern Med. 2008;19:e29–e31. doi: 10.1016/j.ejim.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Yoon B, Shim YS, Chung SW. Central pontine and extrapontine myelinolysis after alcohol withdrawal. Alcohol Alcohol. 2008;43:647–649. doi: 10.1093/alcalc/agn050. [DOI] [PubMed] [Google Scholar]
- 91.Germiniani FM, Roriz M, Nabhan SK, Teive HA, Werneck LC. Central pontine and extra-pontine myelinolysis in an alcoholic patient without hydro-electrolyte disturbances: case report. Arq Neuropsiquiatr. 2002;60:1030–1033. doi: 10.1590/s0004-282x2002000600028. [DOI] [PubMed] [Google Scholar]
- 92.Nagaishi A, Yukitake M, Eriguchi M, Kuroda Y. A case of alcoholic with vitamin B12 deficiency presenting central pontine and extrapontine myelinolysis on MRI. Rinsho shinkeigaku. 2007;47:173–176. [PubMed] [Google Scholar]
- 93.Georgy V, Mullhi D, Jones AF. Central pontine myelinolysis following 'optimal' rate of correction of hyponatraemia with a good clinical outcome. Ann Clin Biochem. 2007;44:488–490. doi: 10.1258/000456307781646067. [DOI] [PubMed] [Google Scholar]
- 94.Savasta S, Sepe V, Scagnelli P, Cisternino M, Libetta C, Soccio G, Marchi MA. Severe hyponatremia followed by extrapontine myelinolysis. Kidney Int. 2006;69:423. doi: 10.1038/sj.ki.5000173. [DOI] [PubMed] [Google Scholar]
- 95.Dellabarca C, Servilla KS, Hart B, Murata GH, Tzamaloukas AH. Osmotic myelinolysis following chronic hyponatremia corrected at an overall rate consistent with current recommendations. Int Urol Nephrol. 2005;37:171–173. doi: 10.1007/s11255-004-4770-9. [DOI] [PubMed] [Google Scholar]
- 96.Pradhan S, Jha R, Singh MN, Gupta S, Phadke RV, Kher V. Central pontine myelinolysis following 'slow' correction of hyponatremia. Clin Neurol Neurosurg. 1995;97:340–343. doi: 10.1016/0303-8467(95)00060-w. [DOI] [PubMed] [Google Scholar]
- 97.Alam NH, Yunus M, Faruque AS, Gyr N, Sattar S, Parvin S, Ahmed JU, Salam MA, Sack DA. Symptomatic hyponatremia during treatment of dehydrating diarrheal disease with reduced osmolarity oral rehydration solution. JAMA. 2006;296:567–573. doi: 10.1001/jama.296.5.567. [DOI] [PubMed] [Google Scholar]
- 98.Keating JP, Schears GJ, Dodge PR. Oral water intoxication in infants. An American epidemic. Am J Dis Child. 1991;145:985–990. doi: 10.1001/archpedi.1991.02160090037018. [DOI] [PubMed] [Google Scholar]
- 99.Moritz ML. Fluid replacement for severe hyponatremia. JAMA. 2007;297:41–42. doi: 10.1001/jama.297.1.41-a. [DOI] [PubMed] [Google Scholar]
- 100.Sasaki S. Nephrogenic diabetes insipidus: update of genetic and clinical aspects. Nephrol Dial Transplant. 2004;19:1351–1353. doi: 10.1093/ndt/gfh172. [DOI] [PubMed] [Google Scholar]
- 101.Ho VB, Fitz CR, Yoder CC, Geyer CA. Resolving MR features in osmotic myelinolysis (central pontine and extrapontine myelinolysis) AJNR Am J Neuroradiol. 1993;14:163–167. [PMC free article] [PubMed] [Google Scholar]
- 102.Wright DG, Laureno R, Victor M. Pontine and extrapontine myelinolysis. Brain. 1979;102:361–385. doi: 10.1093/brain/102.2.361. [DOI] [PubMed] [Google Scholar]
- 103.Kumar SR, Mone AP, Gray LC, Troost BT. Central pontine myelinolysis: delayed changes on neuroimaging. J Neuroimaging. 2000;10:169–172. doi: 10.1111/jon2000103169. [DOI] [PubMed] [Google Scholar]
- 104.Adler S, Verbalis JG, Williams D. Effect of rapid correction of hyponatremia on the blood-brain barrier of rats. Brain Res. 1995;679:135–143. doi: 10.1016/0006-8993(95)00245-l. [DOI] [PubMed] [Google Scholar]
- 105.Ayus JC, Armstrong DL, Arieff AI. Effects of hypernatraemia in the central nervous system and its therapy in rats and rabbits. J Physiol. 1996;492:243–255. doi: 10.1113/jphysiol.1996.sp021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ayus JC, Krothapalli RK, Armstrong DL. Rapid correction of severe hyponatremia in the rat: histopathological changes in the brain. Am J Physiol. 1985;248:F711–F719. doi: 10.1152/ajprenal.1985.248.5.F711. [DOI] [PubMed] [Google Scholar]
- 107.Ayus JC, Krothapalli RK, Armstrong DL, Norton HJ. Symptomatic hyponatremia in rats: effect of treatment on mortality and brain lesions. Am J Physiol. 1989;257:F18–F22. doi: 10.1152/ajprenal.1989.257.1.F18. [DOI] [PubMed] [Google Scholar]
- 108.Baker EA, Tian Y, Adler S, Verbalis JG. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp Neurol. 2000;165:221–230. doi: 10.1006/exnr.2000.7474. [DOI] [PubMed] [Google Scholar]
- 109.Kleinschmidt-DeMasters BK, Norenberg MD. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981;211:1068–1070. doi: 10.1126/science.7466381. [DOI] [PubMed] [Google Scholar]
- 110.Adler S, Verbalis JG, Meyers S, Simplaceanu E, Williams DS. Changes in cerebral blood flow and distribution associated with acute increases in plasma sodium and osmolality of chronic hyponatremic rats. Exp Neurol. 2000;163:63–71. doi: 10.1006/exnr.2000.7376. [DOI] [PubMed] [Google Scholar]
- 111.Soupart A, Penninckx R, Stenuit A, Perier O, Decaux G. Treatment of chronic hyponatremia in rats by intravenous saline: comparison of rate versus magnitude of correction. Kidney Int. 1992;41:1662–1667. doi: 10.1038/ki.1992.239. [DOI] [PubMed] [Google Scholar]
- 112.Verbalis JG, Martinez AJ. Neurological and neuropathological sequelae of correction of chronic hyponatremia. Kidney Int. 1991;39:1274–1282. doi: 10.1038/ki.1991.161. [DOI] [PubMed] [Google Scholar]
- 113.Sterns RH, Thomas DJ, Herndon RM. Brain dehydration and neurologic deterioration after rapid correction of hyponatremia. Kidney Int. 1989;35:69–75. doi: 10.1038/ki.1989.9. [DOI] [PubMed] [Google Scholar]
- 114.Soupart A, Penninckx R, Namias B, Stenuit A, Perier O, Decaux G. Brain myelinolysis following hypernatremia in rats. J Neuropathol Exp Neurol. 1996;55:106–113. doi: 10.1097/00005072-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 115.Ayus JC, Arieff AI. Pathogenesis and prevention of hyponatremic encephalopathy. Endocrinol Metab Clin North Am. 1993;22:425–446. [PubMed] [Google Scholar]
- 116.Goldszmidt MA, Iliescu EA. DDAVP to prevent rapid correction in hyponatremia. Clin Nephrol. 2000;53:226–229. [PubMed] [Google Scholar]
- 117.Perianayagam A, Sterns RH, Silver SM, Grieff M, Mayo R, Hix J, Kouides R. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol. 2008;3:331–336. doi: 10.2215/CJN.03190807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bernstein SA, Williford SL. Intranasal desmopressin-associated hyponatremia: a case report and literature review. J Fam Pract. 1997;44:203–208. [PubMed] [Google Scholar]
- 119.Das P, Carcao M, Hitzler J. DDAVP-induced hyponatremia in young children. J Pediatr Hematol Oncol. 2005;27:330–332. doi: 10.1097/01.mph.0000168728.49519.4a. [DOI] [PubMed] [Google Scholar]
- 120.Ayus JC, Arieff AI. Therapy of dDAVP-associated hyponatremia can lead to permanent brain damage. J Am Soc Nephrol. 2002;13:PUB002. [Google Scholar]
- 121.Hasegawa H, Okubo S, Ikezumi Y, Uchiyama K, Hirokawa T, Hirano H, Uchiyama M. Hyponatremia due to an excess of arginine vasopressin is common in children with febrile disease. Pediatr Nephrol. 2009;24:507–511. doi: 10.1007/s00467-008-1053-1. [DOI] [PubMed] [Google Scholar]
- 122.Don M, Valerio G, Korppi M, Canciani M. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol. 2008;23:2247–2253. doi: 10.1007/s00467-008-0910-2. [DOI] [PubMed] [Google Scholar]
- 123.Hoorn EJ, Geary D, Robb M, Halperin ML, Bohn D. Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics. 2004;113:1279–1284. doi: 10.1542/peds.113.5.1279. [DOI] [PubMed] [Google Scholar]
- 124.Armon K, Riordan A, Playfor S, Millman G, Khader A. Hyponatraemia and hypokalaemia during intravenous fluid administration. Arch Dis Child. 2008;93:285–287. doi: 10.1136/adc.2006.093823. [DOI] [PubMed] [Google Scholar]
- 125.Wattad A, Chiang ML, Hill LL. Hyponatremia in hospitalized children. Clin Pediatr (Phila) 1992;31:153–157. doi: 10.1177/000992289203100305. [DOI] [PubMed] [Google Scholar]
- 126.Cansick J, Rees L, Koffman G, Van't Hoff W, Bockenhauer D. A fatal case of cerebral oedema with hyponatraemia and massive polyuria after renal transplantation. Pediatr Nephrol. 2009;24:1231–1234. doi: 10.1007/s00467-008-1100-y. [DOI] [PubMed] [Google Scholar]
- 127.Neville KA, Verge CF, O'Meara MW, Walker JL. High antidiuretic hormone levels and hyponatremia in children with gastroenteritis. Pediatrics. 2005;116:1401–1407. doi: 10.1542/peds.2004-2376. [DOI] [PubMed] [Google Scholar]
- 128.Mehta S, Kumar P, Narang A. A randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. J Pediatr. 2005;147:781–785. doi: 10.1016/j.jpeds.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 129.Neville KA, Verge CF, Rosenberg AR, O'Meara MW, Walker JL. Isotonic is better than hypotonic saline for intravenous rehydration of children with gastroenteritis: a prospective randomized study. Arch Dis Child. 2006;91:226–232. doi: 10.1136/adc.2005.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dearlove OR, Ram AD, Natsagdoy S, Humphrey G, Cunliffe M, Potter F. Hyponatraemia after postoperative fluid management in children. Br J Anaesth. 2006;97:897–898. doi: 10.1093/bja/ael298. [DOI] [PubMed] [Google Scholar]
- 131.Stewart PC, McGrath K. Paediatric maintenance fluids. Br J Anaesth. 2007;98:406. doi: 10.1093/bja/ael381. [DOI] [PubMed] [Google Scholar]
- 132.Coulthard MG, Cheater LS, Long DA. Perioperative fluid therapy in children. Br J Anaesth. 2007;98:146–147. doi: 10.1093/bja/ael297. [DOI] [PubMed] [Google Scholar]
- 133.Singhi S, Jayashre M. Free water excess is not the main cause for hyponatremia in critically ill children receiving conventional maintenance fluids. Indian Pediatr. 2009;46:577–583. [PubMed] [Google Scholar]
- 134.Singhi S, Jayashre M. Free water excess is not the main cause for hyponatremia in critically ill children receiving conventional maintenance fluids. Indian Pediatr. 2009;46:577–583. [PubMed] [Google Scholar]