Abstract
Understanding regulatory pathways involved in melanoma development and progression has advanced significantly in recent years. It is now appreciated that melanoma is the result of complex changes in multiple signaling pathways that affect growth control, metabolism, motility and the ability to escape cell death programs. Here we review the major signaling pathways currently known to be deregulated in melanoma with an implication to its development and progression. Among these pathways are Ras, B-Raf, MEK, PTEN, phosphatidylinositol-3 kinase (PI3Ks) and Akt which are constitutively activated in a significant number of melanoma tumors, in most cases due to genomic change. Other pathways discussed in this review include the [Janus kinase/signal transducer and activator of transcription (JAK/STAT), transforming growth factor-β pathways which are also activated in melanoma, although the underlying mechanism is not yet clear. As a paradigm for remodeled signaling pathways, melanoma also offers a unique opportunity for targeted drug development.
THE RAS–RAF–MEK–ERK PATHWAY
The breakthrough finding in 2002 that B-Raf is mutated in a large percentage of melanomas (1) triggered a substantial number of new studies that focused on mitogen-activated protein kinase (MAPK) signaling in melanoma. These studies established the notion that constitutive activation of the extracellular signal-regulated protein kinase (Ras–Raf–MEK–ERK) signaling cascade is a hallmark of cutaneous malignant melanoma (Fig. 1). Alterations in other components within this pathway were known beforehand, and are best represented by the finding that Ras is mutated in approximately 15–20% of human melanomas (2,3). The Ras proteins regulate cell proliferation, survival and differentiation by activating a number of effector proteins, including the Ral guanine nucleotide dissociation stimulator (GDS) exchange factors, the phosphatidylinositol-3 kinase (PI3Ks), and the three Raf protein kinases (A-Raf, B-Raf and C-Raf) (4). Most Ras mutations are present in codon 61 of N-Ras, with K-Ras and H-Ras mutations being relatively rare (2,5).
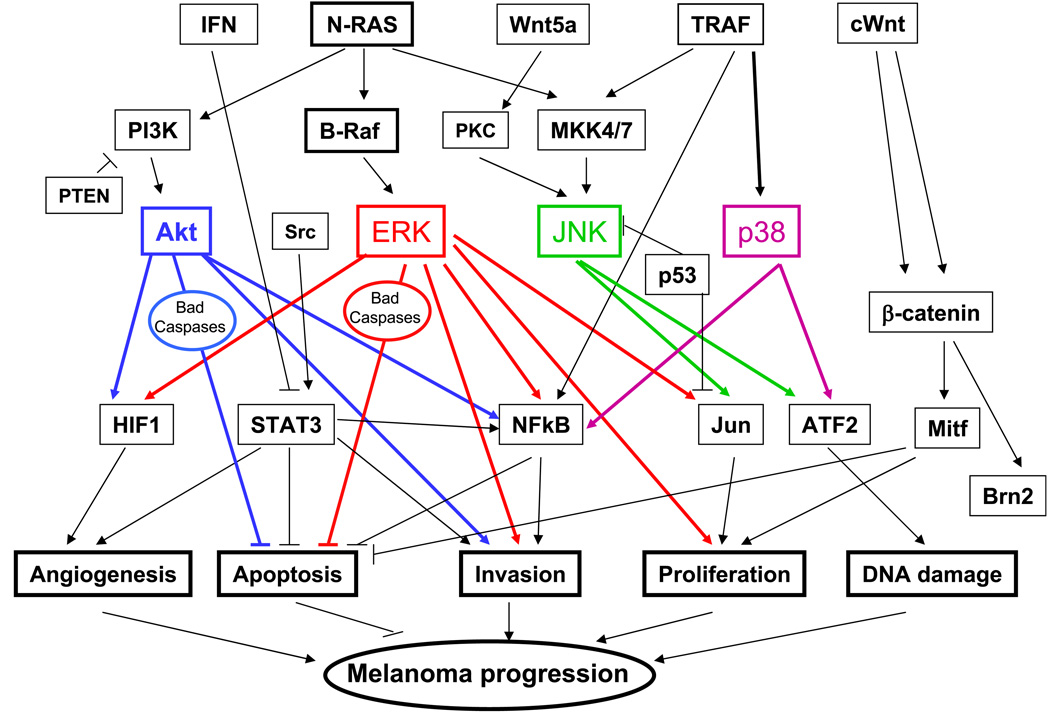
Figure 1.
Outline depicting the major signaling pathways that are deregulated in melanoma.
B-Raf was found to be mutated in up to 82% of cutaneous melanocyte nevi (6), 66% of primary melanomas (1) and 40–68% of metastatic melanomas (7,8). More than 80% of the oncogenic B-Raf alleles described to date consist of the missense exchange from valine to glutamic acid in residue 599 (V599E). The mutation engenders constitutive and maximal activation of B-Raf kinase activity, likely by mimicking phosphorylation of S598/T601 in native B-Raf (1). In vitro studies demonstrated that transfection of V599E B-Raf resulted in a several fold induction of both MEK-ERK and transforming activity (1). Interestingly, B-Raf and N-Ras mutations are mutually exclusive (1,3,9), which is consistent with the finding that active ERK is found in almost all late-stage melanoma cell lines and in tumor tissues. This is in contrast to normal melanocytes and several early-stage radial growth phase melanoma cell lines (10). Constitutive activation of the Ras–Raf–MEK–ERK signaling cascade has been shown to contribute to melanoma tumorigenesis by increasing cell proliferation, tumor invasion and metastasis, and by inhibiting apoptosis (11). The importance of constitutive activation of this pathway for the maintenance of melanoma phenotypes has been demonstrated by specific targeting of the B-Raf and MEK pathways using kinase inhibitors such as CI1040, U0126 and BAY43-9006 (12,13) or B-Raf siRNA (13–15) in in vitro and xenotransplantation models. In all cases perturbation of these pathways suffice to significantly impact growth of melanoma tumors in xenograft mouse models.
Earlier studies revealed that the presence of B-Raf/N-Ras mutations can be associated with a poorer prognosis of melanoma (8,16). However, more recent studies raised several questions regarding the significance of B-Raf and N-Ras mutations in this disease. For example, Akslen et al. found no association between mutations and tumor cell proliferation, tumor thickness, microvessel density, vascular invasion or patient survival (17). In another study, Chang et al. compared patients with and without B-Raf mutations and found no significant differences in age, gender, location of primary melanoma, stage at diagnosis and depth of primary tumor. Interestingly melanomas harboring B-Raf mutations were more likely to metastasize to liver and multiple organs, although there was no clear association with survival (18). The finding that the V599E B-Raf allele could be detected in as many as 80% of benign nevi pointed to a possible role of oncogenic B-Raf in nevus formation and melanoma initiation (6,19). However, to date, no evidence exists to directly support the possibility that benign nevi harboring V599E B-Raf actually progresses to a malignant tumor. In fact, most nevi may represent nonprogressing terminally differentiated lesions (20,21) formed by senescent cells characterized by p16(INK4a) expression (22). Moreover, it has been suggested that this oncogene-induced senescence represents a genuine protective physiologic process (22). These data suggest that although B-Raf and N-Ras mutations are likely to be important for the initiation and maintenance of most melanomas, additional mutations or modifications are required to support melanoma progression to the invasive type. Along these lines, two studies identified association of mutated V599E B-Raf with p16/ARF loss and TP53 and PTEN (phosphatase and tensin homolog deleted on chromosome 10) mutations (23,24). It has been proposed that a possible cooperation between B-Raf activation and loss of either p16/ARF or PTEN contributes to melanoma development (23). Another line of evidence suggests that B-Raf mutation may not be essential in all forms of melanocyte neoplasia, and that distinct pathways of melanoma formation exist (25–27). In agreement with such a possibility, B-Raf mutations were not detected in mucosal or vulvar melanomas, or in more than 90% of sinonasal and uveal melanomas (28–31). Along these lines, through analysis of RNA expression profiles, Shields et al. found a molecularly distinct melanoma subtype characterized by lack of mutations of N-Ras and B-Raf, p53 inactivation, reduced ERK activity and increased expression of epithelial markers (32). These data suggest that despite sharing a common progenitor cell (the neural crest-derived melanocytes), different genetic, and possibly epigenetic, programs impact the diversity of melanoma forms that are formed, explaining their distinct characteristics (25,26).
How does activation of B-Raf affect the oncogenic behavior of melanoma tumors? Alterations in MAPK signaling appear to play a major role in the pathogenesis of most melanomas. The melanoma-relevant effectors of ERK activation however, are largely unknown. Mechanistically, these effects are mediated both by posttranscriptional modification of proteins and by an increase in the transcription of specific genes. With the advent of microarray technology, genes whose expression is governed by MAPK signaling are being identified. More so, specific gene-expression signatures in addition to genes expressed by common MAPK activation are being uncovered for Q61K N-Ras and B-Raf V599E (33,34). Recently through supervised analysis of RNA expression profiles, 82 transcriptional targets in melanoma were identified (including TWIST1, hypoxia-inducible factor-1α [HIF-1α] and interleukin-8 [IL-8]; 33). Activated ERK plays a pivotal role in cell proliferation by controlling the G1-phase to S-phase transition by negative regulation of the p27/Kip1 inhibitor (35,36) and upregulation of c-Myc activity (36). Inhibition of ERK activity is associated with reduced proliferation (36) and G1-phase cell cycle arrest, mediated by upregulation of p27/Kip1 (cyclin-dependent kinase inhibitor) mRNA and hypophosphorylation of retinoblastoma protein (35,37). Additional regulation of p27/Kip1 is provided by B-Raf- and cyclin D1-dependent Skp2 (S-phase kinase-associated protein) proteolysis (37). Interestingly, the combination of B-Raf (V599E) and Skp-2 siRNA resulted in a greater inhibition of matrigel invasive ability than the single suppression of each protein (38).
Another target of ERK is the Brn-2 POU domain transcription factor, which is highly expressed in melanoma cell lines but not in melanocytes or melanoblasts. Expression of Brn-2 is positively regulated by B-Raf and MAPK signaling. Overexpression of Brn-2 in melanocytes results in increased proliferation; depletion of Brn-2 in melanoma cells expressing activated B-Raf leads to decreased proliferation (39). Another mechanism by which constitutive active ERK stimulates cell proliferation is via its regulation of c-Jun, increasing both c-Jun transcription and stability, which are mediated by cyclic adenosine monophosphate response element-binding (CREB) and glycogen synthase kinase 3 (GSK3), respectively (40).
ERK is believed to also play a role in increased proliferation by inhibiting differentiation. During differentiation of melanocytes, an increase in intracellular cAMP leads to stimulation of the Ras–MEK–ERK pathway and expression of microphthalmia-associated transcription factor (MITF). MITF induces expression of the melanogenic enzyme tyrosinase, among other targets. Conversely, constitutively active ERK limits differentiation in melanoma by targeting MITF for degradation (41–43). The constitutively activated ERK pathway mediates melanoma-specific survival signaling by differentially regulating RSK-mediated phosphorylation and inactivation of the proapoptotic protein Bad (44). ERK-mediated inhibition of JAK–STAT (45) is another mechanism by which MAPK affects melanoma cell survival.
ERK contributes to tumor invasion and metastasis by regulating expression of proteins, such as matrix metalloproteinases (MMPs) and integrins. A critical step in the process of metastasis is degradation of the extracellular matrix to allow extravasation and migration of the metastatic cells. Two families of proteases are secreted by the invading cells, the urokinase plasminogen activation and MMPs, which are involved in matrix remodeling. The expression and activity of MMPs and urokinase are tightly controlled by MAPKs (46–48). The Ras–Raf–MEK–ERK pathway is constitutively active in melanoma and is the dominant pathway driving the production of collagenase-1 (MMP-1) (49–51). Furthermore, blocking MEK–ERK activity inhibits melanoma cell proliferation and abrogates collagen degradation, decreasing their metastatic potential (52). Constitutive activation of this MAPK pathway not only promotes increased proliferation of melanoma cells but is also important in the acquisition of an invasive phenotype (52). It has been demonstrated that sustained, and not transient, activation of the Raf–MEK–ERK signaling pathway specifically controls the expression of integrin subunits and may participate in changes in cell adhesion and migration that accompany the process of oncogenic transformation (53). In addition, novel functions for activated MAPK pathway are being discovered. For example, ERK activity was found to play a role in immune evasion by melanoma cells, since targeting of B-Raf and MEK decreased production of the immunosuppressive soluble factors IL-10, vascular endothelial growth factor (VEGF) or IL-6 (54).
Due to its important role in melanoma tumorigenesis, supported by extensive preclinical validation and epidemiologic studies, the B-Raf/MEK pathway represents an attractive therapeutic target (55,56). Consistent with these expectations, promising results were obtained in mouse models (12,13,57) and several new small-molecule inhibitors of B-Raf kinase are currently undergoing clinical evaluation, with others due to enter clinical assessment in the near future. Clinical trials of these inhibitors are also expected to include a combination of drugs that affect different signaling pathways (i.e. in combination with PI3K inhibitors) to maximize the impact on diverse signaling pathways that are deregulated in this tumor type (58,59).
In any case, more original and interesting preclinical data is being generated to help rationale drug development. Sharma et al. found significant differences between the effects of B-Raf and MEK inhibitors. The B-Raf inhibitor BAY 43-9006 (which was found ineffective in clinical trials) did not decrease metastasis in a mouse model, whereas inhibition of MEK using U0126 decreased cellular proliferative capacity, thereby effectively reducing the number and size of lung metastases (60). Inhibition of metastasis was mediated through reduction in melanoma cell extravasation through the endothelium and decreased proliferative capacity (60). The latter is consistent with the growing notion that treatment of melanoma would require combined targeting of distinct signaling pathways (61,62). Altogether, these data show that advances made during the recent few years allow us to better understand the complexity of metastatic melanoma. While devoting substantial efforts to further understand changes underlying the development and progression of different subtypes of melanomas, progress is being made in developing therapeutic modalities to treat this tumor type.
THE PI3K/AKT PATHWAY
The PI3K/Akt pathway was shown to be activated in various cancers, mostly due to mutations in the tumor suppressor gene PTEN (63). In melanoma, the loss of chromosome 10 was first reported by Parmiter et al. (64) and since then has been studied extensively (65,66). The PTEN gene encodes a phosphatase whose primary function is to degrade the products of PI3K by dephosphorylating phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate at the 3 position (67). Loss of functional PTEN from tumor cells causes accumulation of these critical second messenger lipids, which in turn increase Akt phosphorylation and activity, leading to decreased apoptosis and/or increased mitogenic signaling (68). A PTEN mutation rate of 30–50% in melanoma cell lines has been reported by several groups. Among cell lines with a PTEN mutation, 57–70% showed homozygous deletion of the PTEN gene (69,70). On the other hand, PTEN mutations in metastatic melanoma samples are rare (5–20%) (71–73). Importantly, PTEN protein levels were found to be altered in metastatic melanoma in the absence of genetic alterations. Zhou et al. found no PTEN protein expression in 15% (5/34) and low expression in 50% (17/34) of melanoma samples (4 primary and 30 metastatic) (74). Surprisingly, among the five melanomas with no PTEN protein expression, four showed no deletion or mutation of the PTEN gene, indicating the action of an epigenetic mechanism of biallelic functional inactivation of PTEN (74). These observations have led to the conclusion that in addition to PTEN mutation, other mechanisms, such as epigenetic silencing (75–78), altered subcellular localization (79) or ubiquitination (80) are important in PTEN inactivation; collectively these changes could be as frequent as in 40–50% of sporadic melanomas (74). The role of PTEN in melanoma was confirmed by functional studies. Ectopic expression of PTEN was demonstrated to suppress melanoma cell growth (81) and melanoma tumorigenicity and metastasis (82). Moreover, the PI3K inhibitors wortmannin and LY294002 have antitumor activity in vitro, inhibiting proliferation and sensitizing cell lines to chemotherapy and radiation treatment (83). Collectively, these data suggest that PTEN and PI3K play an important role in melanoma tumorigenesis.
Because PTEN functions as an antagonist of PI3K-mediated signaling, a consequence of PTEN loss is the increase in Akt activity. Akt/protein kinase B (PKB), a serine/threonine kinase, is a core component of the PI3K-signaling pathway activated through phosphorylation of Ser-473/474 and Thr-308/309 (84,85). Several studies have shown that Akt/PKB activates the transcription of a wide range of genes, especially those involved in immune activation, cell proliferation, apoptosis and cell survival (85). Considering the frequency of PTEN inactivation in melanoma, dysregulation of Akt activity is expected. Accordingly, several studies documented Akt activation in melanoma. Using an antibody developed against phospho-Akt Ser-473 Dhawan et al. found no significant pAkt levels in normal and slightly dysplastic nevi in marked contrast to the dramatic pAkt immunoreactivity seen in severely dysplastic nevi and melanomas (66.3% positive) (86). More recently, in a 292 sample study, Dai et al. analyzed pAkt levels using tissue microarray and immunohistochemistry. Strong pAkt expression was observed in 17%, 43%, 49% and 77% of the biopsies in normal nevi, dysplastic nevi, primary melanoma and melanoma metastases, respectively. Increasing pAkt expression was inversely correlated with both overall and disease-free survival, and it was a poor prognostic factor for patients with melanomas less than 1.5 mm in thickness (87). Similar results were obtained by Slipicevic et al., except for a higher incidence of pAkt (54%) in benign nevi (88). Activation of Akt was also observed in Spitz nevi (89).
The finding that Akt is activated in melanomas without genetic aberrations in PTEN or Ras prompted several groups to search for possible mutations in other components of the pathway. A study by Samuels et al. showed that the PIK3CA gene, which encodes the p110α catalytic subunit of PI3Ks, is mutated in human cancers (90). However, from a series of 101 melanoma metastases, only three were identified to carry missense mutations in PIK3CA (91). Other studies failed to observe protein overexpression of PI3K (92) or amplification of PI3K genes in melanoma (93). An alternative mechanism for activation of the PI3K/Akt pathway is activation of Akt itself. Screening of the pleckstrin homology domain (94) and codons 308 and 473 (95) of Akt did not identify mutations. However, selective activation of Akt3 was shown in 43–60% of sporadic melanomas, occurring as a result of a combination of increased Akt3 expression accompanying copy number increases in the Akt3 gene and decreased PTEN protein activity caused by loss or haploinsufficiency of the PTEN gene (96). Consistent with these observations, targeted decrease in Akt3 activity using siRNA stimulated apoptotic signaling, which reduced cell survival and inhibited melanoma tumor development (96).
The positive effect of PI3K/Akt pathway on melanoma development was shown to be mediated by several mechanisms including inhibition of apoptosis, increase in cell survival and cell cycle regulation. Recently, a study by Gomez-Gutierrez showed that a triple mutant of FKHRL1, which cannot be phosphorylated by Akt, induced apoptosis in melanoma cells (97). Active Akt was shown to upregulate the cell adhesion protein MelCAM which plays a critical role in cell–cell interactions during melanoma development and whose increased expression has been associated with acquisition of malignancy by human melanoma (98,99). Similarly, PI3K and Akt have recently been shown to induce expression of MMP-2 and MMP-9 by a mechanism involving Akt activation of nuclear factor-kappa B (NF-κB) binding to the MMP promoter (100,101). Overexpression of an active form of Akt led to upregulation of VEGF, increased production of superoxide ROS, and made the switch to a more pronounced glycolytic metabolism. Moreover, subcutaneous implantation of WM35 cells overexpressing Akt led to rapidly growing tumors in vivo, while vector control cells did not form tumors (102). PI3K/Akt can also contribute to tumorigenesis by positively regulating cyclin D3 which contributes to G1-S progression (103). The mechanisms associated with the ability of Akt to suppress apoptosis include phosphorylation and inactivation of many proapoptosis proteins such as Bad (104) and caspase-9 (105). Downstream effects of Akt activation are also mediated by inactivation of the forkhead family of transcription factors (106), and activation of NF-κB (107). Overall, the PI3K pathway emerges as a central axis which is deregulated in melanoma, and in conjunction with the constitutively active MAPK signaling cascade, makes key contributions to melanoma development and progression. Targeting PI3K signaling in conjunction with MAPK is expected to offer an important therapeutic modality for the treatment of this tumor type.
THE WNT PATHWAY
Wnts are secreted glycoproteins that act as ligands to stimulate receptor-mediated signal transduction pathways involved in cell proliferation, survival, behavior and fate. Wnt proteins activate at least three different intracellular signaling pathways—the Wnt/β-catenin, the Wnt/Ca2+ and the Wnt/planar polarity pathways (108). The first, termed canonical, involves stabilization of β-catenin; the other two involve activation of protein kinase C (PKC) and c-Jun N-terminal kinases (JNKs), respectively. In the canonical Wnt pathway, in the absence of a Wnt signal, cytoplasmic β-catenin is phosphorylated at serine and threonine residues through the action of casein kinase Iα and GSK3β and degraded in a complex that also includes adenomas polyposis coli (APC) and axin (109). The pathway becomes activated when Wnt binds to its receptor Frizzled and to a low-density lipoprotein receptor-related protein-5 or -6 (LRP5 or 6) coreceptors. This ternary complex ultimately leads to activation of the cytoplasmic phosphoprotein Dishevelled, which blocks the degradation of β-catenin. This is followed by nuclear translocation of β-catenin where it interacts with specific transcription factors T cell factor/lymphoid-enhancing factor (TCF/LEF) leading to regulation of target genes (110,111). Among the Wnt family members, Wnt 1, 2, 3, 3a, 7a and 8 are involved in the Wnt/β-catenin pathway (112).
Mutations of genes encoding members of the Wnt-signaling cascade, in particular CTNNB1 and APC, are frequent in various types of human cancer. This includes, among others, colorectal carcinoma, hepatocellular carcinoma and hepatoblastoma, as well as primitive neuroectodermal tumors (113). Because activation of β-catenin appeared to be frequent in melanoma (114), in recent years several groups studied the occurrence of genetic modifications and changes in expression of CTNNB1 and APC. APC mutations were found in sporadic cases of primary melanoma (114–116) whereas hypermethylation of APC promoter 1A was present in 13% of cell lines and in 17% of melanoma biopsies (116).
Oncogenic activation of β-catenin by amino acid substitutions or deletions has been demonstrated in 23% of melanoma cell lines (115). Conversely, β-catenin mutations are rare in primary melanoma (114,117). Nonetheless, almost one third of primary human melanoma specimens display aberrant nuclear accumulation of β-catenin, although generally without evidence of direct mutations within the β-catenin or APC gene (114,117,118). Metastatic melanomas were found to contain nuclear and cytoplasmic accumulation of β-catenin and increased TCF/LEF-dependent transcription (119). These observations are consistent with the hypothesis that the Wnt pathway contributes to the behavior of melanoma cells and might be inappropriately deregulated in the genesis of this disease. Moreover, evaluation of activated β-catenin using phosphoantibodies revealed that nuclear phospho β-catenin was more common in metastatic lesions and that high levels of nuclear phospho β-catenin are associated with significantly worse overall survival (120). The finding that more melanomas have nuclear and/or cytoplasmic β-catenin accumulation than those carrying detectable mutations in CTNNB1 or APC suggests that the pathway may be activated in such tumors through aberrations in other genes. ICAT (inhibitor of β-catenin and T cell factor) was identified as a gene that negatively regulates the Wnt-signaling pathway by inhibiting the association of β-catenin with TCF-4 in the cell nucleus and represses transactivation of β-catenin–TCF-4 target genes (121). Messenger RNA expression analyses revealed ICAT transcript levels reduced to 20% or less relative to normal skin and benign nevi in more than two-thirds of melanomas. This suggests that loss of ICAT expression may contribute to melanoma progression and metastasis by virtue of altered β-catenin–TCF-4 regulation in the cell nucleus (118). The mechanism underlying the markedly reduced ICAT mRNA levels in melanomas is unclear at present.
Regardless of the underlying mechanism, constitutive activation of the Wnt/β-catenin signaling pathway is a notable feature of malignant melanoma. The identification of target genes downstream from this pathway is therefore crucial to our understanding of the disease. The POU domain transcription factor, Brn-2, has been found to be directly controlled by the Wnt/β-catenin signaling pathway in melanoma cell lines and in transgenic mice (122). Strikingly, expression of Brn-2 is not only upregulated by β-catenin but is also elevated in response to MAPK (39). Consistent with upregulation of these two pathways, Brn-2 expression is strongly upregulated in melanoma. Overexpression of this gene has been associated with increased proliferation and tumorigenicity in melanoma (122,123). A key role of Wnt signaling in melanocyte development is the activation of the promoter for the gene encoding MITF (124,125). MITF (126,127) is essential for development of the melanocyte lineage and has key functions in control of cell proliferation and survival and in differentiation (41). It was also demonstrated that β-catenin’s contribution to growth of melanoma cells depends on its downstream target, MITF. Moreover, suppression of melanoma clonogenic growth by disruption of the β-catenin–TCF/LEF complex is rescued by constitutive MITF. β-catenin regulation of MITF expression thus represents a tissue-restricted pathway that significantly influences the growth and survival behavior of this notoriously treatment-resistant neoplasm (128). Interestingly, it was recently shown that MITF can interact directly with β-catenin and redirect its transcriptional activity away from canonical Wnt signaling-regulated MITF-specific target genes (129).
Wnt induction is blocked by five classes of proteins—Dkk, Wise, Sfrp, Wif and Cerberus—competing for the Wnt ligand or for the Lrp-Frz-receptor (130). Interestingly, whereas Dickkopf-1 protein secretion was documented in breast, prostate and lung cancer lines, it was negligible in melanoma (131). Kuphal et al. found that DKK-1, -2 and -3 were downregulated or lost in all cell lines and in most of the melanoma tumor samples analyzed (132). Overexpression of Dkk-1 (133) and WIF-1 (134) inhibited melanoma tumor growth in a xenograft mouse model. Similarly, it has been recently shown that an anti-Wnt-2 monoclonal antibody induced apoptosis in malignant melanoma cells and inhibited tumor growth (135).
In recent years it has become clear that Wnt signaling can also function via β-catenin-independent pathways. These noncanonical pathways include: (1) calcium/calmodulin-dependent kinase II (CAMKII), and PKC, (2) phospholipase C (PLC) and phosphodiesterase (PDE), and (3) a pathway termed convergent extension, which is similar to the planar polarity in Drosophila that activates the Jun-N-terminal kinase (136). Activation of these noncanonical Wnt pathways is mediated by Wnt 4, Wnt11 and mainly Wnt 5a. Wnt signaling has been shown to be important not only in development but also in tumorigenesis. Wnt5a is upregulated in cancers of the lung, breast and prostate, and is downregulated in pancreatic cancer (137,138). Expression profiling studies aimed at identifying molecular subclasses of tumors found a series of genes whose expression differed in cutaneous melanomas with differing invasive phenotypes (139,140). Among genes that created the distinct classes were those important in cell motility and invasive ability. WNT5a was identified as a particularly robust marker of highly aggressive behavior (139). Unlike that of other Wnt family members (e.g. Wnt1 and Wnt8), Wnt5a expression does not profoundly affect β-catenin stabilization. Instead, Wnt5a stimulates intracellular Ca2+ release and activates CAMKII and PKC in a G-protein-dependent manner (141). The study by Bittner et al. confirmed early studies of Wnt5a RNA expression in tumors and indicated that overall, many tumors showed increased Wnt5a expression relative to their normal tissue of origin, and accordingly that melanomas showed increased Wnt5a expression relative to skin (139). Furthermore, Wnt5a protein expression in human melanoma biopsies directly correlates with increasing tumor grade, cell motility and invasion of metastatic melanoma (142), and inversely correlates with patient survival (139). Importance of the Wnt5a–Frizzled pathway in melanoma was confirmed by analysis of serial analysis of gene expression (SAGE) libraries from melanoma tissues. This study allowed identification of several genes associated with changes in calcium flux and PKC signaling (PLC gamma, inositol-1,4,5-triphosphate-3 kinase B and C, PKC θ) (143), consistent with increased PKCα/βII and PKCμ activity and increased motility and invasiveness of melanoma cells expressing Wnt5a (142). As additional support for the notion that this pathway contributes to the invasive phenotype of the melanoma cells, the authors demonstrated that inhibition of this pathway by desensitization of the Wnt5a receptor, Frizzled5, by an antibody that interfered with activation by Wnt5a resulted in decreased activation of the PKC pathway and inhibition of in vitro motility and invasion phenotype of the melanoma cells (142). By using overexpression and downregulation of Wnt5a, it was recently found that Wnt5a/PKC stimulates melanoma cell motility via induction of genes involved in the epithelial to mesenchymal transition (EMT) of carcinomas including upregulation of vimentin and Snail (a repressor of E-cadherin), and downregulation of E-cadherin (144). Increased expression of Wnt5a in melanoma tumors is localized, occurring in cells at the site of active invasion and in cells showing morphologic features associated with aggressive tumor behavior. In connection with Wnt5a, it is noteworthy that PKC has been identified as a contributing factor in skin tumorigenesis. In models of melanoma, PKC-α activation is typically associated with increased tumor cell proliferation and invasiveness, and decreased differentiation (145). Of interest, it has also been shown that increase or inhibition of PKC activity results in corresponding changes in Wnt5a expression (146). These observations suggest the possibility that the activities of Wnt5a and PKC drive a positive feedback loop, perhaps a Wnt5a autocrine loop, and that increase in the activity of either may result in increased melanoma motility.
THE JNK/c-JUN PATHWAY
Investigation into the JNKs has focused typically on their activation in response to diverse stresses. In more recent studies these kinases are recognized for their importance in the regulation of mammalian physiology, including: cell proliferation, cell survival, cell death, DNA repair and metabolism. Activation of JNK is induced by a variety of extracellular stimuli, growth factors, cytokines, tumor promoters, UV radiation and hormones (147). JNK is activated by sequential protein phosphorylation through a MAP kinase module, i.e. MAP3K-MAP2K-MAPK (148). Two MAP2Ks (JNKK1/MKK4/SEK1 and JNKK2/MKK7) have been identified for JNK. Phosphorylation of JNK by these dual-specificity protein kinases on Thr183 and Tyr185 is necessary for its activation (147). Several MAP3Ks, including members of the map-erk kinase kinase (MEKK) family, activator of S-phase kinase 1 (ASK1), mixed lineage kinase (MLK), TGF-beta-activated kinase 1 (TAK1) and Tumor progression loci-2 (TPL 2), have been reported to act as MAP3Ks for JNK (149).
JNK has been shown to elicit both positive and negative effects on tumor development depending upon the cellular context (150). JNK activation is required for Ras-mediated transformation (151) and has been found to mediate proliferation and tumor growth (152,153). These observations are consistent with the finding of constitutively active JNK in tumor samples and derived cell lines (40,150,154). On the other hand, studies using JNK−/− fibroblasts revealed that JNK is not required for Ras-dependent tumor development in vivo and that JNK1−/− JNK2−/− fibroblasts are more tumorigenic than wild-type cells (155). The role of JNK in tumorigenesis was also studied in the fly using three different tumor models of increasing malignancy. Similar to that found in mammals, JNK was found to either promote or eradicate tumors depending on the genetic context (156).
Among the many proteins phosphorylated following JNK activation, the one which has been better studied in context of cancer development is the transcription factor c-Jun. JNK-mediated phosphorylation at serines 63 and 73 enhances the ability of c-Jun, a component of the AP-1 transcription complex, to activate transcription in response to a plethora of extracellular stimuli (157). The JNK activation leads to induction of AP-1-dependent target genes involved in cell proliferation, cell death and inflammation. Members of the AP-1 transcriptional complex include c-Jun, JunB, JunD, c-Fos, FosB, Fra-1 and Fra-2, all of which contain a leucine zipper and form either homodimers or heterodimers through this domain. The different dimer combinations recognize different response elements in the promoters and enhancers of target genes (158,159). AP-1 target genes are differentially regulated by distinct AP-1 dimers. The dynamic changes in AP1 composition after stress-stimuli balance discrete signals that play a key role in determining whether cells undergo apoptosis, survival or senescence (160). Among other c-Jun heterodimeric partners that influence the AP1 transcriptional readout is ATF2, which has been shown to play a key role in melanoma development (161).
Whereas the role of JNK in oncogenesis is emerging, c-Jun is a well-defined oncogene in several malignancies. c-Jun is highly amplified and overexpressed in undifferentiated and aggressive human sarcomas (162), and has been associated with proliferation and angiogenesis in invasive breast (163) and lung cancer (164). Moreover, c-Jun phosphorylation is required for Ras-induced transformation of fibroblasts in vitro and Ras-induced skin tumorigenesis in vivo (165). c-Jun has been shown to contribute to the early stages of carcinogen-induced hepatocellular carcinoma by antagonizing the action of p53 (166). Along these lines, a recent article by Das et al. showed that JNK negatively regulates p53-dependent senescence in MEF (167). These studies suggest that the JNK/c-Jun pathway may contribute to cellular transformation by downregulating the p53 tumor suppressor. In the last several years, the JNK/c-Jun pathway has been shown to play an important role in melanoma development. Of note, the relevance of this pathway in melanoma was already recognized in the early 1990s (168,169). c-Jun, Jun-B and c-fos genes have been shown to play a role in the transformation of melanocytes into malignant melanoma (168). Further changes in the composition of AP-1 components studied during progression of melanoma were revealed in mouse melanoma B16 tumor models (169). These data suggest a potential role for AP-1 in the transformation of melanocytes into malignant melanoma. Constitutive activation of JNK in melanoma cell lines and melanoma tumor samples was recently described by our laboratory (40), and by Jørgensen et al. (170). Interestingly, activation of JNK during tumor progression is associated with cell proliferation and shorter relapse-free period for patients with superficial spreading melanomas (170).
The possible role of the JNK/c-Jun pathway in tumorigenesis has led several groups to study the potential clinical relevance of interfering with this pathway. Inhibition of JNK signaling by chemical inhibitors or siRNA inhibited proliferation in non-small cell lung cancer (NSCLC) (154) and breast (171) cell lines and induced apoptosis in prostate cancer cells (172). c-Jun and JunB knockdown in B16-F10 melanoma cells by short hairpin RNA resulted in cell cycle arrest and apoptosis mediated by apoptosis inducing factor and extended survival of mice inoculated with these modified tumor cells (173). These results suggest that in the absence of c-Jun, JunB can act as a tumor promoter; therefore, inactivation of both c-Jun and JunB may provide a valuable strategy for antitumor intervention (173).
Despite an increasing body of evidence implicating the JNK/c-Jun pathway in cancer, little is known about the genetic and mechanistic basis for these findings. Genetic alterations have not been described in JNK or in its upstream kinases. Worthy of mention is that Ras, which requires JNK and c-Jun for transformation, is activated in 30% of human cancers (174). A possible mechanism explaining JNK activation involves the tumor suppressor p16(INK4a) which is frequently deleted in melanoma (175). It was shown that p16(INK4a) can bind to the glycine-rich loop of the N-terminal domain of JNK, inhibiting c-Jun phosphorylation, thus interfering with cell transformation promoted by the H-Ras-JNK-c-Jun-AP-1 signaling axis (176). Recently, a new link between the constitutively active MEK/ERK and JNK pathway was demonstrated. Through its positive effect on c-Jun, ERK enforces a feed-forward mechanism by which c-Jun partially contribute to increased JNK activity (40). Tumor necrosis factor (TNF) receptor associated factor 2 (TRAF2), as well as other members of the TRAF family (TNF receptor [TNFR]-associated factors), is upregulated in various tumors including melanomas (177), and through its effect on MEKK1–MKK4/7 can efficiently activate JNK-c-Jun. Stimulation of TNFR results in receptor trimerization and the recruitment of TNFR associated factors (TRAFs) and/or TNFR-associated death domain protein to the cytoplasmic regions of the receptors (178). TRAF2 plays a critical role in the regulation of most stress kinases, including ASK1, MEKK1 and I-kappa-B kinase (IKK) (179–181). Indeed, expression of a RING finger-deleted TRAF2, which serves as a dominant negative, resulted in sensitization of metastatic melanoma to apoptosis and coincided with upregulation of p38 and tumor necrosis factor-α (TNF-α) and downregulation of NF-κB activities (182).
JNK/c-Jun and PKC
PKC has long been identified as a contributing factor in skin tumorigenesis. In models of melanoma, PKCα expression and activation was associated with increased tumor cell proliferation, invasiveness and metastasis (145,183–187). This is in agreement with the upregulation of several PKC isoforms seen in melanoma cells compared to melanocytes (188). A classic effect of PKC activation is the transcriptional activation of AP-1 target genes (189). Recently it has been described in melanoma cultures that PKC can phosphorylate JNK and enhance JNK activation by MKK4/MKK7 (190). Similar to that described for JNK, the upstream events involved in PKC activation in melanoma are not clear. One interesting possibility is that PKC is activated in response to activation of the noncanonical Wnt pathway (142, see also The Wnt Pathway section). The recently established link between PKC and JNK raises the possibility that the noncanonical Wnt pathway might be also partially involved in the activation of JNK. JNK activation downstream of Wnt is firmly established as part of the planar cell polarity pathway (see Wnt section) but whether or not these pathways are connected in melanoma will require further studies.
JNK/ATF2
Another JNK target also implicated in melanoma is ATF2. ATF2 is activated by JNK and p38 which phosphorylates residues 69 and 71 on ATF2. p38 is a member of the MAPK signaling network and is activated by TRAF2/ASK1/MKK3/6 signaling. ATF2 is a member of the bZIP family of transcription factors, which elicits its transcriptional activities after heterodimerization with c-Jun, as with Rb, CREB and p65/NF-κB following its phosphorylation by the stress kinases, JNK or p38-MAPK (191,192). ATF2 has been implicated in the regulation of TNF-α, transforming growth factor-β (TGF-β), IL-6, cyclin A and E-selectin (192–194). ATF2 activities have also been associated with tumor development and progression (195). Activation of ATF2 by hepatocyte growth factor/scatter factor (HGF/SF) through p38-MAPK and SAPK/JNK mediates proliferation signals in melanoma cells (196). ATF2 plays an important role in the acquisition of resistance to chemotherapy and radiation therapy in human melanoma (197,198). As a transcription factor, ATF2 is active within the nuclear compartment, where it elicits its transcriptional activities. Of particular interest is the finding that nuclear ATF2 expression is more frequently found in metastatic sites (lymph nodes, bone metastases or visceral metastases) than in primary cutaneous specimens, and that it correlates with poor prognosis (199). Strong nuclear staining in a melanoma tumor specimen (199) suggests that ATF2 is subject to constitutive activity. Of importance, ATF2 activities are not limited to transcription control as it was also shown to play an important role in DNA damage response upon its phosphorylation by ataxia telangiectasia mutated (ATM)/ATM and Rad3-related (ATR) (200). ATF2 that is phosphorylated by ATM is found within DNA damage repair foci and contributes to the intra-S phase checkpoint response following the formation of double strand breaks (200). Inhibition of ATF2 activities, either by expression of its dominant negative forms or via short ATF2-driven peptides that out-compete the endogenous protein, has been found to be efficient in sensitizing human and mouse melanoma cells to apoptosis (161,197,201). Significantly, expression of the ATF2-driven peptide in mouse melanoma models prone to metastasis (B16F10 and SW1) resulted in inhibition of melanoma growth and metastasis in the corresponding syngeneic mouse model (202). Mechanistically, when ATF2 is inhibited, JunD was found to cooperate with c-Jun and increase AP1 activities, which elicited a proapoptotic signaling in melanoma (203). Screening for natural compounds that could mimic the ATF2 peptide has led to the identification of Celasterol and gambogic acid that were found to efficiently inhibit melanoma both in culture and in mouse tumor models (204). Intriguingly, while ATF2 elicits oncogenic activities in melanoma, it appears to elicit tumor suppressor activities in nonmalignant skin tumors (205). The nature of tissue-specific differences is currently being elucidated. In all, ATF2 appears to serve one of the important functional arms for the ERK and JNK pathways in melanoma, through which it contributes to tumor development and progression.
THE NF-κB Pathway
The mammalian NF-κB family contains five members—p105/p50 (NF-κB1), p100/p52 (NF-κB2), RelA (p65), RelB and cRel (206,207). NF-κB1 and NF-κB2 are synthesized as the inactive cytosolic precursors p105 and p100, respectively. The canonical activation of NF-κB pathway involves TNF-α stimuli resulting in the activation of TNFR and association of TRAF2/MAP3K module with subsequent phosphorylation/activation of IKK. In turn, IKK-mediated phosphorylation of IκB leads to IκB ubiquitination and proteasomal degradation, releasing an active NF-κB complex (208,209). The composition of activated NF-κB complexes will determine the type of genes that will be trans-activated. For example, NF-κB complexes containing cRel generally activate proapoptotic genes such as DR4/DR5 and Bcl-X, and inhibit anti-apoptotic genes such as cellular inhibitor of apoptosis (cIAP)1, cIAP2 and survivin after TNF-related apoptosis-inducing ligand treatment. Conversely, RelA inhibits expression of DR4/DR5, and up-regulates caspase-8, cIAP1 and cIAP2 (210).
NF-κB is often activated in tumors including melanomas (211). Numerous distinct mechanisms were proposed to be responsible for the elevated level of NF-κB activity in malignant melanoma. For example, sustained NF-κB activation results in induction of chemokines CXCL1 and CXCL8. CXCL1, in turn, is capable of activating IKK and NF-κB demonstrating the presence of a feed-forward mechanism that could contribute to the constitutive activation of NF-κB in melanoma cells (212). The CXC chemokine MGSA/GROα, which is constitutively expressed in melanoma, is also able to induce NF-κB (RelA) activation in a manner dependent on Ras-MEKK1-MEK3/6-p38 pathway (213). Activation of NF-κB activity in melanoma was also linked to loss of E-cadherin expression seen frequently during malignant transformation of melanocytes (214) and was also associated with increased cytoplasmic β-catenin coupled with p38-dependent NF-κB activation (215). In contrast, UV-induced activation of ASK1-p38 disrupts IκBα phosphorylation and decreases transcriptional activity of NF-κB (216), suggesting that in melanoma multiple factors are cooperating, in concert, in the activation of NF-κB.
Consistent with the notion that multiple signaling can contribute to the activation of NF-κB in melanoma, several studies had pointed to the role of the Ras–Raf–MEK–ERK cascade in activation of NF-κB. NIK, an activator of IKK, is highly expressed in melanoma cells, and IKK-associated NIK activity is enhanced in these cells compared with normal cells. It was shown that expression of kinase-dead NIK blocked constitutive NF-κB promoter activity in melanoma cells, but not in control normal human melanocytes. Importantly, overexpression of wild-type NIK results in increased phosphorylation of ERK1/2, and overexpression of a dominant negative ERK construct causes decreased NF-κB promoter activity. These data suggest that ERK acts upstream of NF-κB and regulates NF-κB DNA binding activity (217). Activation of NF-κB by TNF-α prevented the induction of apoptosis following inhibition of B-Raf signaling, further supporting a regulatory link between ERK and TRAF2 signaling (218).
Another important variable in the control of NF-κB availability in melanoma, but also other tumors, is the level and activity of the IκB ligase. Inhibition of β-Trcp (aka HOS), the ubiquitin ligase which efficiently targets IκB for ubiquitination-dependent degradation (219), efficiently sensitizes melanoma cells to apoptosis in response to treatment (220). Interestingly, melanocytes expressing the oncogenic form B-RafV600E exhibit enhanced expression of β-Trcp (221), thereby pointing to the possible use of this ligase as a selective target in melanoma.
The PI3K/Akt pathway was also implicated in activation of NF-κB through different mechanisms. Thus, full transcriptional activation of RelA requires phosphorylation in the transactivation domain, which might be mediated by Akt (222,223). Along these lines, PI3K/Akt pathway-dependent phosphorylation of p50 was shown to increase the binding of NF-κB to DNA (224). Of note, in melanoma cells, inhibitors of PI3K blocked the transcriptional activity of the endogenous NF-κB, without attenuating IKK-mediated phosphorylation of IkBα (86), indicating that PI3K/Akt may affect NF-κB independent of IKK/IκBα (222). Overall, signaling mediated by constitutively active Akt kinase in melanoma could contribute to high transcriptional activity of NF-κB dimers that specifically include RelA, and, thus to enhance the anti-apoptotic properties of melanoma.
It is generally accepted that a major contribution of NF-κB in the development and progression of melanoma relates to its function as a regulator of survival and apoptosis. Meyskens et al. showed that in metastatic melanoma cells, increase in DNA-binding activity of NF-κB is paralleled by increased expression of p50 and RelA—antiapoptotic regulators—and its inhibitor IkBα. However, expression of cRel—transcriptional activator of proapoptotic genes—is markedly decreased in melanoma cells compared with normal melanocytes (225). Consistent with these observations, strong p50 nuclear staining correlated with poor prognosis in melanoma patients (226). In addition to eliciting its antiapoptotic activities NF-κB mediates transcription of MMP2 (227,228) and MMP9 (229,230) whose overexpression was associated with tumor invasion, angiogenesis and metastasis.
Taken together, the emerging theme is that independent pathways which are activated in melanoma (Ras/Raf/MEK/ERK, PI3K/Akt, p38, p16INK4a) contribute not only to increased NF-κB activity, thereby assuring its role in proliferation and survival, but also to tumor progression and invasiveness.
THE JAK/STAT PATHWAY
STAT proteins include a family of transcription factors involved in the activation of target genes in response to cytokines and growth factors (231,232). Tyrosine-phosphorylated STATs undergo homodimerization or heterodimerization, followed by translocation to the nucleus where they contribute to gene transcription (233). Four mammalian JAKs (234) and seven STAT members (235) provide different patterns of gene transcription upon specific stimulation. A variety of mechanisms controlling the level and duration of STAT activation contributes to the complexity of the pathway. These include dephosphorylation of the receptor complex or nuclear STAT dimers by PTPases, interaction of activated STATs with inhibitory molecules from the protein inhibitor of activated STAT family, and feedback inhibition of the pathway by suppressor of cytokine signaling (SOCS) proteins through inhibition and/or degradation of JAKs (236,237). Also, different kinases are able to regulate STAT activity (45,238,239).
Two mechanisms have been suggested to mediate STAT’s effect on carcinogenesis: their impact on immune surveillance (240) and control of growth factor signaling, apoptosis and angiogenesis (241); both are likely to also impact melanoma development and progression. Among the different STATs, STAT3 was shown to play an important role in melanoma development. Expression of a STAT3 dominant-negative variant, STAT3β, was found to induce cell death in murine B16 melanoma cells and to cause inhibition of tumor growth and tumor regression associated with increased apoptosis (242). STAT3 mediates IL-6-induced growth inhibition of normal melanocytes and early-stage melanoma cells, and promotes growth of advanced melanomas (243). In addition to regulation of apoptosis and proliferation STAT3 directly interacts with MMP2 promoter in melanoma cells, causing overexpression of the MMP2 protein in metastatic melanomas. Blockage of activated STAT3 in metastatic cells suppresses invasiveness of the tumor cells, inhibits tumor growth and prevents metastasis in nude mice (244). Active STAT3 upregulates the activity of basic fibroblast growth factor and VEGF promoters in melanoma cells, suggesting its possible role in angiogenesis (245).
Interestingly, overexpression of mutated STAT3 which cannot be phosphorylated/activated increases expression of oncogenes N-Ras and c-MET (246), suggesting the possible existence of a feedback inhibition loop between those two pathways. Combined activity of STAT3 and c-Jun in human melanoma cells mediates suppression of Fas transcription, suggesting that STAT3 oncogenic activities could be mediated through its cooperation with c-Jun, resulting in downregulation of Fas expression, which is implicated in melanoma’s ability to resist apoptosis (247). Interestingly, inhibition of PI3K/Akt signaling disrupts cooperation between c-Jun and STAT3, which is required for silencing of the FasR promoter, resulting in increased expression of FasR and concomitant sensitization to FasL-mediated apoptosis (239).
In addition, constitutive Src kinase activity, but not EGFR or JAK activity, was shown to contribute to increased activation of STAT3 in human melanoma cells (248). Inhibition of Src or STAT3 activity caused downregulation of the antiapoptotic genes Bcl-XL and Mcl-1, and increased apoptosis (248). Src and JAK1 kinases were implicated in STAT5 phosphorylation in malignant melanoma contributing to its survival (249). Another component important in the regulation of STATs is SOCS3, a member of the SOCS family of endogenous inhibitors of STATs. SOCS3’s promoter region was found to be aberrantly methylated in three out of five malignant melanomas, which correlated with low expression of SOCS3 protein (250). In agreement with this observation Fojtova et al. (251) reported that progression of melanoma cells from interferon (IFN) sensitivity to IFN insensitivity associates with increase in SOCS3 expression, lower SOCS1–3 activation following IFN treatment and increased STAT1 activation. Taken together, these data suggest that the highly metastatic, resistant to IFN therapy phenotype of malignant melanoma can be attributed at least in part to changes in SOCS expression.
The contribution of JAK/STAT signaling to IFN resistance of melanoma cells is widely recognized, however the mechanism is not well understood. Wong et al. found low expression of STAT1 and STAT2 in resistant cells. Transfection of IFN-resistant cells STAT1 partially restored IFN responsiveness (252) and it was possible to abrogate IFN-γ-induced growth inhibition by overexpression of dominant negative STAT1 (253), suggesting that STAT1 plays a role in the antiproliferative response. Interestingly, IFN-α activation of STAT5 in melanoma cells attenuates the activation of STAT1. In a separate study, IFN-α was found to upregulate pSTAT1, whereas it downregulated pSTAT3 and total STAT3 levels in tumor cells (254). Thus the balance between STAT5, STAT3 and STAT1 is expected to impact their heterodimerization and transcriptional programs (255).
As outlined in this section, members of the STAT protein family are directly involved in distinct cellular mechanisms resulting in increased melanoma cell proliferation, its resistance to apoptotic stimuli, enhanced invasiveness and angiogenesis. Although attractive (243), further clarification of the precise STAT members which elicits these activities is required before consideration for their selective targeting can be considered.
EMERGING PATHWAYS
HIF-1α
An important factor in tumor development and progression is its surrounding microenvironment. Among key microenvironmental factors is oxygen tension. The skin has been shown to be mildly hypoxic (256), which requires cells to activate programs to enable their survival under hypoxia. HIF-1 is among the key regulatory components of the cellular ability to adapt to hypoxia. HIF-1, which is stabilized under hypoxia, serves as a potent transcription factor which activates a multitude of O2-responsive genes involved in survival, apoptosis, glucose metabolism and angiogenesis (257). This genetic reprogramming was shown to play major roles in tumor development and progression (258).
Nuclear HIF-1α staining has been shown in normal skin (259), suggesting HIF-1α activity in melanocytes. This finding is consistent with the finding that HIF-1α is a MITF transcriptional target in melanocytes (260). Moreover, HIF-1α transcription increases as a consequence of ERK pathway activation (261). A role for hypoxia and HIF-1α expression in melanoma has also been recently suggested. Bedogni et al. showed that the hypoxic microenvironment in the skin contributes to melanocyte transformation and tumor growth induced by Akt (262). Along this line, inhibition of HIF-1α decreases Akt transformation capacity under hypoxia and tumor growth in vivo (262).
The angiogenic factor VEGF is among HIF-1α targets and is an important mediator of HIF-1α effects in tumor growth and metastatic capacity. Production of VEGF has been shown to be induced in melanoma following hypoxia (263). As shown in other tumor models, expression of VEGF was shown to increase tumor growth, angiogenesis, invasiveness and metastasis of melanoma cells (264,265). Work from our laboratory has identified the role of the ubiquitin ligase Siah2 in the regulation of HIF-1α availability, through its ubiquitination-dependent degradation of prolyl hydroxylase 3 (PHD3; 266). PHD3 modification of HIF-1α is required for its recognition by the ubiquitin ligase pVHL, leading to its degradation under both normoxia and physiologic hypoxic conditions (267). More recent studies using melanoma cell lines have identified the role of Akt in the regulation of Siah2 transcription and HIF-1 stability (N. Singha, G. Hogg, P. Hu, P. Lopez-Bergami, K. Liu, G. Boyi, J.H. Paik, K. Nakayama, S.H. Lecker, R.A. DePinho, D. Bowtell, C. Hauser, R. Bodmer and Z. Ronai, unpublished data). Further, inhibition of Siah2 activity effectively blocks melanoma tumorigenesis and metastasis via two distinct pathways—HIF and Ras signaling (J. Qi, K. Nakayama, S. Gaitonde, J. Goydos, S. Krajewski, R. Cardiff, A. Eroshkin, D. Bar-Sagi, D. Bowtell and Z. Ronai, unpublished data). These observations link Akt and HIF signaling and the role of the ubiquitin ligase Siah in control of both HIF and Ras pathways, which are subject to major changes in melanoma.
While the emerging evidence clearly points to the important role of HIF-1a in melanoma development and progression and as an attractive target for development of novel cancer therapeutics (266), additional studies are required to better understand the impact of rewired signaling on the hypoxia response in this tumor type.
The TGF-β pathway
The TGF-β family includes multiple factors that have dual tumor suppressor and oncogenic effects (268). TGF-β binds to membrane receptors that have a cytoplasmic serine/threonine kinase domain. Binding of the ligand causes the assembly of a receptor complex that phosphorylates proteins of the SMAD family that bind DNA and regulates transcription of several genes resulting in diverse effects (269). TGF-β1 is overexpressed in human melanoma cells and stimulates the neighboring stroma cells through increased production and deposition of extracellular matrix proteins. The activation of stroma leads to a tumor cell survival advantage and increased metastasis (270).
Although melanoma cells are resistant to the tumor-suppressive effects of TGF-β, there are no detectable defects at the receptor/SMAD level as seen in other tumors (271). Instead, inhibition of the TGF-β pathway in melanoma is mediated by expression of related genes such as SKI, filamin, endoglin and follistatin (272). For example, SKI inhibits TGF-β signaling through its association with the Smad proteins. However, recently identified novel functions imply that SKI can act as oncogen (273).
The Notch pathway
Notch is an evolutionarily conserved signaling mechanism that participates in a variety of cellular processes—cell fate specification, differentiation, proliferation, apoptosis, adhesion, EMT, migration and angiogenesis. Both Notch and its ligands, Delta and Jagged, are transmembrane proteins. The ligand is expressed on an adjacent cell and activates Notch signaling through a direct cell–cell interaction. Upon ligand binding, Notch intracellular domain is cleaved and translocated to the nucleus, with subsequent activation of target gene transcription (274).
Involvement of Notch signaling in several cancers is well known (275). Its participation in melanoma was first described by Hoek et al. following comparison of gene expression patterns in normal human melanocytes and melanoma cell lines using microarrays (276). Later, expression of Notch-1 and Notch-2, as well as Notch ligands, was found to be upregulated in “dysplastic nevi” and melanomas compared with common melanocytic nevi (277). Notch inhibitor studies implicate Notch signaling as a requirement for melanoma survival. Inhibition of γ-secretase, an enzymatic complex involved in cleavage, and release of Notch induced apoptosis in melanoma cell lines but not in control melanocytes. Apoptosis was mediated by upregulation of BH3-only members Bim and Noxa and was independent of p53 (278). The oncogenic effect of Notch-1 were described to be also mediated by β-catenin, which was upregulated following Notch-1 activation (279) and by activation of the MAPK and Akt pathways (280). However, a more complex signaling is foreseen as Notch signaling was shown to cross-talk with other signaling molecules including Sonic hedgehog (Shh) and p63 (281).
CONCLUDING REMARKS
In this review, we have summarized some of the major changes in signal transduction pathways that contribute to melanoma development and progression. As we better understand the relative contribution of each signaling pathway, we also appreciate the cross-talk among the pathways which is responsible for the modified blueprint of signal transduction cascades in this tumor type. Our better understanding of key signaling pathways that underwent modification in melanoma has spurred the development of drugs that selectively target these pathways. Along these lines several inhibitors of the B-Raf/MEK/ERK pathway have been produced, including the MEK1/2 inhibitors AZD6244, PD0325901 or CI-1040 (282,283) and the B-Raf inhibitor 43-9006 (Sorafenib) (284). The latter is actually a multikinase inhibitor as it also inhibits receptor tyrosine kinases. Currently, only preliminary results of small to medium-sized Phase II clinical trials are available for metastatic melanoma, and initial promising results have been reported for the combination of sorafenib and chemotherapy (285,286). It is expected that ongoing trials in combination with inhibitors to other pathways (i.e. PI3K/AKT; 83) will have a significant impact in the treatment of melanoma. Overall, exciting development in understanding melanoma biology over the past few years combined with growing efforts to further decipher the complexity of the genetic and epigenetic changes is expected to result in better therapeutic modalities that would help treat malignant melanoma, while providing a paradigm for other tumor types in which rewired signaling is so predominant.
Footnotes
This paper is part of a special issue dedicated to Professor Hasan Mukhtar on the occasion of his 60th birthday.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the B-Raf gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.van Elsas A, Zerp S, van der Flier S, Kruse-Wolters M, Vacca A, Ruiter DJ, Schrier P. Analysis of N-ras mutations in human cutaneous melanoma: Tumor heterogeneity detected by polymerase chain reaction/single-stranded conformation polymorphism analysis. Recent Results Cancer Res. 1995;139:57–67. doi: 10.1007/978-3-642-78771-3_5. [DOI] [PubMed] [Google Scholar]
- 3.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. N-Ras and B-Raf mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin. Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- 4.Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J. Biol. Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 5.Carr J, MacKie RM. Point mutations in the N-Ras oncogene in malignant melanoma and congenital naevi. Br. J. Dermatol. 1994;131:72–77. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 6.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of B-Raf mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 7.Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, Houghton AN, Busam K, Polsky D. Analysis of B-Raf and N-Ras mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–3957. [PubMed] [Google Scholar]
- 8.Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, Pyrhonen S, Hemminki K. B-Raf mutations in metastatic melanoma: A possible association with clinical outcome. Clin. Cancer Res. 2003;9:3362–3368. [PubMed] [Google Scholar]
- 9.Ball NJ, Yohn JJ, Morelli JG, Norris DA, Golitz LE, Hoeffler JP. Ras mutations in human melanoma: A marker of malignant progression. J. Invest. Dermatol. 1994;102:285–290. doi: 10.1111/1523-1747.ep12371783. [DOI] [PubMed] [Google Scholar]
- 10.Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both B-Raf mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 11.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int. J. Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 12.Collisson EA, De A, Suzuki H, Gambhir SS, Kolodney MS. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res. 2003;63:5669–5673. [PubMed] [Google Scholar]
- 13.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R. B-Raf is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 14.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of B-Raf(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–5202. [PubMed] [Google Scholar]
- 15.Sumimoto H, Miyagishi M, Miyoshi H, Yamagata S, Shimizu A, Taira K, Kawakami Y. Inhibition of growth and invasive ability of melanoma by inactivation of mutated B-Raf with lentivirus-mediated RNA interference. Oncogene. 2004;23:6031–6039. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- 16.Houben R, Becker JC, Kappel A, Terheyden P, Brocker EB, Goetz R, Rapp UR. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J. Carcinog. 2004;3:6. doi: 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akslen LA, Angelini S, Straume O, Bachmann IM, Molven A, Hemminki K, Kumar R. B-Raf and N-Ras mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J. Invest. Dermatol. 2005;125:312–317. doi: 10.1111/j.0022-202X.2005.23788.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang DZ, Panageas KS, Osman I, Polsky D, Busam K, Chapman PB. Clinical significance of B-Raf mutations in metastatic melanoma. J. Transl. Med. 2004;2:46. doi: 10.1186/1479-5876-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, Redman B, Thomas NE, Gruber SB. B-Raf and N-Ras mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 20.Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S, Niitsu Y. Analysis of K-ras, APC, and β-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599–611. doi: 10.1053/gast.2001.27203. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K-Ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology. 1995;108:434–440. doi: 10.1016/0016-5085(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 22.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. B-RafE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 23.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative reciprocity of N-Ras and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800–1804. [PubMed] [Google Scholar]
- 24.Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, Torre GD, Perrone F, Luoni C, Suardi S, Frattini M, Pilotti S, Anichini A, Tragni G, Parmiani G, Pierotti MA, Rodolfo M. B-Raf alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23:5968–5977. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- 25.Tomicic J, Wanebo HJ. Mucosal melanomas. Surg. Clin. North Am. 2003;83:237–252. doi: 10.1016/S0039-6109(02)00100-7. [DOI] [PubMed] [Google Scholar]
- 26.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- 27.Saldanha G, Purnell D, Fletcher A, Potter L, Gillies A, Pringle JH. High B-Raf mutation frequency does not characterize all melanocytic tumor types. Int. J. Cancer. 2004;111:705–710. doi: 10.1002/ijc.20325. [DOI] [PubMed] [Google Scholar]
- 28.Cohen Y, Goldenberg-Cohen N, Parrella P, Chowers I, Merbs SL, Pe’er and J, Sidransky D. Lack of B-Raf mutation in primary uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003;44:2876–2878. doi: 10.1167/iovs.02-1329. [DOI] [PubMed] [Google Scholar]
- 29.Edmunds SC, Cree IA, Di Nicolantonio F, Hungerford JL, Hurren JS, Kelsell DP. Absence of B-Raf gene mutations in uveal melanomas in contrast to cutaneous melanomas. Br. J. Cancer. 2003;88:1403–1405. doi: 10.1038/sj.bjc.6600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards RH, Ward MR, Wu H, Medina CA, Brose MS, Volpe P, Nussen-Lee S, Haupt HM, Martin AM, Herlyn M, Lessin SR, Weber BL. Absence of B-Raf mutations in UV-protected mucosal melanomas. J. Med. Genet. 2004;41:270–272. doi: 10.1136/jmg.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilic E, Bruggenwirth HT, Verbiest MM, Zwarthoff EC, Mooy NM, Luyten GP, de Klein A. The Ras-B-Raf kinase pathway is not involved in uveal melanoma. Melanoma Res. 2004;14:203–205. doi: 10.1097/01.cmr.0000130006.46885.a0. [DOI] [PubMed] [Google Scholar]
- 32.Shields JM, Thomas NE, Cregger M, Berger AJ, Leslie M, Torrice C, Hao H, Penland S, Arbiser J, Scott G, Zhou T, Bar-Eli M, Bear JE, Der CJ, Kaufmann WK, Rimm DL, Sharpless NE. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- 33.Pavey S, Johansson P, Packer L, Taylor J, Stark M, Pollock PM, Walker GJ, Boyle GM, Harper U, Cozzi SJ, Hansen K, Yudt L, Schmidt C, Hersey P, Ellem KA, O’Rourke MG, Parsons PG, Meltzer P, Ringner M, Hayward NK. Microarray expression profiling in melanoma reveals a B-Raf mutation signature. Oncogene. 2004;23:4060–4067. doi: 10.1038/sj.onc.1207563. [DOI] [PubMed] [Google Scholar]
- 34.Johansson P, Pavey S, Hayward N. Confirmation of a B-Raf mutation-associated gene expression signature in melanoma. Pigment Cell Res. 2007;20:216–221. doi: 10.1111/j.1600-0749.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 35.Kortylewski M, Heinrich PC, Kauffmann ME, Bohm M, MacKiewicz A, Behrmann I. Mitogen-activated protein kinases control p27/Kip1 expression and growth of human melanoma cells. Biochem. J. 2001;357:297–303. doi: 10.1042/0264-6021:3570297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefevre G, Calipel A, Mouriaux F, Hecquet C, Malecaze F, Mascarelli F. Opposite long-term regulation of c-Myc and p27Kip1 through overactivation of Raf-1 and the MEK/ERK module in proliferating human choroidal melanoma cells. Oncogene. 2003;22:8813–8822. doi: 10.1038/sj.onc.1207099. [DOI] [PubMed] [Google Scholar]
- 37.Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-Raf signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene. 2007;26:1056–1066. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- 38.Sumimoto H, Hirata K, Yamagata S, Miyoshi H, Miyagishi M, Taira K, Kawakami Y. Effective inhibition of cell growth and invasion of melanoma by combined suppression of B-Raf (V599E) and Skp2 with lentiviral RNAi. Int. J. Cancer. 2006;118:472–476. doi: 10.1002/ijc.21286. [DOI] [PubMed] [Google Scholar]
- 39.Goodall J, Wellbrock C, Dexter TJ, Roberts K, Marais R, Goding CR. The Brn-2 transcription factor activated B-Raf to melanoma proliferation. Mol. Cell. Biol. 2004;24:2924–2932. doi: 10.1128/MCB.24.7.2923-2931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S, Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goding CR. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 42.Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–312. [PMC free article] [PubMed] [Google Scholar]
- 43.Kim D-S, Hwang E-S, Lee J-E, Kim S-Y, Kwon S-B, Park K-C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003;116:1699–1706. doi: 10.1242/jcs.00366. [DOI] [PubMed] [Google Scholar]
- 44.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo H-M. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein Bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
- 45.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: Implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- 46.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santibanez JF, Iglesias M, Frontelo P, Martinez J, Quintanilla M. Involvement of the Ras/MAPK signaling pathway in the modulation of urokinase production and cellular invasiveness by transforming growth factor-β(1) in transformed keratinocytes. Biochem. Biophys. Res. Commun. 2000;273:521–527. doi: 10.1006/bbrc.2000.2946. [DOI] [PubMed] [Google Scholar]
- 48.Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: Involvement of Ras-dependent and -independent pathways. J. Cell Sci. 2000;113:4319–4330. doi: 10.1242/jcs.113.23.4319. [DOI] [PubMed] [Google Scholar]
- 49.Tower GB, Coon CC, Benbow U, Vincenti MP, Brinckerhoff CE. ERK 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim. Biophys. Acta. 2002;1586:265–274. doi: 10.1016/s0925-4439(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 50.Ishii Y, Ogura T, Tatemichi M, Fujisawa H, Otsuka F, Esumi H. Induction of matrix metalloproteinase gene transcription by nitric oxide and mechanisms of MMP-1 gene induction in human melanoma cell lines. Int. J. Cancer. 2003;103:161–168. doi: 10.1002/ijc.10808. [DOI] [PubMed] [Google Scholar]
- 51.Ramos MC, Steinbrenner H, Stuhlmann D, Sies H, Brenneisen P. Induction of MMP-10 and MMP-1 in a squamous cell carcinoma cell line by ultraviolet radiation. Biol. Chem. 2004;385:75–86. doi: 10.1515/BC.2004.010. [DOI] [PubMed] [Google Scholar]
- 52.Huntington JT, Shields JM, Der CJ, Wyatt CA, Benbow U, Slingluff CL, Jr, Brinckerhoff CE. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: Role of B-Raf mutation and fibroblast growth factor signaling. J. Biol. Chem. 2004;279:33168–33176. doi: 10.1074/jbc.M405102200. [DOI] [PubMed] [Google Scholar]
- 53.Woods D, Cherwinski H, Venetsanakos E, Bhat A, Gysin S, Humbert M, Bray PF, Saylor VL, McMahon M. Induction of {β}3-integrin gene expression by sustained activation of the Ras-regulated Raf-MEK-extracellular signal-regulated kinase signaling pathway. Mol. Cell. Biol. 2001;21:3192–3205. doi: 10.1128/MCB.21.9.3192-3205.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The B-Raf-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin. Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 56.Sosman JA, Puzanov I. Molecular targets in melanoma from angiogenesis to apoptosis. Clin. Cancer Res. 2006;12:2376s–2383s. doi: 10.1158/1078-0432.CCR-05-2558. [DOI] [PubMed] [Google Scholar]
- 57.Hoeflich KP, Gray DC, Eby MT, Tien JY, Wong L, Bower J, Gogineni A, Zha J, Cole MJ, Stern HM, Murray LJ, Davis DP, Seshagiri S. Oncogenic B-Raf is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 58.Wang JY, Wilcoxen KM, Nomoto K, Wu S. Recent advances of MEK inhibitors and their clinical progress. Curr. Top. Med. Chem. 2007;7:1364–1378. doi: 10.2174/156802607781696837. [DOI] [PubMed] [Google Scholar]
- 59.Li N, Batt D, Warmuth M. B-Raf kinase inhibitors for cancer treatment. Curr. Opin. Investig. Drugs. 2007;8:452–456. [PubMed] [Google Scholar]
- 60.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, Dong C, Robertson GP. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–8209. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smalley KSM, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol. Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 62.Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, Herlyn M, Schittek B. Combined targeting of MAPK and Akt signalling pathways is a promising strategy for melanoma treatment. Br. J. Dermatol. 2007;156:1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 63.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: Mutation, marker and potential target for therapeutic intervention. Expert. Opin. Ther. Targets. 2004;8:537–550. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 64.Parmiter AH, Balaban G, Clark WH, Jr, Nowell PC. Possible involvement of the chromosome region 10q24–q26 in early stages of melanocytic neoplasia. Cancer Genet. Cytogenet. 1988;30:313–317. doi: 10.1016/0165-4608(88)90200-2. [DOI] [PubMed] [Google Scholar]
- 65.Herbst RA, Weiss J, Ehnis A, Cavenee WK, Arden KC. Loss of heterozygosity for 10q22-10qter in malignant melanoma progression. Cancer Res. 1994;54:3111–3114. [PubMed] [Google Scholar]
- 66.Healy E, Rehman I, Angus B, Rees JL. Loss of heterozygosity in sporadic primary cutaneous melanoma. Genes Chromosomes Cancer. 1995;12:152–156. doi: 10.1002/gcc.2870120211. [DOI] [PubMed] [Google Scholar]
- 67.Simpson L, Parsons R. PTEN: Life as a tumor suppressor. Exp. Cell Res. 2003;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 68.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 69.Guldberg P, Thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- 70.Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397–3402. doi: 10.1038/sj.onc.1201881. [DOI] [PubMed] [Google Scholar]
- 71.Birck A, Ahrenkiel V, Zeuthen J, Hou-Jensen K, Guldberg P. Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma biopsies. J. Invest. Dermatol. 2000;114:277–280. doi: 10.1046/j.1523-1747.2000.00877.x. [DOI] [PubMed] [Google Scholar]
- 72.Reifenberger J, Wolter M, Boström J, Schulte K, Ruzicka T, Reifenberger G. Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour suppressor gene in primary and metastatic malignant melanomas. Virchows Arch. 2000;436:487–493. doi: 10.1007/s004280050477. [DOI] [PubMed] [Google Scholar]
- 73.Celebi JT, Shendrik I, Silvers DN, Peacocke MJ. Identification of PTEN mutations in metastatic melanoma specimens. J. Med. Genet. 2000;37:653–657. doi: 10.1136/jmg.37.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am. J. Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahia PLM, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C, Eng C. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997;57:4710–4713. [PubMed] [Google Scholar]
- 76.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl Acad. Sci. USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salvesen HB, MacDonald N, Ryan A, Jacobs IJ, Lynch ED, Akslen LA, Das S. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int. J. Cancer. 2001;91:22–26. doi: 10.1002/1097-0215(20010101)91:1<22::aid-ijc1002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 78.Mirmohammadsadegh A, Marini A, Nambiar S, Hassan M, Tannapfel A, Ruzicka T, Hengge UR. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res. 2006;66:6546–6552. doi: 10.1158/0008-5472.CAN-06-0384. [DOI] [PubMed] [Google Scholar]
- 79.Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int. J. Cancer. 2002;99:63–67. doi: 10.1002/ijc.10294. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4-1 is a protooncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robertson GP, Furnari FB, Miele ME, Glendening MJ, Welch DR, Fountain JW, Lugo TG, Huang HJS, Cavenee WK. In vitro loss of heterozygosity targets the PTEN/MMAC1 gene in melanoma. Proc. Natl Acad. Sci. USA. 1998;95:9418–9423. doi: 10.1073/pnas.95.16.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang PH, Yi HK, Kim DS, Nam SY, Kim JS, Lee DY. Suppression of tumorigenicity and metastasis in B16F10 cells by PTEN/MMAC1/TEP1 gene. Cancer Lett. 2001;172:83–91. doi: 10.1016/s0304-3835(01)00632-2. [DOI] [PubMed] [Google Scholar]
- 83.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/Akt pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 84.Harlan JE, Yoon HS, Hajduk PJ, Fesik SW. Structural characterization of the interaction between a pleckstrin homology domain and phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1995;34:9859–9864. doi: 10.1021/bi00031a006. [DOI] [PubMed] [Google Scholar]
- 85.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 86.Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- 87.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: A clinicopathologic study of 292 cases. J. Clin. Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 88.Slipicevic A, Holm R, Nguyen MT, Bøhler PJ, Davidson B, Flørenes VA. Expression of activated Akt and PTEN in malignant melanomas: Relationship with clinical outcome. Am. J. Clin. Pathol. 2005;124:528–536. doi: 10.1309/YT58WWMTA6YR1PRV. [DOI] [PubMed] [Google Scholar]
- 89.Kantrow SM, Boyd AS, Ellis DL, Nanney LB, Richmond A, Shyr Y, Robbins JB. Expression of activated Akt in benign nevi, Spitz nevi and melanomas. J. Cutan. Pathol. 2007;34:593–596. doi: 10.1111/j.1600-0560.2006.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 91.Omholt K, Kröckel D, Ringborg U, Hansson J. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res. 2006;16:197–200. doi: 10.1097/01.cmr.0000200488.77970.e3. [DOI] [PubMed] [Google Scholar]
- 92.Singh RS, Diwan AH, Zhang PS, Prieto VG. Phosphoinositide 3-kinase is not overexpressed in melanocytic lesions. J. Cutan. Pathol. 2007;34:220–225. doi: 10.1111/j.1600-0560.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- 93.Curtin JA, Stark MS, Pinkel D, Hayward NK, Bastian BC. PI3-kinase subunits are infrequent somatic targets in melanoma. J. Invest. Dermatol. 2006;126:1660–1663. doi: 10.1038/sj.jid.5700311. [DOI] [PubMed] [Google Scholar]
- 94.Waldmann V, Wacker J, Deichmann M. Absence of mutations in the pleckstrin homology (PH) domain of protein kinase B (PKB/Akt) in malignant melanoma. Melanoma Res. 2002;12:45–50. doi: 10.1097/00008390-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Waldmann V, Wacker J, Deichmann M. Mutations of the activation-associated phosphorylation sites at codons 308 and 473 of protein kinase B are absent in human melanoma. Arch. Dermatol. Res. 2001;293:368–372. doi: 10.1007/s004030100236. [DOI] [PubMed] [Google Scholar]
- 96.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 97.Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Lun R, Dong YB, Zhou HS, McMasters KM. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol. Ther. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 98.Li G, Kalabis J, Xu X, Meier F, Oka M, Bogenrieder T, Herlyn M. Reciprocal regulation of MelCAM and Akt in human melanoma. Oncogene. 2003;22:6891–6899. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 99.Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- 100.Kim D, Kim S, Koh H, Yoon S-O, Chung A-S, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 101.Park BK, Zeng X, Glazer RI. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–7653. [PubMed] [Google Scholar]
- 102.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martinez-Diez M, Cuezva JM, Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spofford LS, Abel EV, Boisvert-Adamo K, Aplin AE. Cyclin D3 expression in melanoma cells is regulated by adhesion-dependent phosphatidylinositol 3-kinase signaling and contributes to G1-S progression. J. Biol. Chem. 2006;281:25644–25651. doi: 10.1074/jbc.M600197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 105.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 106.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 107.Romashkova JA, Makarov SS. NF-B is a target of Akt in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 108.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 109.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 110.Bejsovec A. Wnt pathway activation: New relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 111.Cadigan KM, Liu YI. Wnt signaling: Complexity at the surface. J. Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 112.Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007;7:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of β-catenin without exon 3 mutations in malignant melanoma. Am. J. Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 116.Worm J, Christensen C, Gronbaek K, Tulchinsky E, Guldberg P. Genetic and epigenetic alterations of the APC gene in malignant melanoma. Oncogene. 2004;23:5215–5226. doi: 10.1038/sj.onc.1207647. [DOI] [PubMed] [Google Scholar]
- 117.Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of β-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int. J. Cancer. 2001;92:839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- 118.Reifenberger J, Knobbe CB, Wolter M, Blaschke B, Schulte KW, Pietsch T, Ruzicka T, Reifenberger G. Molecular genetic analysis of malignant melanomas for aberrations of the WNT signaling pathway genes CTNNB1, APC, ICAT and BTRC. Int. J. Cancer. 2002;100:549–556. doi: 10.1002/ijc.10512. [DOI] [PubMed] [Google Scholar]
- 119.Murakami T, Toda S, Fujimoto M, Ohtsuki M, Byers HR, Etoh T, Nakagawa H. Constitutive activation of Wnt/β-catenin signaling pathway in migration-active melanoma cells: Role of LEF-1 in melanoma with increased metastatic potential. Biochem. Biophys. Res. Commun. 2001;288:8–15. doi: 10.1006/bbrc.2001.5719. [DOI] [PubMed] [Google Scholar]
- 120.Kielhorn E, Provost E, Olsen D, D’Aquila TG, Smith BL, Camp RL, Rimm DL. Tissue microarray-based analysis shows phospho-β-catenin expression in malignant melanoma is associated with poor outcome. Int. J. Cancer. 2003;103:652–656. doi: 10.1002/ijc.10893. [DOI] [PubMed] [Google Scholar]
- 121.Tago K-i, Nakamura T, Nishita M, Hyodo J, Nagai S-i, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, Akiyama T. Inhibition of Wnt signaling by ICAT, a novel β-catenin-interacting protein. Genes Dev. 2000;14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 122.Goodall J, Martinozzi S, Dexter TJ, Champeval D, Carreira S, Larue L, Goding CR. Brn-2 expression controls melanoma proliferation and is directly regulated by {β}-catenin. Mol. Cell. Biol. 2004;24:2915–2922. doi: 10.1128/MCB.24.7.2915-2922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomson JA, Murphy K, Baker E, Sutherland GR, Parsons PG, Sturm RA. The brn-2 gene regulates the melanocytic phenotype and tumorigenic potential of human melanoma cells. Oncogene. 1995;11:690–700. [PubMed] [Google Scholar]
- 124.Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zeB-Rafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article] [PubMed] [Google Scholar]
- 125.Takeda K, Yasumoto K-i, Takada R, Takada S, Watanabe K-i, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- 126.Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 127.Hughes MJ, Lingrel JB, Krakowsky JM, Anderson KP. A helix-loop-helix transcription factor-like gene is located at the mi locus. J. Biol. Chem. 1993;268:687–690. [PubMed] [Google Scholar]
- 128.Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, He X, Fisher DE. {β}-Catenin-induced melanoma growth requires the downstream target microphthalmia-associated transcription factor. J. Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, Steingrimsson E, Hecht A. The microphthalmia-associated transcription factor Mitf interacts with {β}-catenin to determine target gene expression. Mol. Cell. Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 131.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br. J. Cancer. 2007;96:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 133.Mikheev AM, Mikheeva SA, Rostomily R, Zarbl H. Dickkopf-1 activates cell death in MDA-MB435 melanoma cells. Biochem. Biophys. Res. Commun. 2007;352:675–680. doi: 10.1016/j.bbrc.2006.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin YC, You L, Xu Z, He B, Yang CT, Chen JK, Mikami I, Clement G, Shi Y, Kuchenbecker K, Okamoto J, Kashani-Sabet M, Jablons DM. Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum. Gene Ther. 2007;18:379–386. doi: 10.1089/hum.2006.005. [DOI] [PubMed] [Google Scholar]
- 135.You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, McCormick F, Jablons DM. An anti-Wnt-2 mono-clonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 136.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell. Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55:3495–3499. [PubMed] [Google Scholar]
- 138.Crnogorac-Jurcevi T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, Jones M, Tyson K, Bassi C, Scarpa A, Lemoine NR. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 139.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 140.Carr KM, Bittner M, Trent JT. Gene-expression profiling in human cutaneous melanoma. Oncogene. 2003;22:3076–3080. doi: 10.1038/sj.onc.1206448. [DOI] [PubMed] [Google Scholar]
- 141.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 142.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 143.Weeraratna AT, Becker D, Carr KM, Duray PH, Rosenblatt KP, Yang S, Chen Y, Bittner M, Strausberg RL, Riggins GJ, Wagner U, Kallioniemi OP, Trent JM, Morin PJ, Meltzer PS. Generation and analysis of melanoma SAGE libraries: SAGE advice on the melanoma transcriptome. Oncogene. 2004;23:2264–2274. doi: 10.1038/sj.onc.1207337. [DOI] [PubMed] [Google Scholar]
- 144.Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J. Biol. Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lahn MM, Sundell KL. The role of protein kinase C-alpha (PKC-alpha) in melanoma. Melanoma Res. 2004;14:85–89. doi: 10.1097/00008390-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 146.Jonsson M, Smith K, Harris AL. Regulation of Wnt5a expression in human mammary cells by protein kinase C activity and the cytoskeleton. Br. J. Cancer. 1998;78:430–438. doi: 10.1038/bjc.1998.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 148.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 149.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 150.Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- 151.Nielsen C, Thastrup J, Bottzauw T, Jaattela M, Kallunki T. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res. 2007;67:178–185. doi: 10.1158/0008-5472.CAN-06-2801. [DOI] [PubMed] [Google Scholar]
- 152.Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, Depatie C, Mercola D. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin. Cancer Res. 2003;9:391–401. [PubMed] [Google Scholar]
- 153.Cui J, Han SY, Wang C, Su W, Harshyne L, Holgado-Madruga M, Wong AJ. c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells. Cancer Res. 2006;66:10024–10031. doi: 10.1158/0008-5472.CAN-06-0136. [DOI] [PubMed] [Google Scholar]
- 154.Khatlani TS, Wislez M, Sun M, Srinivas H, Iwanaga K, Ma L, Hanna AE, Liu D, Girard L, Kim YH, Pollack JR, Minna Wistuba JD, II, Kurie JM. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene. 2007;26:2658–2666. doi: 10.1038/sj.onc.1210050. [DOI] [PubMed] [Google Scholar]
- 155.Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629–637. doi: 10.1101/gad.1062903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc. Natl Acad. Sci. USA. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adler V, Schaffer A, Kim J, Dolan L, Ronai Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J. Biol. Chem. 1995;270:26071–26077. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- 158.Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438–2452. doi: 10.1038/sj.onc.1204385. [DOI] [PubMed] [Google Scholar]
- 159.Vogt PK. Fortuitous convergences: The beginnings of JUN. Nat. Rev. Cancer. 2002;2:465–469. doi: 10.1038/nrc818. [DOI] [PubMed] [Google Scholar]
- 160.Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- 161.Bhoumik A, Huang T-G, Ivanov V, Gangi L, Qiao RF, Woo SLC, Chen S-H, Ronai Ze. An ATF2-derived peptide sensitizes melanomas to apoptosis and inhibits their growth and metastasis. J. Clin. Invest. 2002;110:643–650. doi: 10.1172/JCI16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mariani O, Brennetot C, Coindre JM, Gruel N, Ganem C, Delattre O, Stern MH, Aurias A. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell. 2007;11:361–374. doi: 10.1016/j.ccr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 163.Vleugel MM, Greijer AE, Bos R, van der Wall E, van Diest PJ. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006;37:668–674. doi: 10.1016/j.humpath.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 164.Maeno K, Masuda A, Yanagisawa K, Konishi H, Osada H, Saito T, Ueda R, Takahashi T. Altered regulation of c-Jun and its involvement in anchorage-independent growth of human lung cancers. Oncogene. 2006;25:271–277. doi: 10.1038/sj.onc.1209018. [DOI] [PubMed] [Google Scholar]
- 165.Behrens A, Jochum W, Sibilia M, Wagner EF. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene. 2000;19:2657–2663. doi: 10.1038/sj.onc.1203603. [DOI] [PubMed] [Google Scholar]
- 166.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 167.Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, Davis RJ. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc. Natl Acad. Sci. USA. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamanishi DT, Buckmeier JA, Meyskens FL., Jr Expression of c-Jun, JunB, and c-Fos protooncogenes in human primary melanocytes and metastatic melanomas. J. Invest. Dermatol. 1991;97:349–353. doi: 10.1111/1523-1747.ep12480698. [DOI] [PubMed] [Google Scholar]
- 169.Rutberg SE, Goldstein IM, Yang YM, Stackpole CW, Ronai Z. Expression and transcriptional activity of AP-1, CRE, and URE binding proteins in B16 mouse melanoma subclones. Mol. Carcinog. 1994;10:82–87. doi: 10.1002/mc.2940100205. [DOI] [PubMed] [Google Scholar]
- 170.Jørgensen K, Davidson B, Flørenes VA. Activation of c-Jun N-terminal kinase is associated with cell proliferation and shorter relapse-free period in superficial spreading malignant melanoma. Mod. Pathol. 2006;19:1446–1455. doi: 10.1038/modpathol.3800662. [DOI] [PubMed] [Google Scholar]
- 171.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- 172.Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64:6190–6199. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- 173.Gurzov EN, Bakiri L, Alfaro JM, Wagner EF, Izquierdo M. Targeting c-Jun and JunB proteins as potential anticancer cell therapy. Oncogene. 2007 doi: 10.1038/sj.onc.1210690. 2007 Jul 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 174.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 175.Grafström E, Egyházi S, Ringborg U, Hansson J, Platz A. Biallelic deletions in INK4 in cutaneous melanoma are common and associated with decreased survival. Clin. Cancer Res. 2005;11:2991–2997. doi: 10.1158/1078-0432.CCR-04-1731. [DOI] [PubMed] [Google Scholar]
- 176.Choi BY, Choi HS, Ko K, Cho YY, Zhu F, Kang BS, Ermakova SP, Ma WY, Bode AM, Dong Z. The tumor suppressor p16(INK4a) prevents cell transformation through inhibition of c-Jun phosphorylation and AP-1 activity. Nat. Struct. Mol. Biol. 2005;12:699–707. doi: 10.1038/nsmb960. [DOI] [PubMed] [Google Scholar]
- 177.Ivanov VN, Kehrl JH, Ronai Z. Role of TRAF2/GCK in melanoma sensitivity to UV-induced apoptosis. Oncogene. 2000;19:933–942. doi: 10.1038/sj.onc.1203415. [DOI] [PubMed] [Google Scholar]
- 178.Arch RH, Gedrich RW, Thompson CB. Translocation of TRAF proteins regulates apoptotic threshold of cells. Biochem. Biophys. Res. Commun. 2000;272:936–945. doi: 10.1006/bbrc.2000.2873. [DOI] [PubMed] [Google Scholar]
- 179.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 180.Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 181.Liu H, Su YC, Becker E, Treisman J, Skolnik EY. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr. Biol. 1999;9:101–104. doi: 10.1016/s0960-9822(99)80023-2. [DOI] [PubMed] [Google Scholar]
- 182.Ivanov VN, Fodstad O, Ronai Z. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFalpha and suppression of NF-kappaB activities. Oncogene. 2001;20:2243–2253. doi: 10.1038/sj.onc.1204314. [DOI] [PubMed] [Google Scholar]
- 183.Coppock DL, Tansey JB, Nathanson L. 12-O-tetradecanoylphorbol-13-acetate induces transient cell cycle arrest in G1 and G2 in metastatic melanoma cells: Inhibition of phosphorylation of p34cdc2. Cell Growth Differ. 1992;3:485–494. [PubMed] [Google Scholar]
- 184.Mapelli E, Banfi P, Sala E, Sensi M, Supino R, Zunino F, Gambetta RA. Effect of protein kinase C inhibitors on invasiveness of human melanoma clones expressing different levels of protein kinase C isoenzymes. Int. J. Cancer. 1994;57:281–286. doi: 10.1002/ijc.2910570225. [DOI] [PubMed] [Google Scholar]
- 185.Arita Y, O’Driscoll KR, Weinstein IB. Growth inhibition of human melanoma-derived cells by 12-O-tetradecanoyl phorbol 13-acetate. Int. J. Cancer. 1994;56:229–235. doi: 10.1002/ijc.2910560215. [DOI] [PubMed] [Google Scholar]
- 186.La Porta CA, Comolli R. Activation of protein kinase C-alpha isoform in murine melanoma cells with high metastatic potential. Clin. Exp. Metastasis. 1997;15:568–579. doi: 10.1023/a:1018447531813. [DOI] [PubMed] [Google Scholar]
- 187.Dennis JU, Dean NM, Bennett CF, Griffith JW, Lang CM, Welch DR. Human melanoma metastasis is inhibited following ex vivo treatment with an antisense oligonucleotide to protein kinase C-alpha. Cancer Lett. 1998;128:65–70. doi: 10.1016/s0304-3835(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 188.Oka M, Kikkawa U. Protein kinase C in melanoma. Cancer Metastasis Rev. 2005;24:287–300. doi: 10.1007/s10555-005-1578-8. [DOI] [PubMed] [Google Scholar]
- 189.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 190.Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol. Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 192.van Dam H, Castellazzi M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene. 2001;20:2453–2464. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- 193.Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol. Cell. Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shimizu M, Nomura Y, Suzuki H, Ichikawa E, Takeuchi A, Suzuki M, Nakamura T, Nakajima T, Oda K. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp. Cell Res. 1998;239:93–103. doi: 10.1006/excr.1997.3884. [DOI] [PubMed] [Google Scholar]
- 195.Huguier S, Baguet J, Perez S, van Dam H, Castellazzi M. Transcription factor ATF2 cooperates with v-Jun to promote growth factor-independent proliferation in vitro and tumor formation in vivo. Mol. Cell. Biol. 1998;18:7020–7029. doi: 10.1128/mcb.18.12.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Recio JA, Merlino G. Hepatocyte growth factor/scatter factor activates proliferation in melanoma cells through p38 MAPK, ATF-2 and cyclin D1. Oncogene. 2002;21:1000–1008. doi: 10.1038/sj.onc.1205150. [DOI] [PubMed] [Google Scholar]
- 197.Ronai Z, Yang YM, Fuchs SY, Adler V, Sardana M, Herlyn M. ATF2 confers radiation resistance to human melanoma cells. Oncogene. 1998;16:523–531. doi: 10.1038/sj.onc.1201566. [DOI] [PubMed] [Google Scholar]
- 198.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 199.Berger AJ, Kluger HM, Li N, Kielhorn E, Halaban R, Ronai Z, Rimm DL. Subcellular localization of activating transcription factor 2 in melanoma specimens predicts patient survival. Cancer Res. 2003;63:8103–8107. [PubMed] [Google Scholar]
- 200.Bhoumik A, Takahashi S, Breitweiser W, Shiloh Y, Jones N, Ronai Z. ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol. Cell. 2005;18:577–587. doi: 10.1016/j.molcel.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bhoumik A, Ivanov V, Ronai Z. Activating transcription factor 2-derived peptides alter resistance of human tumor cell lines to ultraviolet irradiation and chemical treatment. Clin. Cancer Res. 2001;7:331–342. [PubMed] [Google Scholar]
- 202.Bhoumik A, Gangi L, Ronai Z. Inhibition of melanoma growth and metastasis by ATF2-derived peptides. Cancer Res. 2004;64:8222–8230. doi: 10.1158/0008-5472.CAN-04-0714. [DOI] [PubMed] [Google Scholar]
- 203.Bhoumik A, Jones N, Ronai Z. Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity. Proc. Natl Acad. Sci. USA. 2004;101:4222–4227. doi: 10.1073/pnas.0400195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abbas S, Bhoumik A, Dahl R, Vasile S, Krajewski S, Cosford N, Ronai Z. Preclinical studies of celastrol and acetyl isogambogic acid in melanoma. Clin. Cancer Res. 2007;13:6769–6778. doi: 10.1158/1078-0432.CCR-07-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bhoumik A, DeRossi C, Breitwieser W, Kluger H, Davis S, Subtil A, Meltzer P, Krajewski S, Jones N, Ronai Z. Suppressor role of activating transcription factor-2 (ATF2) in skin cancer. Proc. Natl Acad. Sci. USA. doi: 10.1073/pnas.0706057105. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 207.Ueda Y, Richmond A. NF-κB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dixit V, Mak TW. NF-kappaB signaling. Many roads lead to Madrid. Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 209.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl.:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 210.Chen X, Kandasamy K, Srivastava RK. Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappa B in tumor necrosis factor-related apoptosis-inducing ligand signaling. Cancer Res. 2003;63:1059–1066. [PubMed] [Google Scholar]
- 211.Karin M, Cao Y, Greten R, Li Z. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 212.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 213.Wang D, Richmond A. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 2001;276:3650–3659. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J. Biol. Chem. 2001;276:24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 215.Kuphal S, Poser I, Jobin C, Hellerbrand C, Bosserhoff AK. Loss of E-cadherin leads to upregulation of NFkappaB activity in malignant melanoma. Oncogene. 2004;23:8509–8519. doi: 10.1038/sj.onc.1207831. [DOI] [PubMed] [Google Scholar]
- 216.Ivanov V, Ronai Z. p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene. 2000;19:3003–3012. doi: 10.1038/sj.onc.1203602. [DOI] [PubMed] [Google Scholar]
- 217.Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J. Biol. Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gray-Schopfer VC, Karasarides M, Hayward R, Marais R. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when B-Raf signaling is inhibited. Cancer Res. 2007;67:122–129. doi: 10.1158/0008-5472.CAN-06-1880. [DOI] [PubMed] [Google Scholar]
- 219.Fuchs SY, Chen A, Xiong Y, Pan ZQ, Ronai Z. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and β-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- 220.Soldatencov VA, Dritschilo A, Ronai Z, Fuchs SY. Inhibition of homologue of Slimb (HOS) function sensitizes human melanoma cells for apoptosis. Cancer Res. 1999;59:5085–5088. [PubMed] [Google Scholar]
- 221.Liu J, Suresh Kumar KG, Yu D, Molton SA, McMahon M, Herlyn M, Thomas-Tikhonenko A, Fuchs SY. Oncogenic B-Raf regulates β-Trcp expression and NF-kappaB activity in human melanoma cells. Oncogene. 2007;26:1954–1958. doi: 10.1038/sj.onc.1209994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24:301–313. doi: 10.1007/s10555-005-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 224.Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFkappaB activation without interfering with the IkappaB degradation pathway. J. Biol. Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- 225.Meyskens FL, Jr, Buckmeier JA, McNulty SE, Tohidian NB. Activation of nuclear factor-kappa B in human metastatic melanoma cells and the effect of oxidative stress. Clin. Cancer Res. 1999;5:1197–1202. [PubMed] [Google Scholar]
- 226.Gao K, Dai DL, Martinka M, Li G. Prognostic significance of nuclear factor-kappa B p105/p50 in human melanoma and its role in cell migration. Cancer Res. 2006;66:8382–8388. doi: 10.1158/0008-5472.CAN-05-4402. [DOI] [PubMed] [Google Scholar]
- 227.Philip S, Kundu GC. Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J. Biol. Chem. 2003;278:14487–14497. doi: 10.1074/jbc.M207309200. [DOI] [PubMed] [Google Scholar]
- 228.Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. Mda-9/Syntenin regulates the metastatic phenotype in 304 human melanoma cells by activating nuclear factor-kappaB. Cancer Res. 2007;67:1812–1822. doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- 229.Rangaswami H, Bulbule A, Kundu GC. Nuclear factor inducing kinase: A key regulator in osteopontin-induced MAPK/IkappaB kinase dependent NF-kappaB-mediated promatrix metalloproteinase-9 activation. Glycoconj. J. 2006;23:221–232. doi: 10.1007/s10719-006-7927-1. [DOI] [PubMed] [Google Scholar]
- 230.Rangaswami H, Kundu GC. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol. Rep. 2007;18:909–915. [PubMed] [Google Scholar]
- 231.Ihle JN, Kerr IM. Jaks and STATs in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 232.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 233.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J. Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 234.Ihle JN. The STAT family in cytokine signaling. Curr. Opin. Cell Biol. 2001;13:211–217. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 235.Mertens C, Zhong M, Krishnaraj R, Zou W, Chen X, Darnell JE., Jr Dephosphorylation of phosphotyrosine on STAT1 dimers requires extensive spatial reorientation of the monomers facilitated by the N-terminal domain. Genes Dev. 2006;20:3372–3381. doi: 10.1101/gad.1485406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 238.Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 239.Ivanov VN, Krasilnikov M, Ronai Z. Regulation of Fas expression by STAT3 and c-Jun is mediated by phosphatidylinositol 3-kinase-Akt signaling. J. Biol. Chem. 2002;277:4932–4944. doi: 10.1074/jbc.M108233200. [DOI] [PubMed] [Google Scholar]
- 240.Bromberg J. STAT proteins and oncogenesis. J. Clin. Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG. The role of STATs in lung carcinogenesis: An emerging target for novel therapeutics. J. Mol. Med. 2007;85:427–436. doi: 10.1007/s00109-006-0152-3. [DOI] [PubMed] [Google Scholar]
- 242.Niu G, Heller R, Catlett-Falcone R, Coppolac D, Jaroszeski M, Dalton W, Jove R, Yu H. Gene therapy with dominant-negative STAT3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59:5059–5063. [PubMed] [Google Scholar]
- 243.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 244.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. STAT3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 245.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 246.Yang JM, Chatterjee-Kishore SM, Staugaitis H, Nguyen K, Schlessinger D, Levy E, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 247.Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Z. Cooperation between STAT3 and c-Jun suppresses Fas transcription. Mol. Cell. 2001;7:517–528. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- 248.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and STAT3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 249.Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S, Tannapfel A, Bojar H, Ruzicka T, Hengge UR. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J. Invest. Dermatol. 2006;126:2272–2280. doi: 10.1038/sj.jid.5700385. [DOI] [PubMed] [Google Scholar]
- 250.Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, Masuda T. Methylation STATus of the SOCS3 gene in human malignant melanomas. Int. J. Oncol. 2007;30:689–694. [PubMed] [Google Scholar]
- 251.Fojtova M, Boudny V, Kovarik A, Lauerova L, Adamkova L, Souckova K, Jarkovsky J, Kovarik J. Development of IFN-γ resistance is associated with attenuation of SOCS genes induction and constitutive expression of SOCS 3 in melanoma cells. Br. J. Cancer. 2007;97:231–237. doi: 10.1038/sj.bjc.6603849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- 253.Kortylewski M, Komyod W, Kauffmann ME, Bosserhoff A, Heinrich PC, Behrmann I. Interferon-gamma-mediated growth regulation of melanoma cells: Involvement of STAT1-dependent and STAT1-independent signals. J. Invest. Dermatol. 2004;122:414–422. doi: 10.1046/j.0022-202X.2004.22237.x. [DOI] [PubMed] [Google Scholar]
- 254.Wang W, Edington HD, Rao UN, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin. Cancer Res. 2007;13:1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- 255.Wellbrock C, Weisser C, Hassel JC, Fischer P, Becker J, Vetter CS, Behrmann I, Kortylewski M, Heinrich PC, Schartl M. STAT5 contributes to interferon resistance of melanoma cells. Curr. Biol. 2005;15:1629–1639. doi: 10.1016/j.cub.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 256.Stewart FA, Denekamp J, Randhawa VS. Skin sensitization by misonidazole: A demonstration of uniform mild hypoxia. Br. J. Cancer. 1982;45:869–877. doi: 10.1038/bjc.1982.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Semenza GL. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 258.Harris AL. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 259.Distler O, Distler JHW, Scheid A, Acker T, Hirth A, Rethage J, Michel BA, Gay RE, Muller-Ladner U, Matucci-Cerinic M, Plate KH, Gassmann M, Gay S. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ. Res. 2004;95:109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 260.Busca R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B, Thyss R, Fitsialos G, Larribere L, Bertolotto C, Virolle T, Barbry P, Pouyssegur J, Ponzio G, Ballotti R. Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. J. Cell Biol. 2005;170:49–59. doi: 10.1083/jcb.200501067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, Acs G, Herlyn M, Xu X. Mutant V600E B-Raf increases hypoxia inducible factor-1{alpha} expression in melanoma. Cancer Res. 2007;67:3177–3184. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- 262.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 263.Rofstad EK, Danielsen T. Hypoxia-induced angiogenesis and vascular endothelial growth factor secretion in human melanoma. Br. J. Cancer. 1998;77:897–902. doi: 10.1038/bjc.1998.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo K-T, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 265.Lacal PM, Ruffini F, Pagani E, D’Atri S. An autocrine loop directed by the vascular endothelial growth factor promotes invasiveness of human melanoma cells. Int. J. Oncol. 2005;27:1625–1632. [PubMed] [Google Scholar]
- 266.Jones DT, Harris AL. Identification of novel small-molecule inhibitors of hypoxia-inducible factor-1 transactivation and DNA binding. Mol. Cancer Ther. 2006;5:2193–2202. doi: 10.1158/1535-7163.MCT-05-0443. [DOI] [PubMed] [Google Scholar]
- 267.Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, Bowtell DD, Ronai Z. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1[alpha] abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 268.Piek E, Roberts AB. Suppressor and oncogenic roles of transforming growth factor-β and its signaling pathways in tumorigenesis. Adv. Cancer Res. 2001;83:1–54. doi: 10.1016/s0065-230x(01)83001-3. [DOI] [PubMed] [Google Scholar]
- 269.Miyazono K, ten Dijke P, Heldin CH. TGF-β signaling by Smad proteins. Adv. Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 270.Berking C, Takemoto R, Schaider H, Showe L, Satyamoorthy K, Robbins P, Herlyn M. Transforming growth factor-{β}1 increases survival of human melanoma through stroma remodeling. Cancer Res. 2001;61:8306–8316. [PubMed] [Google Scholar]
- 271.Kang SH, Bang YJ, Im YH, Yang HK, Lee DA, Lee HY, Lee HS, Kim NK, Kim SJ. Transcriptional repression of the transforming growth factor-β type I receptor gene by DNA methylation results in the development of TGF-β resistance in human gastric cancer. Oncogene. 1999;18:7280–7286. doi: 10.1038/sj.onc.1203146. [DOI] [PubMed] [Google Scholar]
- 272.Hussein MR. Transforming growth factor-β and malignant melanoma: Molecular mechanisms. J. Cutan. Pathol. 2005;32:389–395. doi: 10.1111/j.0303-6987.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 273.Reed JA, Lin Q, Chen D, Mian IS, Medrano EE. SKI pathways inducing progression of human melanoma. Cancer Metastasis Rev. 2005;24:265–272. doi: 10.1007/s10555-005-1576-x. [DOI] [PubMed] [Google Scholar]
- 274.Ehebauer M, Hayward P, Martinez-Arias A. Notch Signaling Pathway. Sci. STKE. 2006;2006 doi: 10.1126/stke.3642006cm7. cm7. [DOI] [PubMed] [Google Scholar]
- 275.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr. Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 276.Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, Wu T, Niinobe M, Yoshikawa K, Hannigan GE, Halaban R. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 277.Massi D, Tarantini F, Franchi A, Paglierani M, Di Serio C, Pellerito S, Leoncini G, Cirino G, Geppetti P, Santucci M. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod. Pathol. 2006;19:246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 278.Qin J-Z, Stennett L, Bacon P, Bodner B, Hendrix MJC, Seftor REB, Seftor EA, Margaryan NV, Pollock PM, Curtis A, Trent JM, Bennett F, Miele L, Nickoloff BJ. p53-independent NOXA induction overcomes apoptotic resistance of malignant melanomas. Mol. Cancer Ther. 2004;3:895–902. [PubMed] [Google Scholar]
- 279.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu Z-J. Activation of Notch1 signaling is required for {b}-catenin-mediated human primary melanoma progression. J. Clin. Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu Z-J, Xiao M, Balint K, Smalley KSM, B-Rafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 281.Okuyama R, Tagami H, Aiba S. Notch signaling: Its role in epidermal homeostasis and in the pathogenesis of skin diseases. J. Dermatol. Sci. doi: 10.1016/j.jdermsci.2007.05.017. (In press). [DOI] [PubMed] [Google Scholar]
- 282.LoRusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, Hanson L, DeLuca P, Bruzek L, Piens J, Asbury P, Van Becelaere K, Herrera R, Sebolt-Leopold J, Meyer MB. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J. Clin. Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 283.Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, Cockerill M, Cartlidge S, Smith PD. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: Mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol. Cancer Ther. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 284.Richly H, Henning BF, Kupsch P, Passarge K, Grubert M, Hilger RA, Christensen O, Brendel E, Schwartz B, Ludwig M, Flashar C, Voigtmann R, Scheulen ME, Seeber S, Strumberg D. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann. Oncol. 2006;17:866–873. doi: 10.1093/annonc/mdl017. [DOI] [PubMed] [Google Scholar]
- 285.Becker JC, Kirkwood JM, Agarwala SS, Dummer R, Schrama D, Hauschild A. Molecularly targeted therapy for melanoma. Cancer. 2006;107:2317–2327. doi: 10.1002/cncr.22273. [DOI] [PubMed] [Google Scholar]
- 286.Hersey P. Apoptosis and melanoma: How new insights are effecting the development of new therapies for melanoma. Curr. Opin. Oncol. 2006;18:189–196. doi: 10.1097/01.cco.0000208794.24228.9f. [DOI] [PubMed] [Google Scholar]