Abstract
Memory B and plasma cells (PCs) are generated in the germinal center (GC). As PD-1 is highly expressed in T follicular helper cells (TFH), we investigated the role of PD-1 signaling in the humoral response. We found that PD-L1 and PD-L2 are upregulated on GC B cells. Pdcd1lg2−/−, CD274−/−Pdcd1lg2−/− and Pdcd1−/− mice had reduced numbers of long-lived PCs. The mechanism involved increased GC cell death and decreased TFH cytokine production in the absence of PD-1; the effect was selective, as remaining PCs had higher affinity. PD-1 expression on T cells and PD-L2 expression on B cells controlled TFH and PC numbers. Thus, PD-1 regulates selection and survival in the GC, impacting the quantity and quality of long-lived PCs.
Introduction
Adaptive immune responses generate long-lived, antigen (Ag)-experienced cells that can protect the host during subsequent infections. This immune system “memory” consists of cells derived from both activated B and T cells. Various intrinsic and extrinsic signals that control the survival, differentiation, function and maintenance of T cell subsets have been elucidated1. In contrast to this extensive body of work, the signals regulating the formation of long-lived PCs and memory B cells remain largely unknown.
Long-lived, high-affinity PCs and memory B cells are mainly generated during the GC response2. GCs are organized sites of proliferating cells, in which Ag-specific B cells undergo extensive multiplication and somatic hypermutation3. During multiple rounds of division, high-affinity GC cells are selected and differentiate into either memory cells or PCs4–6. Dysregulation of proliferation, mutation and differentiation within GCs can lead to detrimental outcomes, including tumorigenesis7, autoimmunity8 or immunodeficiency9. Therefore, regulation of survival, proliferation and differentiation signals within this dynamic environment is important for determining both the quality and size of PC and memory cell populations. Accordingly, many genes identified as upregulated in memory compared to naïve B cells were first upregulated in GC cells10–12. We hypothesized that these genes may be important for regulating the GC to memory cell transition, and thus focused on them for further analysis. Comparison of gene expression between memory and naïve B cells revealed upregulation of the B7-family member PD-L2 (also called B7-DC) in memory cells11. Further analysis reported here demonstrated that PD-L1 (CD274, B7-H1) and PD-L2, and their receptor PD-1 (refs. 13,14), were also upregulated on B cells in the GC. Thus, this set of important immune regulators was being modulated in concert during GC and memory cell development, but their functions had yet to be explored.
PD-1 signaling plays a major role in inhibiting T cell responses13. It is induced upon T cell activation, and markedly elevated expression of PD-1 is associated with “exhausted” memory T cells in chronic viral infections15. While PD-1 has only two known ligands, PD-L1 and PD-L2, it was recently shown that PD-L1 and CD80 interact to transduce bidirectional signals16. These multiple interactions most likely evolved to allow the immune system to constantly adapt, fine-tune, and eventually downregulate the response. PD-L1 is expressed on many cells, including activated B cells, whereas PD-L2 was originally thought to be expressed only on DCs and macrophages13,14,17, and more recently shown to be expressed on B1 B cells18. Accordingly, research on these ligands has focused on DC–T cell interactions, with little known about their roles on B cells. Interestingly, T cells within the follicle prominently express PD-1 (ref. 19). TFH, along with T cells at the T–B border, are a source of extrinsic factors that promote GC formation, expansion and isotype-switching20–22. It has been suggested that PD-1 is elevated during a viral infection to inhibit chronically activated T cells from causing immunopathology or becoming autoreactive23,24. Similarly, during humoral responses, regulation of proliferating GC cells is required for production of high-affinity effector cells without causing expansion of autoreactive or low-affinity clones. Yet, although PD-1 and its ligands are well investigated in T cell responses, their role in regulating GC responses and consequent output of long-lived B cells has not yet been explored.
Here we have used a combination of mutant mice and cell transfer approaches to test the hypothesis that PD-L1 and or PD-L2-expressing B cells interact with PD-1+ TFH cells in the GC to regulate formation of long-lived B cells. Indeed, we find that in the absence of PD-ligands or PD-1, formation of long-lived PCs were significantly diminished, although neither molecule is absolutely required. The decrease in PCs was traced back to the late stages of the GC, in which PD-1 signals substantially influence the rate of GC cell death, a major determinant of GC outcomes such as affinity maturation, selection and differentiation25. The PCs that did form in the absence of PD-1 signaling were of a higher affinity than controls, further suggesting that PD-1 plays a role in selection during the GC. This result was linked to decreased production of interleukin 4 (IL-4) and IL-21 mRNA by TFH in the absence of PD-1. Finally, given the recent identification of the PDL1-CD80 pathway16, it was important to investigate the cell intrinsic requirements of PD-1 and PD-L2. Cell transfers and mixed BM chimeras demonstrated that PD-L1 and PD-L2 expression on B cells and PD-1 expression on T cells determined PC numbers. Therefore, PD-1 controls development and function of TFH, which most likely in turn controls the optimal formation of long-lived PCs, by regulating the survival and selection of B cells in the mature GC.
Results
Expression of PD-L1 and PD-L2 on GC and memory cells
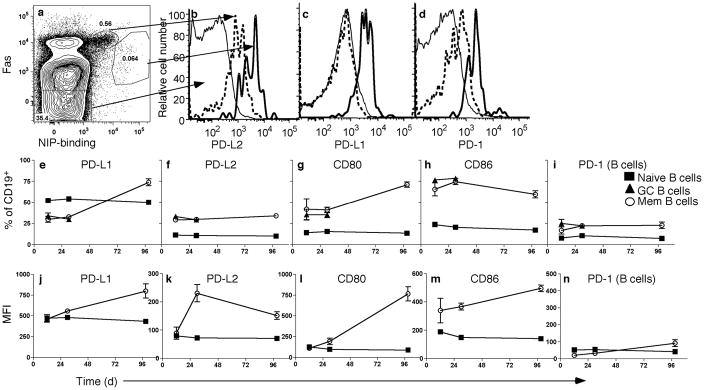
We previously found that murine memory B cells upregulated the mRNA encoding PD-L2 more than 25-fold compared to naïve B cells11. We further determined the expression of PD-L2, as well as PD-L1 and PD-1, on B cell subsets during a primary response in B6 wild-type mice (Fig. 1a–d). Naïve cells expressed PD-L1 (Fig. 1c), but neither PD-L2 (Fig. 1b) nor PD-1 (Fig. 1d). GC cells expressed intermediate levels of all three molecules, although they expressed amounts of PD-L1 comparable to naïve B cells. Emerging memory B cells26 (so called because they express surface markers similar to memory cells 3 months post-immunization) expressed more PD-L1, PD-L2 and PD-1 than naïve or GC cells, similar to long-lived memory B cells and consistent with microarray and quantitative PCR (qPCR) data obtained from memory cells at 12 wks11.
Figure 1. Differential expression of PD-1 and its ligands by B cell subsets.
Representative flow cytometry analysis of B6 mice immunized with NP-CGG in alum (n = 3–5) 12 days post-immunization. Splenic CD19+EMA− cells were analyzed for NIP-binding and expression of Fas (a), and were gated as follows: NIP−Fas− (naïve, thin solid histogram), NIPintFashi (GC, dashed histogram) and NIP+Fas+ (emerging memory, thick solid histogram). B cell populations were then examined for expression of PD-L2 (b), PD-L1 (c) and PD-1 (d). Frequency (e–i) and kinetics (j–n) of B7 family members and PD-1 on B cell subsets 12 days, and 4 and 14 wks post-immunization. The CD19+NIP+IgG1+CD38+kappalo phenotype was used to identify memory B cells 14 wks post-immunization due to the very low frequency of detectable cells. (e–n): points are means and error bars are SEM. Data are representative of at least three independent experiments.
Expression of PD-1 on T cells is modulated during an immune response23. To determine whether expression of B7-family receptors and ligands is also modulated on B cells over the course of a primary response, wild-type mice were immunized with (4-hydroxy-3-nitrophenyl)acetyl-chicken-γ-globulin (NP-CGG) and evaluated during the middle (d12) and late (wk4) phases of the GC response, as well as a memory time point (wk14). Initially, we assessed the percentage of each B cell type positive for the selected marker (Fig. 1e–i). The frequency of cells positive for PD-L1 and CD80 increased over time in memory B cells (Fig. 1e,g), whereas it remained unchanged for other molecules tested. We also measured the mean fluorescence intensity (MFI) of these molecules over time (Fig. 1j–m). Intensity of expression on memory B cells generally increased over time; however, in the case of PD-L2, expression peaked at 4 wks post-immunization, though it remained elevated compared to naïve cells 3 months post-immunization, consistent with microarray data11.
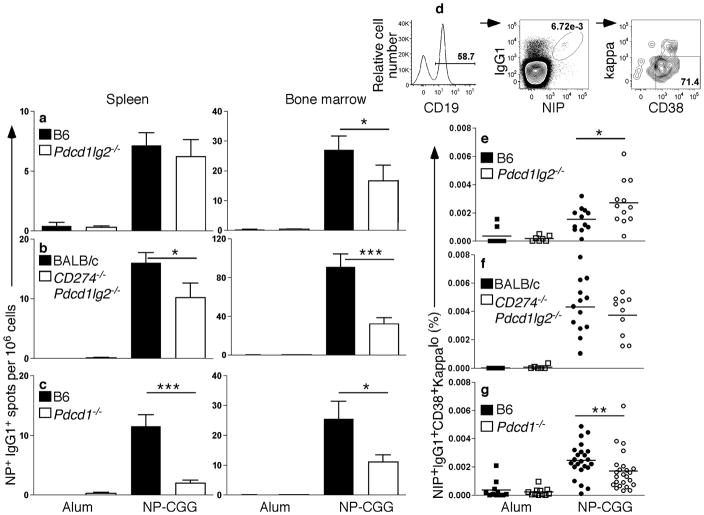
Since PD-1 and its ligands were all expressed by GC B cells, their role in the formation of GCs at d12 was investigated. We examined mice deficient for PD-L2 (abrogating PD-L2–PD-1 interactions; Pdcd1lg2−/−), PD-1 (abrogating PD-1 interactions with both PD-L1 and PD-L2; Pdcd1−/−), or lacking both PD-L1 and PD-L2 (abrogating interactions with both PD-1 and CD80; CD274−/−Pdcd1lg2−/−) mice. Mice appeared to generate a robust GC response at this time point. Sizes of GCs in Pdcd1lg2−/−, CD274−/−Pdcd1lg2−/− and Pdcd1−/− mice were similar to wild-type, and T cells were readily seen in the GCs (Supplementary Fig. 1a). GC B cell numbers were comparable in Pdcd1lg2−/− and Pdcd1−/− mice (Supplementary Fig. 1b,c), indicating that PD-1 signaling was not required for GC or TFH formation, the latter being defined as T cells present within the GC. Pdcd1lg2−/− and Pdcd1−/− mice were able to generate NIP+IgG1+ Ab-forming cells (AFCs), and developed titers of IgM and IgG1 anti-NP that were comparable to B6 wild-type mice (Supplementary Fig. 1d,e). Therefore, PD-1 was not required for the formation of a GC or for the early Ab response.
PD-1 signaling regulates formation of long-lived PCs
GC responses generate long-lived, high affinity PCs and memory B cells. Wild-type or PD-ligand or PD-1-deficient mice were immunized and then rested until the primary response had dissipated, allowing assessment of long-lived B cell-derived populations. After three months, a reduction in NP+IgG1+ AFCs was observed in both spleen and BM, in Pdcd1lg2−/−, CD274−/−Pdcd1lg2−/− and Pdcd1−/− mice (Fig. 2a–c); reductions in spleen in PD-L2-deficient mice did not reach significance. In the absence of PD-1, Ag-specific IgG1+ CD38+ memory B cells were able to form (Fig. 2d–g); however, the percentage of memory cells was modestly decreased compared to the wild-type controls in the absence of PD-1 (Fig. 2g), but not in the ligand-deficient mice (Fig. 2e,f). In the case of Pdcd1lg2−/−, memory B cells numbers were slightly increased, suggesting a possible divergence in the roles of PD-L1 and PD-L2 in memory cell development16, which could relate to broad tissue expression of PD-L1 and or function of CD80 as a second binding partner16. Therefore, in the absence of PD-1 signaling, both long-lived B cell-derived populations were reduced, however PD-1 signaling appears to play only a minor role in memory cell formation relative to PC formation.
Figure 2. Long-lived PCs are decreased in the absence of PD-1 signaling.
Analysis of long-lived PC and memory responses at least 12 weeks post-immunization with alum (n ≥ 5) or NP-CGG. (a–c) ELISpot analysis of spleen and BM from Pdcd1lg2−/− (a; n ≥ 16), CD274−/−Pdcd1lg2−/− (b, n ≥ 10), Pdcd1−/− (c; n ≥ 14) (open bars) or wild-type (WT) controls (black bars). (d) Live splenocytes were gated on CD19+NIP+IgG1+CD38+kappalo to assess memory B cell frequency. The CD38 marker was included to gate out any residual GC B cells (which are CD38−). Because naïve B cells are also CD38+, IgG1 was included to separate memory B cells from naïve B cells, although this precludes analysis of any present IgM+ memory B cells. (e–g) Frequency of memory B cells in Pdcd1lg2−/− (e), CD274−/−Pdcd1lg2−/− (f) and Pdcd1−/− (g) mice (open symbols) compared to WT controls (closed symbols). *P < 0.05, **P < 0.01, ***P < 0.001. Data are combined from two (CD274−/−Pdcd1lg2−/−) or seven (Pdcd1lg2−/− and Pdcd1−/−) independent experiments.
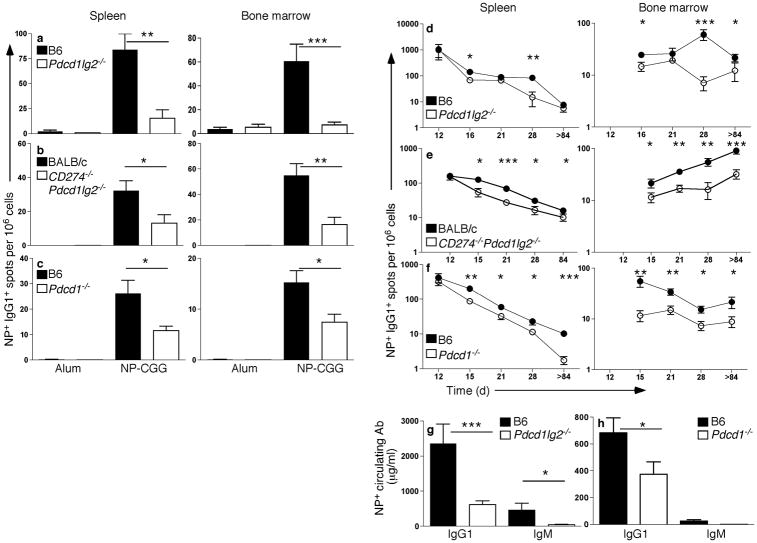
Earlier work revealed that high-affinity, long-lived PCs are formed late in the GC response27. Since AFC numbers were similar in wild-type and PD-L2 and PD-1 strains at d12, but not at later time points, we considered that PD-1 plays a more prominent role in the late GC response. IgG1+ AFCs were significantly reduced in the spleen in the absence of PD-1 signaling 4 wks post-immunization in the Pdcd1lg2−/− (Fig. 3a), CD274−/−Pdcd1lg2−/− (Fig. 3b), and Pdcd1−/− (Fig. 3c) mice. Long-lived PCs in the bone marrow (BM) were also significantly reduced in all three strains (Fig. 3a–c), and differences in frequency of memory phenotype B cells in knockouts compared to controls paralleled the differences seen 3 months post-immunization (not shown). To determine when differences in AFC number between wild-type and PD-deficient mice emerged, mice were immunized and AFCs assessed at multiple additional time points. In Pdcd1lg2−/−, CD274−/−Pdcd1lg2−/− and Pdcd1−/− mice, diminished AFCs are evident by d15–16 (Fig. 3d–f). This reduction was consistent for all time-points assessed after d15–16 for the Pdcd1−/− and CD274−/−Pdcd1lg2−/− mice, however the differences in the Pdcd1lg2−/− mice were variable depending on the time-point. Reduced numbers of AFCs were also concordant with a decrease in both circulating IgG1 and IgM titers (Fig. 3g,h). The decrease in serum IgM further suggests that reduced IgG1 AFCs are not simply due to a defect in isotype switching, but is the result of reduced AFC formation per se.
Figure 3. The reduction in PC numbers occurs during the late GC response, and affects both IgG1 and IgM Ab.
(a–c) ELISpot analysis of NP+IgG1+ AFCs in spleen and BM from WT controls (black bars) or Pdcd1lg2−/− (a, n ≥ 6 for WT and mutant strain), CD274−/−Pdcd1lg2−/− (b, n ≥ 10) or Pdcd1−/− (c, n ≥ 12; open bars) mice immunized with alum (n = 3–9 for all genotypes) or NP-CGG ~4 wks post-immunization. Data is combined from at least two independent experiments. Pdcd1lg2−/− (d), CD274−/−Pdcd1lg2−/− (e), Pdcd1−/− (f) or WT controls were immunized with NP-CGG or alum alone and NP+IgG1+ AFCs in spleen and BM assessed at multiple time-points post-immunization (d28 includes d27-d31, as shown also in a–c; d12 also shown in Supplementary Fig. 1, >d84 in Fig. 2). For all time-points except for CD274−/−Pdcd1lg2−/− d18 and d21, data are combined from multiple independent experiments. (g,h) Circulating IgG1 and IgM in Pdcd1lg2−/− (g; n = 6–8), Pdcd1−/− (h; n = 6) or WT at wk4 and wk3 post-immunization, respectively. *P < 0.05, **P < 0.01, ***P < 0.001. Data are representative of at least two independent experiments from wks 3–4.
Decreased GC B cell survival in the absence of PD-1
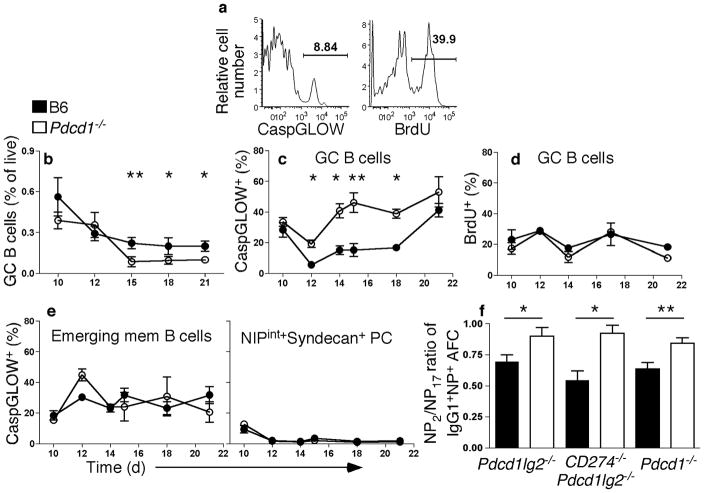
Why are PCs able to form early during the response but are reduced at later time-points? It was possible that a block in differentiation from plasmablast to PC was occurring, allowing short-lived plasmablasts to be generated, but not to terminally differentiate. However, examination of the progression of this process, using several markers (for example, downregulation of surface BCR and expression of CD138) that change during PC differentiation, revealed no difference between wild-type and PD-L2 and PD-1 mice (not shown). Since PC differentiation itself was not affected by absence of PD-1 signals, we suspected a defect in the GC that affected development of these long-lived cells. Therefore, we investigated whether decreased proliferation or increased apoptosis during GC responses in the absence of PD-1 signaling was potentially causing the reduction in long-lived B cells. We assessed this notion by detecting caspase activation using a fluorescent pan-caspase irreversible inhibitor (“CaspGLOW” assay) and measurement of BrdU incorporation, respectively (Fig. 4a). GC B cell frequency was decreased in Pdcd1−/− relative to wild-type mice (Fig. 4b) from d15 on, even though GC formation was comparable to wild-type at d12. Commensurate with decrease of GC B cells, an increase in CaspGLOW+ GC cells was observed in Pdcd1−/− mice (Fig. 4c). A decrease in GC B cell frequency, and increase in CaspGLOW+ GC cells, was also observed in CD274−/−Pdcd1lg2−/− mice (Supplementary Fig. 2). This increase was observed beginning on d12, the height of the GC response and three days before the decrease in AFCs was first noted (Fig. 3). On the other hand, there was no difference in BrdU incorporation by GC cells of B6 and Pdcd1−/− mice (Fig. 4d). In contrast to GC cells, death of emerging memory B cells and of the plasmablast and PC population in Pdcd1−/− mice was comparable to wild-type mice (Fig. 4e), as was proliferation (not shown). Hence, we could narrow the effect of PD-1 signaling to prevention of GC cell death in the mature GC; whereas there was no detectable influence in the early GC, on GC or memory cell proliferation, or PC differentiation. During the GC response, high-affinity variants are selected to differentiate, whereas low-affinity cells undergo cell death. Due to the increase in cell death of GC B cells observed in Pdcd1−/− mice, we assessed the affinity of BM PCs at least 12 wks post-immunization. By assessing the ratio of high-affinity to low-affinity AFCs, we observed an increase in affinity of the remaining PCs in the absence of either PD-ligands or PD-1 (Fig. 4f). Igh VH186.2 sequences from memory B cells from Pdcd1−/− mice and wild-type controls were also compared (Supplementary Fig. 3). Average mutations per sequence were comparable in the two datasets (B6: 8.8 mutations/sequence, 59 sequences analyzed; Pdcd1−/−: 8.5 mutations/sequence, 32 sequences analyzed), as was RCDR/(Stotal+RCDR) (B6: 0.53; Pdcd1−/−: 0.5), and the frequency of Trp to Leu codon replacements at position 33 (B6: 36%, Pdcd1−/−: 41%)28.
Figure 4. Increased cell death but normal proliferation in the GCs of Pdcd1−/− mice.
WT (closed symbols) and Pdcd1−/− B6 (open symbols) mice immunized with NP-CGG, were pulsed with 3 mg/mouse of BrdU 2hr before analysis. Splenic CD19+NIPintFashi cells undergoing cell death were revealed by detection of activated caspases using CaspGLOW immediately after cell harvest at the timepoints: d10, 12 (also shown in Supplementary Fig. 1), 14, 15, 18 and 21 post-immunization. (a) Sample flow cytometry data of CaspGLOW and BrdU staining from an immunized B6 mouse at d12. (b–d) Summary of frequencies derived from flow cytometry analysis of splenic GC B cells (b) that are CaspGLOW+ (c) and BrdU+ (d) over time. (e) Flow cytometry analysis of emerging memory cells and plasmablasts/PCs that are CaspGLOW+; n ≥ 4 per time point. (f) NP2-BSA or NP17-BSA was used as capture Ag for ELISpot and the ratio of NP2 versus NP17-specific IgG1+ BM AFCs for knockout (open histogram; n ≥ 6) and WT mice (closed histogram) were plotted. *P < 0.05, **P < 0.01, ***P < 0.001. Data are combined from, or are representative of, at least two independent experiments.
However, it was noted that although both wild-type B6 and Pdcd1−/− memory B cells show positive selection in the IgH complementarity determining regions, there was significant negative selection in the framework regions in the Pdcd1−/− mice only, suggesting that responding B cells in these mice may be under more stringent selection, and thus are more sensitive to deleterious mutations. As the late GC response is more focused on generating long-lived AFCs27, we propose that excessive cell death in the absence of PD-1 signaling specifically affects the selection of cells entering the long-lived B cell compartments, leading to a deficit in the generation of long-lived PCs and to a lesser extent, memory B cells.
PD-1 affects the function and numbers of TFH cells
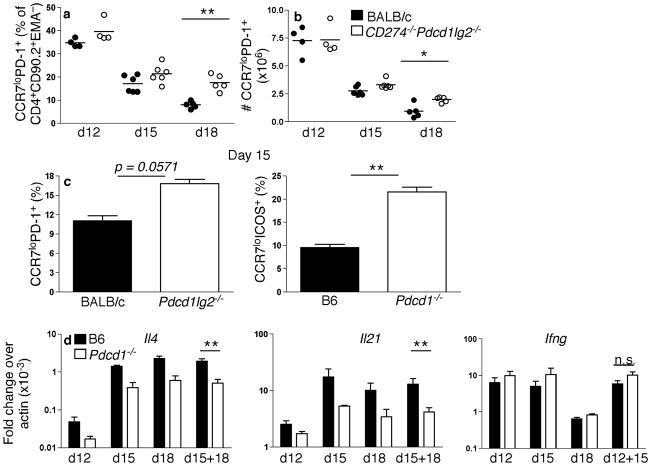
As PD-1 is highly expressed by TFH, we investigated whether the numbers and or function of TFH cells were affected during the GC response in the absence of PD-1 signaling. TFH cells were identified by CCR7 downregulation in combination with PD-1 expression in Pdcd1lg2−/− and CD274−/−Pdcd1lg2−/− mice, or in combination with ICOS expression in Pdcd1−/− mice. Although at d12 TFH frequency (Fig. 5a) and number (Fig. 5b) were comparable in CD274−/−Pdcd1lg2−/− and wild-type mice, this population was increased in CD274−/−Pdcd1lg2−/− mice at later time points. This increase was also seen in both Pdcd1lg2−/− and Pdcd1−/− mice (Fig. 5c). To investigate how PD-1 signaling affected the capacity for cytokine production (Fig. 5d), TFH were sorted and mRNA collected. TFH from Pdcd1−/− mice had reduced amounts of mRNA encoding IL-4 and IL-21, two cytokines secreted by TFH that promote GC development29. Reductions in mRNA abundance were observed for both cytokines at all time points, with greater reductions seen in the later GC, commensurate with the late defects in GC B cell survival. Combining the similar d15 and d18 time-points, these differences reached significance for both Il4 and Il21 (P < 0.01). On the other hand, mRNA encoding interferon-γ (IFN-γ) were comparable between Pdcd1−/− and wild-type mice; although there was a slight increase in transcript abundance in the absence of PD-1, this was less than 2-fold and did not reach significance, even when the two similar time-points of d12 and d15 were combined. mRNA encoding IL-17 was not present in TFH from either wild-type or knockout mice (not shown). Therefore, although the lack of PD-1 signaling increases the quantity of TFH, it impairs the quality of TFH by reducing their capacity to synthesize important cytokines while not promoting the development of an alternatively polarized T cell type.
Figure 5. An increase of cells of a TFH phenotype correlates with a decrease in cytokine production in the absence of PD-1 signaling.
(a–c) CD274−/−Pdcd1lg2−/−, Pdcd1lg2−/−, Pdcd1−/− and WT mice were immunized with NP-CGG and CD4+CD90.2+EMA− cells were gated for the TFH markers of PD-1 expression and CCR7 downregulation, or ICOS expression and CCR7 downregulation for Pdcd1−/− and corresponding WT mice. (a,b) Flow cytometry analysis of TFH frequency among CD4 cells (a) and number (b) in CD274−/−Pdcd1lg2−/− and WT mice d12, d15 and d18 post-immunization. (c) TFH frequency in Pdcd1lg2−/−, Pdcd1−/− and WT mice d15 post-immunization (n = 3–5). Data are representative of at least two independent experiments during the late GC response. (d) Pdcd1−/− and WT mice were immunized with NP-CGG in alum and sorted for ICOS expression and CCR7 downregulation (d12, n = 3), in conjunction with CXCR5 expression (d15, n = 2; d18, n = 3). Il4, Il21 and Ifng expression was assessed by qPCR. Data are shown as fold change as calculated by 2(actin Ct − cytokine Ct). *P < 0.05, **P < 0.01, ***P < 0.001. n.s. = no significance. Each time point is a separate independent experiment.
PD-L1 and PD-L2 on B cells regulates AFC production
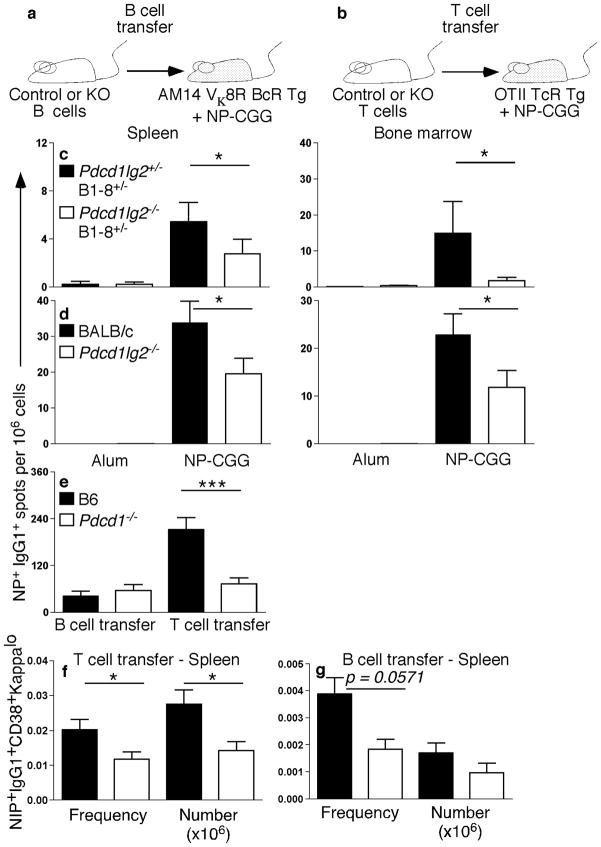
In addition to their expression on B cells, PD-L1 and PD-L2 are expressed on other immune cells. PD-1 is expressed on both B and T cells. Therefore, it was not clear on which cell types expression was critical to promote proper AFC numbers. To investigate, we assessed the cell-intrinsic expression requirements of both PD-L2 and PD-1 by purifying and transferring ligand–receptor-deficient B or T cells (as shown in the schematic representation, Fig. 6a. In wild-type mice, long-lived PCs persist at quite low frequencies (Fig. 2). Therefore, to facilitate sensitivity and interpretation of transfer experiments, Pdcd1lg2−/− mice were crossed to B1-8 Igh knockin mice30, which express an NP-specific BCR, thereby increasing the NP-specific precursor frequency among transferred cells and ensuring that a readout of long-lived PCs from transferred B cells would be possible. It should be noted that, after transfer, there was still a very low precursor frequency of Ag-specific Pdcd1lg2−/− B cells. B cells from either Pdcd1lg2−/−B1-8+/− or Pdcd1lg2−/−B1-8+/− mice on a B6 background were transferred into AM14 Vκ8R transgenic (Tg) recipients. AM14 Vκ8R Tg mice have a virtually homogenous B cell population of irrelevant specificity31, such that host B cells should not respond to NP-CGG (not shown). At 63 or more days post-immunization there was a significant decrease in both splenic and BM PCs in the recipients of deficient vs sufficient B cells (Fig. 6c). Similarly, when B cells from Pdcd1lg2−/− mice lacking the B1-8 knockin allele were transferred into AM14 Vκ8R Tg recipients, AFC numbers were also decreased 3 wks post-immunization compared to wild-type BALB/c B cells (Fig. 6d). Taken together, these results demonstrate that the lack of PD-L2 expression on B cells, rather than DCs or macrophages, results in a decrease in PC generation. To examine the role of PD-1 expression on B cells, Pdcd1−/− or wild-type B6 B cells were transferred into AM14 Vκ8R Tg recipients. Reciprocally, Pdcd1−/− or wild-type B6 T cells were transferred into OT-II TCR Tg mice, again to restrict the host repertoire (Fig. 6b). Control recipient mice that did not receive donor cells were also immunized to confirm that the recipient mice did not respond to NP-CGG (not shown). Transferred Pdcd1−/− and wild-type B6 B cells yielded comparable AFC production 4 wks post-immunization (Fig. 6e). In contrast, when Pdcd1−/− or wild-type B6 T cells were transferred, there was a significant decrease in AFCs (Fig. 6e). Interestingly, in both the Pdcd1−/− B cell and T cell transfer, B cells of a memory phenotype were decreased (Fig. 6f,g).
Figure 6. Decrease in AFCs is due to impaired interactions between PD-ligands on B cells and PD-1 on T cells.
(a,b) Schematic representation of the B cell transfer system (a) and T cell transfer system (b). (c,d) ELISpot analysis of NP+IgG1+ AFCs in spleen and BM from AM14 Vκ8R recipient mice of transferred control B cells (black bar) or B cells from Pdcd1lg2−/−B1-8+/−(c; >day 63; n = 16) or Pdcd1lg2−/− (d; day 26–28; n = 14–15) mice (open bar) immunized with alum or NP-CGG. Data are combined from three (c) or four (d) independent experiments. (e–g) Analysis of Pdcd1−/− B and T cell transfers 4 wks post-immunization: (e) ELISpot analysis of splenic NP+IgG1+ AFCs in either AM14 Vκ8R recipients of transferred B cells (n = 8), or OT-II recipients of transferred T cells (n = 14), from B6 WT mice (black bar) or Pdcd1−/− mice (open bar). (f,g) Frequency and number of NIP+IgG1+CD38+kappalo B cells after transfer of WT (closed symbols) or Pdcd1−/− (open symbols) T cells (f) or B cells (g). *P < 0.05, **P < 0.01, ***P < 0.001. Data are combined from two (B cell transfer) or three (T cell transfer) independent experiments.
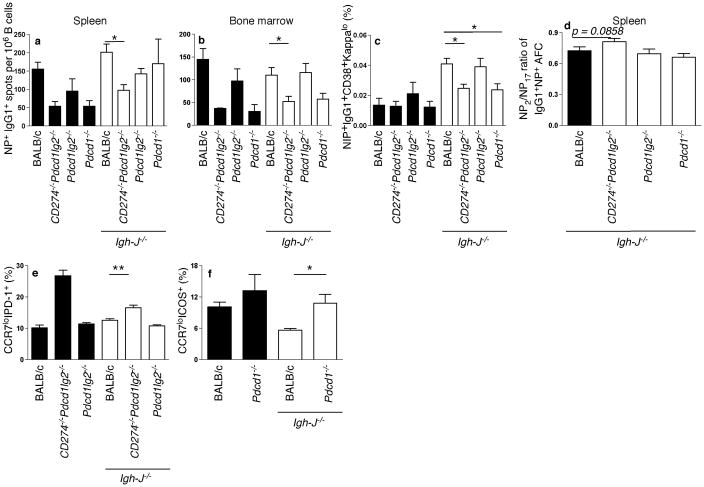
To confirm the results of these transfer systems, we also utilized mixed BM chimeras. BM from Igh-J−/− mice (that lack B cells) was mixed with BM from Pdcd1lg2−/−, CD274−/−Pdcd1lg2−/−, Pdcd1−/− or wild-type mice in a mix of approximately 80% to 20% and transferred into lethally irradiated JH-deficient recipients, which were then immunized after reconstitution. In such chimeras, all B cells would lack the PD-molecule of interest, while the majority of non-B cells would express PD-molecules. As controls 100% BM from gene-deficient mice was transferred. These control mice generally replicated the phenotype seen in the intact gene-deficient animals (Fig. 7), demonstrating that the BM transfer system worked and further showing that most of the phenotype of intact PD-deficient animals is attributable to hematopoietic expression of the relevant PD-molecule. At d25–26 post-immunization, mice lacking PD-L1 and PD-L2 on B cells had significantly decreased numbers of AFCs and memory B cell formation (Fig. 7a–c), and the affinity of AFCs was increased (Fig. 7d). Splenic AFCs were not significantly decreased in chimeras in which only B cells lacked only PD-L2, though there was a trend in this direction (Fig. 7a). There was no decrease in memory cell formation (Fig. 7c), consistent with results from intact Pdcd1lg2−/− mice (Fig. 2). Chimeric mice lacking PD-1 expression on B cells showed no deficiency in splenic AFC numbers, though there was a trend towards a decrease in BM AFCs that did not reach significance; these mice did have significantly decreased memory cell formation (Fig. 7a–c), consistent with the transfer data (Fig. 6). TFH cells were increased in both the CD274−/−Pdcd1lg2−/− and Pdcd1−/− mixed BM chimeras, but not Pdcd1lg2−/− mixed chimeras (Fig. 7e,f). Therefore, we conclude that B cells that express PD-L1 and PD-L2 and T cells expressing PD-1 interact via this pathway to regulate both PC and memory B cell production, whereas B cell-expressed PD-1, that could mediate B-B or B-T interactions (wherein the ligand is expressed on the T cell), affects memory and possibly BM PC formation, but not splenic PC formation.
Figure 7. AFC production, memory B cell formation and TFH are altered in mixed BM chimeras.
Mixed BM chimeras were made by mixing Igh-J−/− BM with Pdcd1lg2−/−, CD274−/− Pdcd1lg2−/−, Pdcd1−/− or BALB/c BM at a 80:20% ratio, and injecting into lethally irradiated BALB/c recipients (white bars). Black bars represent mice that received BM only from WT or knockout mice (without Igh-J−/− BM). Mice were assessed for degree of chimerization, which approximated the above ratio (not shown). Mice were rested for 6 wks before immunization with NP-CGG. Mice were assessed at d25–26 post-immunization (Igh-J−/−:BALB/c: n = 19, Igh-J−/−:Pdcd1lg2−/−: n = 12, Igh-J−/−:CD274−/−Pdcd1lg2−/−: n = 8, Igh-J−/−:Pdcd1−/−: n = 5). (a,b,d) ELISpot analysis of mixed BM chimeras in the spleen (a,c) and BM (b). (c, e–g) flow cytometry analyses of memory B cell frequency (of CD19+ cells; c) and TFH cell frequency (of CD4+CD90.2+ cells; Igh-J−/−: BALB/c: n = 15, Igh-J−/−:Pdcd1lg2−/−: n = 9, Igh-J−/−:CD274−/−Pdcd1lg2−/−: n = 4; Igh-J−/−: Pdcd1−/−: n = 5; e,f) as described in the text. *P < 0.05, **P < 0.01, ***P < 0.001. Data are combined from or are representative of two independent experiments.
Discussion
We report that PD-1, and its ligands PD-L1 and PD-L2, play a previously unknown role in regulating the primary humoral immune response, and provide insight into how this is mediated. The most prominent effect of the loss of PD-1 signaling was a reduction in numbers of long-lived PCs. One element of the complexity of B7 family immunoregulation is the variety of potential interactions. By using mice deficient in PD-1, PD-L2, and both PD-L1 and PD-L2, along with cell transfer and mixed BM chimeras, we were able to delineate some of the key interactions and cell-intrinsic requirements for regulation of the response. In particular, our results directly implicate interactions between PD-1 on T cells and PD-L1 and or PD-L2 on B cells in the formation of long-lived PCs. A pivotal observation is that B cell-intrinsic expression of PD-L2 by itself was required for optimal AFC generation, directly implicating a non-redundant role for this molecule that is not expressed on B cells until they differentiate in the GC. Nonetheless, in many ways the phenotypes of the CD274−/−Pdcd1lg2−/− and Pdcd1−/− mice were more pronounced than those of the Pdcd1lg2−/− animals, possibly reflecting constitutive PD-L1 expression on B cells, which can also contribute to PD-1 signals. On the other hand, our results do not rule out roles for additional interactions mediated by these molecules, such as PD-1 on B cells binding to PD-L1 or PD-L2 on B cells in the promotion of memory cell formation, as suggested by our mixed BM chimera data. Taken together, significant differences in PC numbers between wild-type and PD-1 signaling-deficient animals were observed at multiple time points, in different tissues, and in each type of gene-targeted animal on different backgrounds, establishing that PD-1 signaling regulates the formation of long-lived PCs.
We delineated several aspects of the mechanism by which signals through PD-1 regulate the primary humoral response. In the absence of PD-1 signaling, increased GC B cell death corresponded to a quantitative defect in PC numbers; however, the remaining PC were of higher affinity compared to wild-type. This effect was most evident late in the response, and consistent with this, memory B cell numbers were not as severely affected as PCs, likely due to their formation occurring early in the response26,27,32. How does PD-1 signaling on T cells promote GC B cell survival? The lack of PD-1 signaling resulted in an increase in the number of TFH, but a decrease in Il4 and Il21 cytokine mRNA synthesis by them. Therefore, PD-1 ligation of PD-1 on T cells by PD-L2 and or PD-L1 expressed on B cells appears to qualitatively affect TFH homeostasis and function. Reciprocally, signals in T cells influence the probability of B cell survival, by delivering contact-based signals or cytokines, such as Fas-ligand, IL-4 and IL-21. IL-21 is required for optimal Ab production33, and interestingly, IL-21 is able to induce PD-1 expression34. Both IL-4Rα−/− and IL-21R−/− mice have phenotypes similar to the Pdcd1−/− mice, with both showing reduced GC B cell numbers35. Further, it has recently been shown that IL-21 acts directly on GC B cells through IL-21R to affect GC B cell maintenance and subsequent formation of PC, and is not required for the formation of TFH cells36–38. Together with our data, these findings provide a mechanism by which the reduced amounts of T cell-derived IL-4 and IL-21 in the Pdcd1−/− mice is linked to the inability to sustain GC B cells in the late response, possibly resulting in the competitive survival of higher-affinity AFCs.
Though reduced, AFC responses were not abrogated in the absence of PD-1-signaling, indicating that interactions between PD-1 and its ligands control the extent of the response, rather than whether it happens at all. Reduced production of PCs observed in the absence of PD-1 or other molecules, such as IL-2133, are more consistent with a model of division-linked differentiation, in which multiple survival and differentiation signals are integrated at each division, thereby affecting the probability of different potential outcomes39. Fine-tuning of B cell behavior per division allows small changes, such as those governed by PD-1 or IL-21 signals in the GC, to produce a large cumulative effect on the quality and quantity of humoral responses over time40.
Our data also provide insight into the functional significance of the markedly increased expression of PD-1 on TFH, demonstrating a specific function in the control of GC B cell survival and selection. Interestingly, the data clearly show a “paradoxical role” for PD-1, in that the presence of PD-1 promotes the humoral response, whereas heretofore PD-1 has only been seen as an inhibitor of both autoimmune and immunopathogenic anti-viral responses. Though concepts of PD-1 function in T cells mainly come from CD8 cells23, the role of PD-1 likely depends on the type of T cell41. TFH seem to be very different even from other types of CD4 T cells in terms of their ontogeny and the transcription factors that define them19. Though PD-1 is commonly thought of as a marker of “exhaustion”, TFH cells are not exhausted in that they secrete copious amounts of IL-21 and other cytokines during the humoral response. In the absence of PD-1, TFH did accumulate more in vivo, suggestive of a relief of an “inhibitory” signal. However, this correlated with a decrease in TFH function, notably a reduction in IL-21 production. Therefore, it is possible that high expression of PD-1 may freeze T cells in a TFH state, allowing sustained production of IL-4 and IL-21 in the B cell follicle, whereas lower to negligible levels of PD-1 on T cells may allow proliferation and differentiation into another type of Th effector cell. Though the roles of PD-1 as inhibiting and mediating exhaustion in CD8 T cells while enabling TFH to prevent GC cell death seem in conflict, we propose a unifying view that PD-1 could function to favor aspects of the humoral response over the cellular. This could be an adaptation for certain chronic inflammatory situations; if acute cytotoxic responses prove ineffectual, these are suppressed, perhaps limiting immunopathology, while long-lived Ab responses are enhanced, perhaps limiting further pathogen spread.
Overall, while the roles of PD-1 signaling in the T cell response are the subject of numerous reports (reviewed in23), the role of this signaling pathway in the B cell response has been virtually unstudied. The current report helps to fill this gap, defining an unexpected role for PD-1 signaling in promoting the long-lived PC response. These pathways could have many consequences for both humoral immunity and autoimmunity, the outcomes of which are often determined by the exact balance and timing of the response.
Methods
Mice and immunizations
Pdcd1lg2−/− were generated as described42 and backcrossed onto B6 background at least 10 generations. Pdcd1lg2−/− and CD274−/−Pdcd1lg2−/− mice on BALB/c background were generated as described in43. Pdcd1−/− mice were generated as described and backcrossed onto B644 background or BALB/c45 background. B6 and BALB/c controls, as well as OT-II TcR transgenic mice, were obtained from Jackson Labs. AM14 Vκ8R46,47, Igh-J−/−48 and B1-8 knockin30 mice were generated as described. For primary responses, mice were immunized intraperitoneally with 50μg of alum precipitated NP-CGG, with a ratio of NP to CGG ranging from 25 to 31, or precipitated alum alone as a control. All mice were maintained under SPF conditions and immunized/transferred at 6–12 weeks of age. All animal experiments were approved by the Yale Institutional Animal Care and Use Committee.
Antibodies and detection reagents
The following staining reagents were used: (4-hydroxy-5-iodo-3-nitrophenyl)acetyl (NIP)-binding reagents (NIP-PE and NIP-APC) and mAbs against murine CD3e (2C-11), CD4 (GK1.5), CD90.2 (30H12), CD80 (1610A1) and Kappa (187.1) were prepared as described in49. Abs against IgG1 (A85-1), CD19 (1D3), CD38 (90), CD138 (281-2), CD86 (GL1), CD45R (RA3-6B2), CD95 (Jo-2). CD25 (PC61), CXCR5 (2G8) and SA-PE-Cy7 were purchased from BD Biosciences. Abs against CCR7 (4B12), PD-L1 (MIH5), PD-L2 (TY25), PD-1 (J43) and CD62L (Ly-22) were purchased from eBioscience. Streptavidin-Alexa 680 was purchased from Invitrogen, and Abs against BrdU (PRB-1; Molecular Probes), ICOS (C398.4A; Biolegend), PD-1 (29F.1A12; Biolegend) and IgD (11-26c.2a; Biolegend) were also purchased.
Flow cytometry
Single cell suspensions of splenocytes were stained with appropriate antibodies in PBS containing 3% calf serum, 0.05% sodium azide on ice. Non-specific staining was blocked by the mAb 24G2, or rat serum for intracellular staining. For intracellular staining, samples were fixed and permeabilized before incubation with antibody in cytoperm (BD). Live/dead discrimination was performed using ethidium monoazide (EMA; molecular probes), and gating strategies were utilized for doublet discrimination. For purification of populations, cells were sorted on a FACSAria (BD Immunocytometry Systems). For analysis, samples were fixed with 1% paraformaldehyde before analysis on a BD LSRII. Data was analyzed with FlowJo software (Tree Star).
BrdU detection and apoptosis assays
For BrdU labeling, mice were given intraperitoneal injections of 3 mg of BrdU (Sigma-Aldrich) 2 h before sacrifice. Detection of BrdU in cells was performed as described previously25. For detection of apoptosis in situ, splenocytes were incubated with zVAD-FMK-Fluorescein (Casp-Glow, BioVision) in RPMI for 30–45 min at 37 °C. Cells were washed according to the manufacturer’s protocol, and then labeled with appropriate antibodies for surface phenotyping.
ELISpots and ELISAs
To determine production of AFC using an ELISpot assay or Ab using an ELISA, plates were coated with 5μg of NP-BSA conjugated at the appropriate ratio (NP5-BSA for IgM, NP17-27-BSA for IgG1, and NP2-BSA for IgG1 affinity studies) overnight at 4 °C. Non-specific binding was blocked with 1% BSA in PBS, and samples incubated at 37 °C. Secondary Ab (anti-IgG1- or anti-IgM-alkaline phosphatase; Southern Biotech) was detected using bromo-4-chloro-3-indolyl phosphate substrate (Southern Biotech) or p-nitrophenyl phosphate (Southern Biotech) for ELISpot or ELISA assays, respectively.
Quantitative PCR
Total RNA was isolated from sort-purified populations. cDNA synthesis and qPCR was performed as previously described13. Primer sequences are as follows: Il4 sense: 5′-AAAGACTTCCTGGAAAGCCTA-3′, antisense: 5′-CCTTATGGCCAAATGAAGTGA-3′; Il17 sense: 5′-TCCAGAAGGCCCTCAGACTA-3′, antisense: 5′-TCATGTGGTGGTCCAGCTT-3′; Il21 sense: 5′-TGAAAGCCTGTGGAAGTGCAAACC-3′, anti-sense: 5′-AGCAGATTCATCACAGGACACCCA-3′; Ifng sense: 5′-ATGAACGCTACACACTGCATC-3′, antisense: 5′-CCATCCTTTTGCCAGTTCCTC-3′; and actin as stated in11.
Sequencing analysis
Cell pellets from sort-purified populations were digested overnight as previously described50. VH186.2/JH2 sequences were amplified by nested PCR using external primers, sense: 5′-CCTGACCCAGATGTCCCTTCTTCTCCAGCA-3′, antisense: 5′-GGTGTCCCTAGTCCTTCATGACC-3′; and internal primers, sense: 5′-AGGTCCAACTGCAGCC-3′, antisense: 5′-TGTGAGAGTGGTGCCT-3′. Amplified DNA was cloned, colony PCRs performed, DNA purified, and sequenced as described in50 using the M13 sequencing primers. The Clustal W method of alignment was performed using MegAlign (DNASTAR, Inc.). Selection analysis, including analysis of mutations in the CDR and FW regions, was performed using the methods discussed in Hershberg et al28.
Adoptive transfer
B cells from WT or knockout mice were obtained using the EasySep negative B cell isolation kit from StemCell Technologies, following the manufacturer’s instructions. Single cell suspensions were transferred into tail veins of recipient mice. Approximately 1 × 107 B cells were transferred per mouse for WT transfers, with NIP+ cells accounting for ~0.02–0.05% of B cells. Approximately 1–2 × 105 B cells were transferred per mouse for B1-8 transfers. Purity of B cells was typically 90%. Mice were rested for one to two days and then immunized as outlined above.
BM chimeras
BM from WT or knockout mice and Igh-J−/− mice were mixed in a 20:80% ratio. Cell suspensions were injected into lethally irradiated Balb/c recipients (recipients received total body irradiation, 2x doses of 450 cGy, separated by 3 h, from a 137Cs source). Mice were rested for at least 6 weeks before immunization, and were assessed for extent of chimerism at the time of harvest.
Immunofluorescence histology
Sections were stained with PNA-biotin and anti-TCRβ-FITC, using Streptavidin-Alexa 555 and anti-FITC-Alexa 488 as secondary reagents, respectively. Images were taken at a 20X magnification.
Statistics
Statistical analyses was performed using the Mann-Whitney nonparametric test, with two-tailed P-values indicated throughout as: *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Acknowledgments
We thank K. Rajewsky (Harvard University) for B1-8 knockin mice and T. Honjo (Kyoto University) for Pdcd1−/− mice; E. Song and G. Zuccarino-Catania for technical assistance; U. Hershberg for assistance analyzing memory B cell sequencing data; the staff of the Yale Cell Sorter Facility and the Yale Animal Resource Center for excellent cell sorting and animal care, respectively; members of the Craft and Haberman labs for oligonucleotides; and S. Kerfoot for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI43603 (to M.J.S.), AI40614 (to A.H.S), and K08AI78533 (to M.M.T). K.L.G-J was supported by an NHMRC Biomedical Research Fellowship and an Arthritis Australia Postdoctoral Fellowship.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
Author contributions
K.L.G-J, M.M.T, and M.J.S. designed research; K.L.G-J and C.G.S performed research; L.C and A.S. generated and contributed knockout mice; K.L.G-J and M.J.S. analyzed data and wrote the manuscript.
References
- 1.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 2.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. Journal of Immunology. 1995;155:556–567. [PubMed] [Google Scholar]
- 3.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 4.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, et al. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J Exp Med. 1999;190:399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 8.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen RC, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome.[see comment] Science. 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 10.Klein U, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomayko MM, et al. Systematic comparison of gene expression between murine memory and naïve B cells demonstrates that memory B cells have unique signaling capabilities. J Immunol. 2008;181:27–38. doi: 10.4049/jimmunol.181.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naïve B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 13.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 18.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 19.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 21.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. Journal of Experimental Medicine. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens.[erratum appears in Eur J Immunol 1992 Feb;22(2):615] European Journal of Immunology. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 24.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson SM, et al. Taking advantage: high-affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low-affinity cells. J Immunol. 2009;183:7314–7325. doi: 10.4049/jimmunol.0902452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inamine A, et al. Two waves of memory B-cell generation in the primary immune response. Int Immunol. 2005;17:581–589. doi: 10.1093/intimm/dxh241. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 28.Hershberg U, Uduman M, Shlomchik MJ, Kleinstein SH. Improved methods for detecting selection by mutation analysis of Ig V region sequences. Int Immunol. 2008;20:683–694. doi: 10.1093/intimm/dxn026. [DOI] [PubMed] [Google Scholar]
- 29.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonoda E, et al. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 31.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 32.Blink EJ, et al. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 34.Kinter AL, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 35.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avery DT, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 40.Hodgkin PD, Rush J, Gett AV, Bartell G, Hasbold J. The logic of intercellular communication in the immune system. Immunol Cell Biol. 1998;76:448–453. doi: 10.1046/j.1440-1711.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 41.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin T, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 45.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 46.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J Exp Med. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 49.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. Journal of Experimental Medicine. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.