Abstract
In the last few years, extensive research has been made to elucidate the functional significance of RLIP76. The resulting novel breakthroughs have helped us understand its transport and signaling functions. RLIP76 is a ubiquitously expressed, key stress-defensive, anti-apoptotic, multi-functional protein that transports glutathione-conjugates of electrophilic compounds, thus controlling the intracellular concentration of pro-apoptotic oxidized lipid byproducts and other xenobiotics such as chemotherapeutic agents. These properties place RLIP76 at a very important position in the hierarchy of the stress defense mechanism adopted by the cell. Selective over-expression of RLIP76 in malignant cells of diverse origin is one of the possible mechanisms by which these cells overcome chemotherapy and radiation induced oxidative damage. RLIP76 has also been shown to be an effective transporter of many conventional chemotherapeutic drugs. Such transport, if inhibited, can lead to increased cellular accumulation of drugs which in turn translates to enhanced drug sensitivity. Recent studies have shown that inhibition and/or depletion of RLIP76 by antibodies, siRNA, or antisense can lead to drastic and sustained regression of lung, kidney, melanoma, colon, and prostate cancer xenografts with no observed recurrence of tumors. All these findings converge on the fact that such inhibition/depletion of RLIP76 can be used clinically to terminate cancer growth and progression. In the present review, we will discuss the role of RLIP76 as a multi-drug transporter, its involvement in cancer, and the prospects of using RLIP76 inhibition as an emerging treatment for cancer.
Keywords: RLIP76, cancer, drug-resistance, radiation-resistance, xenografts, chemotherapy
1. Introduction
Current cancer therapy regimens, though effective to some extent, have certain toxic effects on normal cells which manifest clinically as side effects, limiting the escalation of doses for effective control of cancer growth. This limitation of anti-neoplastic therapy of chemical injury to normal cells has encouraged researchers to figure out a different strategy in combating cancer. Many cellular proteins, including receptors, enzymes, etc., have been identified as potential targets for chemotherapy with limited success. Any protein selectively over expressed in cancer cells, and inhibition of which, can reduce the tumor growth is the solution to this problem.
RLIP76, also known as RalBP1, is a ubiquitously expressed protein in the human body and was originally identified to show dinitrophenyl-S-glutathione conjugate-dependent ATPase (DNP-SG ATPase) activity [1,2]. It has been shown to transport lipid peroxidation (LPO) end products and chemotherapeutic drugs with a broader allocrite spectrum compared to other known membrane transporters [1]. Numerous factors contribute to the physiological significance of RLIP76 which include regulation of the concentrations of intracellular LPO end product, 4-hydroxy-t-nonenal (4-HNE), transportation of leukotrienes [3], regulation of signaling processes, and contribution in mitotic spindle formation [4]. Our studies have also shown the stress responsive, anti-apoptotic activity of RLIP76 [5]. Recent studies with RLIP76 knockout (RLIP76−/−) mice showed that radiation injury in these animals got completely reverted back with liposomal delivery of purified RLIP76 into the tissues [6,7]. On the other hand, such liposomal delivery proved to be protective if given to wild-type (RLIP76+/+) animals before radiation exposure, revealing that such delivery could prevent injury during radio-therapy sessions.
Taking into consideration the selective over-expression of RLIP76 in cancer cells which translates to proportional amount of drug-efflux, the signaling function of RLIP76 and the dependence of cancer cells on this protein to overcome chemo/radio-therapy induced oxidant injury makes it one of the key molecules involved in cancer cell survival, proliferation, and metastasis. This emphasizes the importance of RLIP76 as a potential chemotherapeutic agent. The consequences of RLIP76 inhibition/silencing have also been elaborated with contexts to different experimental in-vitro and in-vivo models. Regression of different cancer cell xenografts in mice after inhibiting RLIP76 with antibody, or by silencing with siRNA, or antisense, proves that it is one of the survival mechanisms of cancer cells [8–11]. Here, we briefly discuss the signaling functions of RLIP76 and the mechanisms by which it functions as a signaling regulator and stress responsive protein. In the present review, we focus on the functions of RLIP76 as an endo- and xenobiotic transporter and present evidence from various studies performed in our lab to demonstrate the potential of RLIP76 to become the next generation chemotherapeutic agent.
2. Salient features of RLIP76 and its involvement in various cellular processes
RLIP76, a GTPase-activating protein, was cloned as a Ral effector protein linking Ral GTPase to Rho pathway [12–14]. Numerous functions have been attributed to RLIP76, which will be elaborated in detail in the following sections (Table 1).
Table 1.
Breakthroughs in characterizing the functional significance of RLIP76
| Findings/properties of RLIP76 | References |
|---|---|
| Demonstration of GTPase activity of RLIP76 | Jullien-Flores V et al [12] |
| Cloning, purification and reconstitution of RLIP76 in artificial liposomes | Awasthi S et al [13,14] |
| RLIP76 transports GS-Es and DOX in an ATP-dependent manner | Awasthi S et al [13,14] |
| Identification of ATP binding sequences | Awasthi S et al [14] |
| RLIP76 as a GS-Es and DOX transporter in red blood cells | Sharma R et al [39] |
| Induction of RLIP76 and hGST5.8 as an early stress response | Cheng JZ et al [5] |
| RLIP76 mediated transport of GS-HNE and DNP-SG | Singhal SS et al [51] |
| RLIP76 represents the major DOX transporter in lung cancer | Awasthi S et al [35] |
| RLIP76, an effector of the Ral GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis | Rosse C et al [27] |
| RLIP76 transports leukotriene C4 in cancer cells | Sharma R et al [3] |
| Identification of membrane anchoring domains of RLIP76 | Yadav S et al [45] |
| Transport of vinorelbine and role of drug-resistance in NSCLC | Stuckler D et al [32] |
| POB-1 over-expression inhibits transport function of RLIP76 | Yadav S et al [29] |
| Regression of B16 mouse melanoma model by RLIP76 depletion | Singhal SS et al [8] |
| RLIP76 mediates adhesion dependent Rac activation and cell migration | Goldfinger LE et al [44] |
| RLIP76 is required for mitogenic and drug-resistance mediated effects of PKC α | Singhal SS et al [41] |
| Regression of lung and colon cancer xenografts upon RLIP76 depletion | Singhal SS et al [9] |
| Mass spectroscopic phospho-protein mapping of RLIP76 | Herlevsen MC et al [52] |
| Role of antibodies to C-terminal of RLIP76 in immune mediated vascular diseases and atherosclerosis | Margutti P et al [53] |
| Polymorphisms of RLIP76 causes differential drug responses in Epilepsy | Leschziner GD et al [54] |
| Protective effect of RLIP76 against radiation poisoning | Singhal J et al [7] |
| RLIP76 depletion causes regression of prostate cancer xenografts | Singhal SS et al [11] |
| Inhibition of transport function of RLIP76 by Cdc2 | Singhal SS et al [28] |
| Role of RLIP76 in kidney cancer therapy | Singhal SS et al [10,34] |
| RLIP76 transports sunitinib and sorafenib | Singhal SS et al [34] |
3. RLIP76: Non-conventional transporter
RLIP76 is a non-ABC transporter with no apparent transmembrane or classical walker domain [15–18]. This unconventional nature of the protein helps in its trans-cellular movement during various cellular signaling processes and membrane up-regulation during stressful environments. The unique structural features of RLIP76 might help in the wide spectrum of its functions, ranging from: mitosis signaling, clathrin-coated-pit mediated receptor-ligand endocytosis of epidermal growth factor receptor (EGFR), insulin receptor (IR), transforming growth factor-β (TGF- β), and also as a modulator of stress responsive proteins within the cell [4,15–20].
4. Identity with DNP-SG ATPase
RLIP76 was first cloned as a Ral-binding GTPase activating protein (GAP), a Ral-effector through yeast two-hybrid screen [12]. The nucleotide sequence of DNP-SG ATPase was not known for years because of the difficulty in its purification and degradation of the intact protein except for the consistently appearing 38 kDa peptide. Our lab has cloned RLIP76 independently while searching for transporters of glutathione-electrophile conjugates (GS-Es) and drugs from human bone marrow cDNA library using polyclonal antibodies raised against this peptide [21–23]. Bacterially expressed RLIP76 exhibits similar transport and ATPase activity as that of DNP-SG ATPase, and the protein purified from DNP-SG affinity chromatography has amino acid sequence within 96 % of that expected for RLIP76. This authenticates its similarity with DNP-SG ATPase. Several researchers also have observed the aberrant behavior of RLIP76 in SDS-PAGE which migrates as a major band between molecular weights of 95 to 110 kDa, which can be attributed to the alternative splicing of the protein [12, 24,25]. The bands higher than the predicted ones are the aggregated peptides and those seen in 38 kDa range are the products of proteolytic digestion of the parent protein, most of which include the C-terminal (RLIP76410–655) and N-terminal (RLIP761–367) of the protein [13,14]. All of the above mentioned bands of RLIP76 were recognized by antibodies raised against DNP-SG ATPase in Western blot analyses indicating its structural identity with DNP-SG ATPase.
5. Transport function of RLIP76
RLIP76 is an ATP-dependent non-ABC transporter which actively transports structurally divergent compounds. Ever since the DNP-SG ATPase activity of RLIP76 has been discovered, many studies have shown the importance of transport function of RLIP76 especially of chemotherapeutic agents and anti-epileptic drugs [31], in addition to electrophilic conjugates [13]. Its substrates range from weakly cationic compounds including: doxorubicin (DOX), vinblastine (VBL), vincristine (VCR), vinorelbine (VRL) [1,13,32], colchicine, sunitinib and sorafenib [33–35], to anionic metabolites like glutathione-conjugates of electrophiles [13,15]. The wide substrate specificity, the ubiquitous presence of this transporter in different tissues, especially its over-expression specifically in cancer tissues makes RLIP76 an important regulator of endo- and xenobiotic metabolite end-products.
One of the distinguishing features of RLIP76 in relation to its transport function is that intact RLIP76 is not necessary for its transport function. N-terminal RLIP761–367 and the C-terminal RLIP76410–655, when purified separately and reconstituted into artificial liposomes, exhibited similar transport activity which illustrates that peptides obtained from proteolysis could reconstitute the full transport function. Though both the peptides (N- and C-terminal) showed ATPase activity, N-terminal having greater activity than the C-terminal, neither of them could show the transport function independently [14]. The ATPase activity of the peptides increased 2-fold in the presence of their substrates. This transport activity is temperature dependent and is sensitive to osmolarity with 1:1 stoichiometry between ATP hydrolysis and transport. Absence of the transport function when ATP was replaced by non-hydrolyzable analog, methylene ATP suggests that the transport is ATP-dependent. Further evidence that RLIP76 is a GS-E/drug transporter has been authenticated by studies performed in RLIP76−/− mice which showed 2- to 7-fold increased accumulation of lipid hydroperoxides, aldehydes, and alkenals in tissues as a consequence of RLIP76 loss [6,7,15]. This was accompanied by >80% loss of total transport activity for GS-E as well as anthracycline; this loss translates into greater sensitivity to xenobiotic toxins including traditional chemotherapeutic agents, which are substrates of RLIP76, as well as other alkylating agents and platinum-coordinates that are metabolized to GS-E. Membrane vesicles prepared from tissues obtained from RLIP76−/− mice showed a loss of ~80% of the transport activity. This activity was completely restored after liposomal delivery of purified RLIP76 into these vesicles [6,7,36].
6 Signaling and Interaction with other molecules
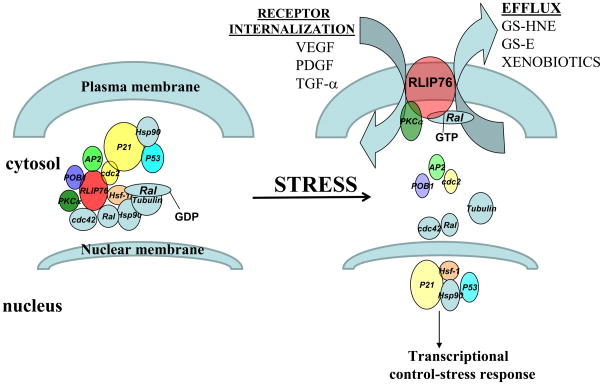
Besides acting as a membrane transporter, RLIP76 also acts as a regulator of various stress responsive proteins. In the normal physiological conditions, RLIP76 is found in a complex with heat shock factor-1 (Hsf-1), tubulin, HSP90 and Ral [19,20]. HSP90 is released from this complex in response to chemical and/or radiation injury-both of which result in oxidative stress and Hsf-1 migrates into the nucleus leading to transcriptional up-regulation of numerous heat shock proteins. It has also been shown that the Ral signal transduction pathway is highly activated by heat shock [19]. Activation of the Ral signaling pathway is associated with conversion of Ral-GDP to Ral-GTP and, because of the high affinity of Ral-GTP for RLIP76, the RLIP76-Hsf1-HSP90-tubulin hetero-complexes can potentially dissociate, leading to release of Hsf-1 and allowing it to translocate into the nucleus (Figure 1). Thus, RLIP76 binding to Hsf-1 serves to inhibit Hsf-1 from increasing heat-shock protein transcription. Elevated amounts (~ 2–5 folds by real time Q-PCR) of heat shock proteins found in the tissues of RLIP76−/− mice confirm this fact [26].
Figure 1.
Model for stress mediated signaling by RLIP76
It is already known that Cell cycle regulatory protein CDK1 (Cdc2) binds to RLIP76 at aa 481–625 and this binding is necessary for the termination of mitosis [27]. There is also an inhibitory effect of Cdc2 on the transport functions of RLIP76. This inhibition is attributed to the fact that after binding to Cdc2 (a complex of RLIP76, Cdc2 and cyclin B), RLIP76 dissociates from the membrane to function as a motor for spindle movement at the mitotic spindle [27,28]. On the other hand, POB1 (partner of Ralbp1), an endocytosis linked protein, binds to Ralbp1 at a similar location as Hsf-1 and is a saturable inhibitor of GS-Es and DOX transport activity. In a series of experiments conducted we were able to demonstrate that Hsf-1 and POB1 inhibited the transport function of RLIP76 [20,29]. Complex formations between RLIP76-POB1 [29,30], and RLIP76-Hsf1 [19,20] have already been demonstrated. We were able to show the trimeric complex formation of RLIP76, Hsf-1 and POB1 by using protein cross-linking and immuno-precipitation [20]. Thus, RLIP76 functions as a regulator for cell response to oxidant injury.
7 Protection against heat shock and oxidative stress
Induction of RLIP76 was an early response to heat shock or oxidant injury even before the appearance of previously known heat shock proteins or antioxidant enzyme [5]. When K562 cells were exposed to mild heat shock (42 °C, 30 min) or oxidative stress (50 μM H2O2, 20 min) and allowed to recover for 2 h, there was an increased level of 4-HNE, a several fold induction of hGST 5.8 (which catalyzes the formation of GS-HNE), and induction of RLIP76, which mediates the efflux of GS-HNE from cells. This was further demonstrated by the fact that these cells transported the end-product, GS-HNE at a 3-fold higher rate. This increased efflux of GS-HNE was blocked by coating the cells with antibodies against RLIP76, confirming that GS-HNE is transported by RLIP76. The stress-preconditioned cells with induced hGST 5.8 and RLIP76 acquired resistance to 4-HNE and H2O2 mediated apoptosis by suppressing sustained activation of JNK and caspase-3. The protective effect of stress preconditioning against H2O2 or 4-HNE induced apoptosis was abrogated by coating the cells with anti-RLIP76 IgG, which inhibited the efflux of GS-HNE. These results showed that the cells acquired resistance to apoptosis by metabolizing and excluding 4-HNE at a higher rate and suggested that the intracellular concentrations of GS-HNE and endogenous GS-E modulated stress-mediated signaling [5].
8 Protection in radiation toxicity
Radiation injury increases lipid peroxidation end-products and as RLIP76 is the major transporter of GS-Es, it can be considered as the key defense mechanism against such injury. Studies have shown toxic effects of inhibition of RLIP76 during radio-therapy in cancer cells and protective effects of RLIP76 over-expression from radiation poisoning in normal cells. This property of RLIP76 can also be used as sensitizer for radio-therapy as part of cancer therapy. RLIP76−/− mice are sensitive to X-irradiation and this sensitivity has been reversed by liposomal delivery of purified RLIP76 [6,7]. These findings suggest the protective role of RLIP76 against radiation toxicity. On the other hand, liposomal delivery of RLIP76 has shown protection against radiation injury even in wild-type and knockout mice, which is directly related to the amount of RLIP76 present in the cell. Similar types of responses have been observed in mouse embryonic fibroblasts (MEFs) derived from these mice. RLIP76 inhibition can be used to augment the sensitivity of radio-therapy which is given to cancer patients. On the other hand, liposomal delivery prior to radio-therapy can drastically reduce the radiation injury in normal cells [7,11,15].
9. Distribution of RLIP76 in normal and malignant cell
Protection from stress-mediated apoptosis is essential for cancer cells, which often have to survive under very adverse conditions and these cells acquire this evasive phenomenon by various mechanisms, especially by reducing/modulating intracellular free radical concentrations, removing check point proteins such as p53, or by over-expressing transporter proteins. RLIP76 expression is found to be increased in a number of malignant cells by gene expression array studies. Determination of RLIP76 expression was done at both transcript and protein levels. RLIP76 protein expression in normal and malignant cells was measured by three assays-Western blots, tissue extract ELISA, and immuno-histochemistry [8,9,11,37–39]. It has been shown that normal human tissues express RLIP76 transcripts, but expression varies considerably with 10- to 12-fold higher levels in ovary and skeletal muscle; 3- to 7-fold higher in thymus, prostate, testis, small intestine, heart, brain, placenta, and kidney; and lower levels in peripheral blood leukocytes, colon, pancreas, spleen, liver, and lung [24]. RLIP76 protein is expressed in all normal tissues; erythrocytes, breast, heart, and liver tissues had greater expression relative to colon and brain tissues as determined by immuno-histochemistry. This difference was even noted in-vitro in the normal and cancer cell lines. Melanoma, ovary, prostate, and lung cancers had higher RLIP76 levels than breast or liver cancer or normal immortalized cultured cells [8–11]. Functional assays of DOX transport were performed on inside-out-vesicles prepared from erythrocytes, cultured immortalized normal cells, and cancer cell lines to find a correlation between RLIP76 expression and its transport activity [8,39]. In most of the tissues, DOX transport rates matched RLIP76 protein content. This was not the case for lung cancer cells and normal erythrocytes. Increased transport rate in non small cell lung carcinoma (NSCLC) relative to small cell lung carcinoma (SCLC) cell lines occurred even in the absence of different RLIP76 protein concentrations and was found to be due to T297 phosphorylation by protein kinase C specifically in NSCLC [35,38,40–42]. Increased transport in the case of erythrocytes may be due to the higher density of membrane protein on the smaller, anucleate cells relative to other normal cells. Because RLIP76 transport provides resistance to multiple chemotherapy agents and radio-therapy, examination of tissue specific RLIP76 content and state of T297 phosphorylation may be useful biomarkers for, and help in prediction of clinical outcomes.
10. Anti-neoplastic effect of RLIP76 in-vitro
After establishing the role of RLIP76 as a versatile transporter and its physiological significance as a key survival protein of the cell, we determined the anti-neoplastic activity of RLIP76 by depletion or blocking its function. We used polyclonal rabbit-anti-human RLIP76 IgG to inhibit RLIP76 mediated transport activity, and siRNA and antisense, to block its cellular function. The cross reactivities of anti-RLIP76 IgG were checked by Ouchterlony double immuno-diffusion assay against Pgp or MRP and found to be specific [15,38]. The specificity of anti-RLIP76 IgG was further checked in RLIP76+/+ and RLIP76−/− mice. In wild-type mouse tissues homogenate, anti-RLIP76 IgG recognized only two peptides of 95 and 38 kDa where as no peptides were recognized in similar homogenate prepared from RLIP76−/− mouse tissues. The striking lack of any recognized band in the RLIP76−/− could not be overcome by increasing protein loaded per well from 150 to 400 μg [6]. RLIP76 siRNA and antisense were designed against the nucleotide nt508–528 starting from the N-terminal region of RLIP76 as the target. Selected DNA sequence was subjected to blast-search (NCBI database) against EST libraries, to ensure that only the selected gene was targeted.
The cytotoxic effects of RLIP76 inhibition by anti-RLIP76 IgG or the depletion of RLIP76 by either siRNA or antisense phosphorothioate were assessed in a panel of malignant cell lines including: human lung, colon, ovary, prostate, melanoma and B16 mouse melanoma, and normal cell lines like Human lung bronchio-epithelial cells (HLBEC), Human umbilical vein endothelial cell (HUVEC) and Human aortic vascular smooth muscle cells (HAVSMC) [8]. The malignant cells were significantly more susceptible than non malignant cells to the cytotoxicity of all three modalities to inhibit RLIP76 function. The non malignant cells showed almost no cytotoxicity after treatment; where as malignant cells on same concentration showed significant cytotoxicity ranging from 38% to 66% [8–11]. In contrast to the results observed in other malignant cells (in which all three modalities gave similar results) antisense was significantly more effective in killing prostate cancer cells than the antibody [11].
11. Anti-neoplastic effect of RLIP76 in- vivo
The ultimate pre-clinical test for the potential utility of any anti-neoplastic agent is its effectiveness in animal model. Encouraged by results from in-vitro studies, we subsequently tested the ability of all three targeting agents (anti-RLIP76 IgG, RLIP76 siRNA and RLIP76 antisense) to cause regression of xenografts of diverse human origin including lung (NSCLC H358 and H520) [9], colon (SW480) [9], prostate (PC3) [11], and kidney (Caki2) [10] (Table II).
Table II.
Effect of RLIP76 inhibition and/or depletion on tumor xenografts
| Cancer type | Estimated deaths in US in 2009 [55] | Cell types used for cancer Xenograft | RLIP76 siRNA | RLIP76 antisense | RLIP76 antibody |
|---|---|---|---|---|---|
| Melanoma [8] | 5,500 male 3,100 female |
B16 mouse melanoma | + | + | + |
| Lung cancer [9] | 88,900 male 70,000 female |
H358 NSCLC H520 NSCLC |
ND | + | + |
| Colon cancer [9] | 5,240 male 4,680 female |
SW480 | ND | + | + |
| Prostate cancer [11] | 27,360 male | PC-3 | + | + | + |
| Kidney cancer [10] | 8,160 male 4,820 female |
Caki-2 | + | + | + |
+, regression of xenografts observed; ND, not determined
The ability of RLIP76 antisense to deplete RLIP76 in mouse tissues was first examined in non tumor-bearing animals by injecting a single 200 μg dose of RLIP76 antisense, i.p. Animals were sacrificed at 1, 2, 4, and 14 days later and crude homogenate fractions of liver, kidney, lung, and brain were assayed for RLIP76 protein by ELISA and for total protein by Bradford assay. RLIP76 represented a remarkably constant 0.4 ± 0.05 percentage of total extractable protein in all four organs. Within 24 h of RLIP76 antisense treatment, RLIP76 protein was depleted to <0.01% of the total extractable protein and a slow partial recovery was seen within a 14-day period of these experiments. Immuno-histochemical studies for RLIP76 expression further confirmed ELISA findings. These results indicated that the antisense regimen used during the studies caused RLIP76 depletion in tissues [9].
In Athymic nude nu/nu mice, 2 ×106 cells were injected subcutaneously to develop xenografts. Treatment was administered when the tumor surface area exceeded ~ 42 mm2 (days 22 to 30 depending upon cell type). Treatment consisted of administration of 200 μg anti-RLIP76 IgG, siRNA or antisense on day 1 and day 15 by i. p. injection. Treated animals had rapid and dramatic reductions in tumors, whereas uncontrolled growth was observed in the control groups. The remarkable contrast in the outcome of tumors in animals treated with RLIP76 antibody, RLIP76 siRNA or RLIP76 antisense versus pre-immune serum, scrambled siRNA or antisense was clearly evident in these cancer cell lines. RLIP76 antibody, RLIP76 siRNA or RLIP76 antisense treated animals survived up to 10 months without evidence of tumor recurrence. Weight gain was comparable to non-tumor bearing controls, and no overt-toxicity was evident. To date, depletion of RLIP76 has successfully cured the xenografts of lung [9], colon [9], prostate [11], kidney [10] and melanoma [8] and has also shown partial regression of those of pancreas (BXPC3) and breast (MCF7) (unpublished observations).
12. Synergistic effects of RLIP76 and conventional chemotherapeutic agents
Current chemotherapeutic strategies use combination therapy to overcome cancer growth. This not only addresses the issue of drug resistance but also reduces chemotherapeutic toxicity because of the low doses of drugs used as compared to single drug regimens. With the central idea to inhibit the growth of cancer cells by inhibiting the transport protein, RLIP76 studies were conducted to look into the feasibility of using RLIP76 inhibition/silencing as a therapeutic strategy. In studies conducted both in in-vitro and in-vivo models, we also looked into the synergistic effects of RLIP76 depletion with other conventional chemotherapeutic drugs, especially those which are known substrates or allocrites of RLIP76.
Our initial in-vitro studies in lung cancer cells show that cells coated with anti-RLIP76 IgG accumulated significantly greater levels of DOX, thus leading to synergistically more cell death as compared to either DOX or anti-RLIP76 IgG alone. The synergy between anti-RLIP76 IgG and DOX (CI 0.36 ± 0.27) was greater than that between Herceptin and DOX (CI 0.75 ± 0.49) where as Herceptin and RLIP76 IgG showed only additive effect [43].
The cisplatin (CDDP)-VRL combination is highly active in lung cancer and has been shown to be synergistic. We observed from our recent studies that RLIP76 can mediate efflux of VRL [32] and predicted that the efficacy of the CDDP-VRL combination could be enhanced by depleting or inhibiting RLIP76. In-vitro studies demonstrated that CDDP-VRL-anti-RLIP76 IgG effect was supra-additive where as VRL-anti-RLIP76 IgG and CDDP-anti-RLIP76 IgG were additive and sub-additive respectively. The predictions from this in-vitro model were tested in a nude mouse xenograft model using NSCLC H358. The animals treated with anti-RLIP76 IgG responded much more quickly than those treated with CDDP, VRL, or CDDP-VRL. Addition of either CDDP or VRL individually to anti-RLIP76 IgG did not result in any increase in the efficacy of the antibody alone. However, treatment with combination of all three agents caused the most rapid remission of tumor in mice [9]. Our studies show that the combination therapy of RLIP76 and other chemotherapy drugs can overcome the drug-resistant mechanism developed by cancer cell lines thus making them more vulnerable to cytotoxicity.
13. Mechanism of tumor growth inhibition by depletion of RLIP76
The above studies establish that RLIP76 depletion causes regression of cancer xenografts but do not distinguish between two possible models of RLIP76 function: (i) the transport activity of RLIP76 is the key element necessary for its protective effects or (ii) it functions through signaling interactions with anti-apoptotic signaling proteins distinct from its transport activity [4,12,24,25,44]. Because RLIP76 antisense depletes virtually all cytosolic and membrane-associated RLIP76, the anti-neoplastic effect would be the same with either of the two models. We reasoned that an experimental approach aimed at specifically inhibiting transport activity of RLIP76 without affecting total cellular RLIP76 would be necessary to distinguish between the two models. We have previously shown that a cell surface epitope of RLIP76 (aa171–185) is recognized by anti-RLIP76 IgG as well as by antibodies generated against the specified peptide, and this recognition is abrogated on sole expression of mutant RLIP76 lacking this peptide [45]. Coating live cells with either antibody causes rapid increase in cellular content of transported substrates including DOX and GS-HNE, whereas total RLIP76 protein content remains unchanged. These findings predict that anti-RLIP76 IgG should function with at least equal anti-tumor efficacy as the antisense if the anti-apoptotic activity of RLIP76 is dependent only on its transport activity.
Since both anti-RLIP76 IgG and RLIP76 antisense DNA/siRNA produce similar effects in most of the cancer cells, our findings support the assertion that the anti-apoptotic and stress-protective effects of RLIP76 are primarily related to its transport activity.RLIP76 is present in cytosol, where it performs its cell regulatory function and can also translocate to the membrane to function as a transporter. Functions attributed to the intracellular RLIP76 (e.g., its signaling role as a Ral or Ras-R GTPase-activating protein) are least likely to be affected by anti-RLIP76 IgG that cannot enter the cells and specifically interact with epitopes on membrane-anchored RLIP76 to inhibit its transport function. The lack of the presence of RLIP76 within cells also contributes to the observed suppression of tumors due to the cessation of some or all of its known cellular functions (e.g., involvement in Ral or Ras-R signaling, endocytosis and mitosis, spindle motor functions, and the regulation of the expression of heat shock proteins), one would expect a more tumor-suppressive activity associated with antisense as compared with anti-RLIP76 IgG [9]. So far we have found that RLIP76 antisense is more effective than anti-RLIP76 IgG only in prostate cancer cells [11]. This difference could be related to factors in prostate cancers that reduce the effectiveness of antibody, or perhaps due to relatively greater role of some intracellular function of RLIP76 in prostate cancers. These findings show that RLIP76 is a unique anticancer target that is a versatile and efficient multi-drug transporter and an anti-apoptotic protein essential for the survival of cancer cells. Through its ability to efficiently transport the GSH-conjugates of endogenous pro-apoptotic molecules (e.g., 4-HNE), as well as chemotherapeutic drugs, RLIP76 provides survival advantage to cancer cells. Future studies in other cell types will expand our knowledge about the mechanisms of tumor growth inhibition by depleting/blocking RLIP76.
14. RLIP76 and drug-resistance
Multi-drug resistance (MDR), a phenotype exhibited by many cancers to develop resistance to the cytotoxic effects of myriad of structurally divergent cytotoxic agents, remains a major obstacle to the eradication of malignancies using drug therapy. Numerous cellular biochemical mechanisms have been identified as contributors to the development of MDR phenotype, though the prototypical MDR is most frequently associated with a decrease in cellular accumulation of drugs due to an active energy-dependent efflux of drugs or metabolites. Accumulation defective MDR is mediated by a diverse array of transporter proteins like MRP and Pgp [46–48]. Because drug-efflux mechanisms mediate drug accumulation defects which translates to resistance against the cytotoxic effects of drugs [49], the function of RLIP76 being a multi-drug transporter would predict that over-expression of this protein should cause resistance to the drugs which are its allocrites. We have reported detailed studies regarding role of RLIP76 in mediating resistance to multiple chemotherapeutic drugs including: CDDP, melphalan (MEL), DOX, daunorubicin (DAU), VCR, VBL, VRL, sunitinib and sorafenib [1,13,32–35,50]. Our studies show that RLIP76 mediated drug resistance against above mentioned drugs is not limited to one cell line but involves cells from different origins like leukemia, lung, prostate and kidney cancer.
Over-expression of RLIP76 in human myelogenous leukemia K562 shows that it can confer resistance up to 2.5 fold against anthracycline (DOX, DAU), vinca alkaloids (VCR, VBL, and VRL), alkylating agents (MEL, mitomycin C) and platinum coordinates (CDDP) [13,50,51]. The higher resistance to DOX in NSCLC as compared with the SCLC cells correlates with a higher RLIP76-mediated efflux of DOX in NSCLC. Coating with RLIP76 antibodies sensitizes NSCLC to DOX by blocking their RLIP76-mediated transport [43]. Our recent studies show that the Caki-2, 786-O and A-498 renal carcinoma cell lines over-express RLIP76 protein as compared to mesangial cells. Due to greater expression of RLIP76 in renal carcinoma cells, there is 2.5 to 5.5 fold increased transport of sunitinib and sorafenib as compared to mesangial cells. We also observed that sunitinib, a generally more effective kinase inhibitor and more active clinically, is a poor substrate for transport by RLIP76 as compared to sorafenib [34]. Taken together, these findings indicate that RLIP76, besides being predominant GS-E transporter in normal cells, functions as a key protein in cancer cells and its inhibition leads to drug sensitivity of these cells.
15. Significance and Concluding Remarks
RLIP76, a multi-drug transporter and an anti-apoptotic protein, is one of the key molecules at which both the chemotherapeutic resistance and cancer cell signaling converge. This offers the evidence to recognize the potential of using RLIP76 as a unique anti-cancer target. This conclusion is also supported by the fact that there is inherent over-expression of RLIP76 in cancer cells and a relatively less dependence of normal cells on RLIP76 for survival. This would result in less toxicity to the normal cells when RLIP76 is inhibited. Triggering the apoptotic pathways by inhibiting RLIP76, the result observed consistently across many of the cancer cell lines and xenografts provides sufficient evidence to use RLIP76 clinically as a novel therapeutic agent. Further studies are required to elaborate full structural elucidation of RLIP76 to develop small molecule inhibitors against this transporter to block its function and use as a chemotherapeutic drug. A feasible method to detect cancer development even before the onset of clinical symptomatology will provide an added advantage to combat cancer.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA 77495 and CA 104661, Cancer Research Foundation of North Texas, Institute for Cancer Research and the Joe & Jessie Crump Fund for Medical Education.
The abbreviations used are
- RLIP76
Ral-interacting protein
- GSH
glutathione
- GS-E
glutathione-electrophile conjugate
- DNP-SG
dinitrophenyl S-glutathione
- DOX
doxorubicin
- 4-HNE
4-hydroxy-t-nonenal
- POB1
partner of RLIP76
- Cdc2
Cell cycle regulatory protein CDK1
- HLBEC
Human lung bronchio-epithelial cells
- HUVEC
Human umbilical vein endothelial cell
- HAVSMC
Human aortic vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Awasthi S, Singhal SS, Srivastava SK, Zimniak P, Bajpai KK, Saxena M, et al. Adenosine-triphosphate-dependent transport of doxorubicin, daunomyicn, and vinblastine in human tissues by a mechanism distinct from the P-glycoprotein. J Clin Invest. 1994;93:958–65. doi: 10.1172/JCI117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBelle EF, Singh SV, Srivastava SK, Awasthi YC. Dinitrophenyl glutathione efflux from human erythrocytes is primary active ATP-dependent transport. Biochem J. 1986;238:443–9. doi: 10.1042/bj2380443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma R, Singhal SS, Wickramarachchi D, Awasthi YC, Awasthi S. RLIP76-mediated transport of leukotriene C4 in cancer cells: implications in drug-resistance. Int J Cancer. 2004;112:934–42. doi: 10.1002/ijc.20516. [DOI] [PubMed] [Google Scholar]
- 4.Quaroni A, Paul EC. Cytocentrin is a Ral-binding protein involved in the assembly and function of the mitotic-apparatus. J Cell Sci. 1999;112:707–18. doi: 10.1242/jcs.112.5.707. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, et al. Accelerated metabolism and exclusion of 4-hydroxy-nonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative-stress. J Biol Chem. 2001;276:41213–23. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, et al. RLIP76 is a major determinant of radiation-sensitivity. Cancer Res. 2005;65:6022–8. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 7.Singhal J, Singhal SS, Yadav S, Warnke M, Yacoub A, Dent P, et al. RLIP76 in defense of radiation and chemical-poisoning. Int J Rad Oncol Biol Phys. 2008;72:553–61. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76-depletion. Cancer Res. 2006;66:2354–60. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 9.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor P, Awasthi YC, et al. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76. Cancer Res. 2007;67:4382–9. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 10.Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, Awasthi S. RLIP76: A target for kidney cancer therapy. Cancer Res. 2009;69:4244–51. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal SS, Roth C, Leake K, Singhal J, Yadav S, Awasthi S. Regression of prostate cancer xenografts by RLIP76 depletion. Biochem Pharmacol. 2009;77:1074–83. doi: 10.1016/j.bcp.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, et al. Bridging Ral-GTPase to Rho-pathways. RLIP76, a Ral-effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem. 1995;270:22473–7. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 13.Awasthi S, Cheng J, Singhal SS, Saini MK, Pandya U, Pikula S, et al. Novel function of human RLIP76: ATP-dependent transport of glutathione-conjugates and doxorubicin. Biochemistry. 2000;39:9327–34. doi: 10.1021/bi992964c. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi S, Cheng J, Singhal SS, Sharma R, Pandya U, Singh SV, et al. Functional reassembly of xenobiotic-transport from the N-terminal and C-terminal domains of RLIP76 and identification of ATP-binding sequences. Biochemistry. 2001;40:4159–68. doi: 10.1021/bi002182f. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione- conjugates and chemotherapeutic drugs by RLIP76: a novel link between G-protein and tyrosine-kinase signaling and drug-resistance. Int J Cancer. 2003;106:635–46. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi YC, Sharma R, Yadav S, Dwivedi S, Sharma A, Awasthi S. The non-ABC drug-transporter RLIP76 plays a major role in the mechanisms of drug-resistance. Curr Drug Metab. 2007;8:315–23. doi: 10.2174/138920007780655414. [DOI] [PubMed] [Google Scholar]
- 17.Yadav S, Zajac E, Singhal SS, Awasthi S. Linking stress-signaling, glutathione-metabolism, signaling pathways and xenobiotic-transporters. Cancer Metas Rev. 2007;26:59–69. doi: 10.1007/s10555-007-9043-5. [DOI] [PubMed] [Google Scholar]
- 18.Awasthi S, Sharma R, Singhal SS, Zimniak P, Awasthi YC. RLIP76, a novel transporter catalyzing ATP-dependent efflux of xenobiotics. Drug Metab Dispos. 2002;30:1300–10. doi: 10.1124/dmd.30.12.1300. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Mivechi NF. HSF-1 interacts with Ral-binding-protein 1 in a stress-responsive, multi-protein complex with HSP90 in vivo. J Biol Chem. 2003;278:17299–306. doi: 10.1074/jbc.M300788200. [DOI] [PubMed] [Google Scholar]
- 20.Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug- sensitivity and apoptosis by inhibiting Ralbp1. J Biol Chem. 2008;283:19714–29. doi: 10.1074/jbc.M708703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma R, Gupta S, Singh SV, Medh RD, Ahmad H, LaBelle EF, et al. Purification and characterization of dinitrophenylglutathione ATPase of human erythrocytes and its expression in other tissues. Biochem Biophys Res Commun. 1990;171:155–61. doi: 10.1016/0006-291x(90)91370-8. [DOI] [PubMed] [Google Scholar]
- 22.Awasthi S, Singhal SS, Srivastava SK, Torman RT, Zimniak P, Pikula J, et al. ATP dependent human erythrocyte glutathione-conjugate-transporter. I. Purification, photoaffinity-labeling, and kinetic characteristics of ATPase-activity. Biochemistry. 1998;37:5231–8. doi: 10.1021/bi972130z. [DOI] [PubMed] [Google Scholar]
- 23.Awasthi S, Singhal SS, Pikula S, Piper JT, Srivastava SK, Torman RT, et al. ATP- dependent human erythrocyte glutathione-conjugate-transporter. II. Functional reconstitution of transport-activity. Biochemistry. 1998;37:5239–48. doi: 10.1021/bi972131r. [DOI] [PubMed] [Google Scholar]
- 24.Cantor SB, Urano T, Feig LA. Identification and characterization of Ral-binding-protein1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–84. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SH, Weinberg RA. A putative effector of Ral has homology to Rho/Rac GTPase-activating proteins. Oncogene. 1995;11:2349–55. [PubMed] [Google Scholar]
- 26.Singhal SS, Awasthi S. Toxicology of glutathione S-transferases. Boca Raton, FL: CRC Press; 2006. Glutathione-conjugate transport and stress-response signaling: role of RLIP76; pp. 231–56. [Google Scholar]
- 27.Rosse C, L’Hoste S, Offner N, Picard A, Camonis JH. RLIP, an effector of the Ral-GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. J Biol Chem. 2003;278:30597–604. doi: 10.1074/jbc.M302191200. [DOI] [PubMed] [Google Scholar]
- 28.Singhal SS, Yadav S, Vatsyayan R, Chaudhary P, Borvak J, Singhal J, et al. Increased expression of cdc2 inhibits transport function of RLIP76 and promotes apoptosis. Cancer Lett. 2009;283:152–8. doi: 10.1016/j.canlet.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav S, Zajac E, Singhal SS, Singhal J, Drake K, Awasthi YC, et al. POB1 over-expression inhibits RLIP76-mediated transport of glutathione-conjugates, drugs and promotes apoptosis. Biochem Biophys Res Commun. 2005;328:1003–9. doi: 10.1016/j.bbrc.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 30.Oosterhoff JK, Penninkhof F, Brinkmann AO, Grootegoed JA, Blok LG. POB1 is down-regulated during human prostate cancer progression and inhibits growth factor signaling in prostate cancer cells. Oncogene. 2003;22:2920–5. doi: 10.1038/sj.onc.1206397. [DOI] [PubMed] [Google Scholar]
- 31.Awasthi S, Hallene KL, Fazio V, Singhal SS, Cucullo L, Awasthi YC, et al. RLIP76, a non-ABC transporter, and drug resistance in epilepsy. BMC Neurosci. 2005;6:61. doi: 10.1186/1471-2202-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuckler D, Singhal J, Singhal SS, Yadav S, Awasthi YC, Awasthi S. RLIP76 transports vinorelbine and mediates drug-resistance in non-small cell lung cancer. Cancer Res. 2005;65:991–8. [PubMed] [Google Scholar]
- 33.Awasthi S, Singhal SS, Pandya U, Gopal S, Zimniak P, Singh SV, et al. ATP-dependent colchicines transport by human erythrocyte glutathione-conjugate-transporter. Toxicol Appl Pharmacol. 1999;155:215–26. doi: 10.1006/taap.1998.8617. [DOI] [PubMed] [Google Scholar]
- 34.Singhal SS, Sehrawat A, Sahu M, Singhal P, Vatsyayan R, Lelsani PC, et al. RLIP76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int J Cancer. 2010 doi: 10.1002/ijc.24767. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awasthi S, Singhal SS, Singhal J, Cheng J, Zimniak P, Awasthi YC. Role of RLIP76 in lung cancer doxorubicin-resistance: II. Doxorubicin-transport in lung cancer by RLIP76. Int J Oncol. 2003;22:713–20. [PubMed] [Google Scholar]
- 36.Singhal SS, Yadav S, Singhal J, Sahu M, Sehrawat A, Awasthi S. Diminished drug transport and augmented radiation sensitivity caused by loss of RLIP76. FEBS Lett. 2008;582:3408–14. doi: 10.1016/j.febslet.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem Pharmacol. 2005;70:481–8. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Singhal SS, Singhal J, Sharma R, Singh SV, Zimniak P, Awasthi YC, et al. Role of RLIP76 in lung cancer doxorubicin-resistance: I The ATPase activity of RLIP76 correlates with doxorubicin and 4HNE-resistance in lung cancer cells. Int J Oncol. 2003;22:365–75. [PubMed] [Google Scholar]
- 39.Sharma R, Singhal SS, Cheng J, Yang Y, Sharma A, Zimniak P, et al. RLIP76 is the major ATP-dependent transporter of glutathione-conjugates and doxorubicin in human erythrocytes. Arch Biochem Biophys. 2001;391:171–9. doi: 10.1006/abbi.2001.2395. [DOI] [PubMed] [Google Scholar]
- 40.Singhal SS, Wickramarachchi D, Singhal J, Yadav S, Awasthi YC, Awasthi S. Determinants of differential doxorubicin-sensitivity between SCLC and NSCLC. FEBS Lett. 2006;580:2258–64. doi: 10.1016/j.febslet.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 41.Singhal SS, Yadav S, Singhal J, Awasthi YC, Awasthi S. Mitogenic and drug-resistance mediating effects of PKCα require RLIP76. Biochem Biophys Res Commun. 2006;348:722–7. doi: 10.1016/j.bbrc.2006.07.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singhal SS, Yadav S, Singhal J, Drake K, Awasthi YC, Awasthi S. The role of PKCα and RLIP76 in transport-mediated doxorubicin-resistance in lung cancer. FEBS Lett. 2005;579:4635–41. doi: 10.1016/j.febslet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Awasthi S, Singhal SS, Singhal J, Yang Y, Zimniak P, Awasthi YC. Role of RLIP76 in lung cancer doxorubicin-resistance: III. Anti-RLIP76-antibodies trigger apoptosis in lung cancer cells and synergistically increase doxorubicin-cytotoxicity. Int J Oncol. 2003;22:721–32. [PubMed] [Google Scholar]
- 44.Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac-activation and cell migration. J Cell Biol. 2006;174:877–88. doi: 10.1083/jcb.200603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav S, Singhal SS, Singhal J, Wickramarachchi D, Kuntson E, Albrecht TB, et al. Identification of membrane-anchoring domains of RLIP76 using deletion mutants analyses. Biochemistry. 2004;43:16243–53. doi: 10.1021/bi0482811. [DOI] [PubMed] [Google Scholar]
- 46.Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide-polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux-transporters. Int J Toxicol. 2006;25:231–59. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- 47.Higgins CF. Multiple molecular mechanisms for multidrug-resistance transporters. Nature. 2007;446:749–57. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 48.Sharom FJ. ABC multidrug-transporters: structure, function and role in chemo-resistance. Pharmacogenomics. 2008;9:105–27. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Paumi CM, Ledford BG, Smitherman PK, Townsend AJ, Morrow CS. Role of multi-drug resistance protein 1 (MRP1) and glutathione S-transferase A1–1 in alkylating agent resistance. Kinetics of glutathione conjugate formation and efflux govern differential cellular sensitivity to chlorambucil versus melphalan toxicity. J Biol Chem. 2001;276:7952–6. doi: 10.1074/jbc.M009400200. [DOI] [PubMed] [Google Scholar]
- 50.Drake KJ, Singhal J, Yadav S, Nadkar A, Singhal SS, Awasthi S. RALBP1/RLIP76 mediates multi-drug resistance. Int J Oncol. 2007;30:139–44. [PubMed] [Google Scholar]
- 51.Singhal SS, Sehrawat A, Metha A, Sahu M, Awasthi S. Functional reconstitution of RLIP76 catalyzing ATP-dependent transport of glutathione-conjugate. Int J Oncol. 2009;34:191–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Herlevsen MC, Theodorescu D. Mass spectroscopic phosphoprotein mapping of Ral-binding-protein1 (RalBP1/Rip1/RLIP76) Biochem Biophys Res Commun. 2007;362:56–62. doi: 10.1016/j.bbrc.2007.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margutti P, Matarrese P, Conti F, Colasanti T, Delunardo F, Capozzi A, et al. Autoantibodies to the C-terminal subunit of RLIP76 induce oxidative-stress and endothelial cell apoptosis in immune-mediated vascular diseases and atherosclerosis. Blood. 2008;111:4559–70. doi: 10.1182/blood-2007-05-092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leschziner GD, Jorgensen AL, Andrew T, Williamson PR, Marson AG, Coffey AJ, et al. The association between polymorphisms in RLIP76 and drug-response in epilepsy. Pharmacogenomics. 2007;8:1715–22. doi: 10.2217/14622416.8.12.1715. [DOI] [PubMed] [Google Scholar]
- 55.Cancer facts and figures. American Cancer Society; 2009. www.cancer.org/downloads/STT/500809web.pdf. [Google Scholar]