Abstract
Background
Invasive aspergillosis (IA) is an important cause of morbidity and mortality in hematopoietic stem cell (HSCT) and solid organ transplant (SOT) recipients. The purpose of this study was to evaluate factors associated with mortality in transplant patients with IA.
Methods
Transplant patients from 23 U.S. centers were enrolled from March 2001 to October 2005 as part of the Transplant Associated Infection Surveillance Network (TRANSNET). IA cases were identified prospectively in this cohort through March 2006, and data were collected. Factors associated with 12-week all-cause mortality were determined by logistic regression analysis and Cox proportional hazards regression.
Results
Six-hundred forty-two cases of proven or probable IA were evaluated, of which 317 (49.4%) died by the study endpoint. All-cause mortality was greater in HSCT (239/415, 57.5%) when compared to SOT patients (78/227, 34.4%; p<0.001). Independent poor prognostic factors in HSCT patients were neutropenia, renal insufficiency, hepatic insufficiency, early-onset IA, proven IA and methylprednisolone use. In contrast, white race was associated with decreased risk of death. Among SOT patients, hepatic insufficiency, malnutrition and CNS disease were poor prognostic indicators; whereas, prednisone use was associated with decreased risk of death. Among HSCT or SOT patients who received antifungal therapy, use of an amphotericin B preparation as part of initial therapy was associated with increased risk of death.
Conclusions
There are multiple variables associated with survival in transplant patients with IA. Understanding these prognostic factors may assist in the development of treatment algorithms and clinical trials.
Keywords: Aspergillosis, transplant, Aspergillus, mortality
Introduction
Invasive aspergillosis (IA) is an important cause of morbidity and mortality in hematopoietic stem cell (HSCT) and solid organ transplant (SOT) recipients. Despite improvements in the antifungal armamentarium and diagnostic modalities, mortality remains unacceptably high [1,2]. Because of the poor outcomes associated with IA in transplant patients, there is much interest in prevention and treatment measures. Identification of prognostic factors may help to determine which patients require risk modification and/or more aggressive treatment.
Risk factors for IA have been studied extensively in transplant patients, but there are few studies which specifically describe variables relate to outcomes [3-7]. These studies are difficult to perform for several reasons. First, studies are limited by small numbers of patients and the low prevalence of IA at individual institutions. Second, defining outcome endpoints (all-cause versus attributable mortality) and the appropriate timing of endpoint determination is controversial. Finally, to predict which variables may contribute to outcomes, given the myriad factors which likely influence response to therapy and mortality, predicting the most important variables is challenging . Several studies document the importance of underlying immunosuppression, co-morbid conditions, and disseminated infection as factors impacting survival [3-6]. Herein we analyzed the Transplant Associated Infection Surveillance Network (TRANSNET) database to determine factors associated with mortality among patients with IA
Methods
TRANSNET is a Center for Diseases Control and Prevention and industry co-sponsored prospective surveillance network comprised of 23 U.S. transplant centers with University of Alabama at Birmingham serving as the coordinating center [8]. Enrollment in to the cohort of transplant recipients occurred between March 2001 and September 2005. The period of surveillance for proven and probable invasive mycoses was March 2001, through March 2006. Patients with proven or probable invasive aspergillosis (IA) based on modified European Organization for Research and Treatment of Cancer/ Mycoses Study Group (EORTC/MSG) criteria were included [9]. Modifications included applying the Ascioglu criteria to solid organ transplant patients and allowing bronchoalveolar lavage galactomannan as a diagnostic modality. There was no specified CMV prophylaxis, fungal prophylaxis, or immunosuppressive regimen as part of the study. From 31,823 screened transplant patients, a total of 2199 mycoses were identified, including 652 IA cases. This study includes 642 IA cases for which 12-week outcome data were available.
Clinical Data and definitions
Patient data included demographics, transplant type, underlying diseases, site(s) of IA, and Aspergillus species. Data were also collected on other potential risk factors including neutropenia, graft rejection or graft-versus-host disease (GVHD), cytomegalovirus (CMV) disease, antifungal prophylaxis and treatment, and other co-morbidities. Early-onset IA was defined as diagnosis < 30 days post-transplantation. Neutropenia was defined as an absolute neutrophil count (ANC) <500/mm3 within 30-days prior to diagnosis of IA, representing pre- or post-engraftment neutropenia. CMV disease was defined as CMV detected in blood (antigen, PCR), or histopathologic evidence of CMV, in association with signs and symptoms consistent with infection [4,5]. GVHD was defined as > grade II. The following definitions of co-morbid conditions were applied at the time of diagnosis of IA: renal insufficiency as a creatinine ≥ 3.0 mg/dl or a creatinine clearance <30 mL/min [4]; corticosteroid use as any systemic use;. hepatic insufficiency as ascites, other clinical stigmata of liver disease, or abnormal laboratory values (prothrombin time, INR, liver enzyme tests);malnutrition as a serum albumin <2g/dl or ≥5% ideal body weight if albumin between 2.1-2.5g/dl. Disseminated IA was defined as extrapulmonary disease, excluding sinus disease. Mould-active antifungal prophylaxis was defined as receipt of any systemic mould-active antifungal agent in the 3 months prior to diagnosis of IA, excluding use for empiric or pre-emptive therapy. Antifungal treatment data included the specific agent and first date of administration. For the purposes of this study, primary combination therapy was defined as the administration of two anti-Aspergillus antifungalswithin 48 hours of initial therapy. The primary outcome endpoint was all-cause mortality at 12-weeks post-diagnosis of IA.
Statistical analysis
For analysis of the relationships of variables to survivors and non-survivors, univariate analyses were performed using the two-group chi-square test, or Fisher's exact test for categorical variables and the two-group t-test for continuous variables. Multivariable analyses for factors associated with mortality were performed using stepwise multiple logistic regression analysis. Models using mortality as the dependent variable were determined separately for HSCT and SOT patients. All variables significant at α=0.20 in univariate analyses were considered as possible predictor variables for multivariable analyses. The criterion for entry into the model was significance at α=0.20, while the criterion for remaining in the model was significance at α=0.05. Odds ratios and corresponding 95% confidence intervals were calculated. Model fit was assessed using the Hosmer-Lemeshow goodness-of-fit statistic and all models fit the data well. A multiple logistic regression model containing the best predictor variables obtained from the stepwise analysis was then run using all available data in order to obtain more robust estimates of the odds ratios, confidence intervals, and p-values. For HSCT patients, a potential interaction between methylprednisone use and GVHD was evaluated by incorporating an interaction term into the final model obtained through multiple logistic regression analysis. For SOT patients, a potential interaction between prednisone use and rejection was evaluated by incorporating an interaction term into the final model obtained through multiple logistic regression analysis.
A time-to-death analysis was performed. Univariate analyses for SOT or HSCT patients were performed using the Kaplan-Meier method and the log-rank test. Multivariable analyses for factors associated with time to death were performed using stepwise Cox regression analysis, separately for HSCT and SOT patients. All variables significant at α=0.20 in univariate analyses were considered as possible predictor variables for the multivariable analyses. The criterion for entry into the model was significance at α=0.20; the criterion for remaining in the model was significance at α=0.05. Hazard ratios and their corresponding 95% confidence intervals were calculated. A multivariable Cox proportional hazards model containing the best predictor variables obtained from the stepwise analysis was then run using all available data in order to obtain more robust estimates of the hazard ratios, confidence intervals, and p-values. For HSCT patients, a potential interaction between methylprednisone use and GVHD was evaluated by incorporating an interaction term into the final model obtained through multivariable Cox regression analysis. For SOT patients, a potential interaction between prednisone use and rejection was evaluated by incorporating an interaction term into the final model obtained through multivariable Cox regression analysis. All statistical tests were two-tailed and were performed using a 5% significance level ((α = 0.05). Statistical analyses were performed using SAS (version 9.1.3; SAS Institute, Inc., Cary, NC).
Results
Clinical characteristics (Tables 1, 2). Of 652 IA patients enrolled in TRANSNET during the study period, 642 were evaluated to determine factors associated with mortality. The 12-month cumulative incidence of IA was 1.6% in HSCT patients and 0.63% in SOT patients. The mean age was 49 years; 61% were male; 84% were Caucasian; 64.6% were HSCT recipients. Twelve week all-cause mortality was 49.4%, and greater among HSCT than SOT recipients (57.5% vs. 34.4%; p<0.001) (Figure 1). Aspergillus fumigatus was the most common species isolated (49.8%), whereas A. niger (7.6%), A. flavus (7.2%) and A. terreus (4.8%) were less common.
Table 1.
Characteristics of 415 HSCT patients with invasive aspergillosis (IA)
| Characteristic | Total N(%) N=415 |
Survivorsa (N=176) |
Non-survivors (N=239) |
P valueb |
|---|---|---|---|---|
| Mean Age (±SD) | 46.9(±15.6) | 47.3(±16.9) | 46.6(±14.6) | 0.68 |
| Male Sex | 260/414(62.8) | 108/176(61.4) | 152/238(63.9) | 0.60 |
| White Race | 343/384(89.3) | 151/163(92.6) | 192/221(86.9) | 0.07 |
| HSCT | ||||
| Allogeneic | 337(81.2) | 131(74.4) | 206(86.1) | 0.002 |
| Matched relatedc | 168/337(49.9) | 71/131(54.2) | 97/206(47.1) | 0.20 |
| Autologous | 78(18.8) | 45(25.6) | 33(13.8) | |
| Myeloablative conditioning | 288(69.4) | 117(66.5) | 171(71.6) | 0.27 |
| Neutropeniad | 224(54.0) | 83(47.1) | 141(59.0) | 0.017 |
| Fevere | 207(49.9) | 84(47.7) | 123(51.5) | 0.45 |
| GVHDf | 135/337(40) | 48/131(39) | 87/206(61.1) | 0.30 |
| CMV Diseaseg | 104(25.1) | 34(19.3) | 70(29.3) | 0.021 |
| Renal Insufficiencyh | 118(28.4) | 31(17.6) | 87(36.4) | <0.001 |
| Hepatic insufficiencyi | 86(20.8) | 11(6.3) | 75(31.4) | <0.001 |
| Malnutritionj | 69(16.6) | 19(10.8) | 50(20.9) | 0.006 |
| Diabetes | 116(28.0) | 43(24.4) | 73(30.5) | 0.17 |
| Early onset IAk | 147/401(36.7) | 70/174(40.2) | 77/227(33.9) | 0.19 |
| Proven IA (vs probable) | 113(27.2) | 30(17.1) | 83(34.7) | <0.001 |
| Aspergillus fumigatus | 183(44.1) | 69(39.2) | 114(47.7) | 0.085 |
| Prednisone usel | 134(32.3) | 64(36.4) | 70(29.3) | 0.13 |
| Methylprednisone usel | 131(31.6) | 37(21.0) | 94(39.3) | <0.001 |
| Site of IA | ||||
| Pulmonary | 385(92.8) | 166(94.3) | 219(91.6) | 0.29 |
| Central nervous system | 25(6.0) | 4(2.3) | 21(8.8) | 0.006 |
| Disseminatedm | 66(15.9) | 16(9.1) | 50(20.9) | 0.001 |
| Mould-active prophylaxis | 115(27.7) | 47(26.7) | 68(28.5) | 0.69 |
| Antifungal Therapy n | ||||
| Combination Therapyo | 100/348(28.7) | 41/151(27.1) | 59/197(29.9) | 0.57 |
| Voriconazole | 158/348(45.4) | 81/151(53.6) | 77/197(39.1) | 0.007 |
| Caspofungin | 143/348(41.1) | 58/151(38.4) | 85/197(43.2) | 0.37 |
| Amphotericin B Preparation | 144/348(41.4) | 50/151(33.1) | 94/197(47.7) | 0.006 |
| Lipid AMB | 134/348(38.5) | 49/151(32.5) | 85/197(43.2) | 0.042 |
| Amphotericin B | 10/348(2.9) | 1/151(0.7) | 9/197(4.6) | 0.048 |
| Itraconazole | 10/348(2.9) | 5/151(3.3) | 5/197(2.5) | 0.75 |
Mortality defined at 12-weeks post IA diagnosis
P-values computed with Chi-square or Fisher's exact testing for categorical variables and Student's T-test for the variable age.
When compared to other (matched unrelated, mismatched related, cord blood)
Defined as absolute neurophil count <500 within 30 days prior to IA diagnosis
Temperature > 100.5 with 7 days of IA diagnosis
Defined as > grade II GVHD
Positive antigen, culture, or histopathology in association with signs and symptoms consistent with CMV
Defined as serum creatinine >3.0 mg/dl or creatine clearance <30cc/min at diagnosis of IA.
Hepatic insufficiency was defined as ascites, stigmata of liver disease, or elevated laboratory values related to the liver (prothrombin time, INR, liver function tests) at time of diagnosis of IA
Serum albumin <2g/dl; or ≤5% ideal body weight if albumin between 2.1-2.5g/dl
IA Diagnosis less than 30 days after transplant
Use at time of diagnosis of IA
Defined as extrapulmonary disease, excluding sinuses
Data available for 348 patients, and refers to drug(s) used in initial regimen (primary therapy).
Combination therapy defined as initiation of two antifungal agents within 48 hours as primary therapy SD=standard deviation; HSCT-hematopoietic stem cell transplant; GVHD=graft-versus-host disease; CMV=cytomegalovirus
Table 2.
Characteristics of 227 SOT patients with invasive aspergillosis (IA)
| Characteristic | Total N(%) (N=227) |
Survivorsa (N=149) |
Non-survivors (N=78) |
P valueb |
|---|---|---|---|---|
| Mean Age (±SD) | 52.9 (±13.3) | 52.6 (±12.8) | 53.4(±14.3) | 0.67 |
| Male Sex | 130/224(58.0) | 79/146(54.2) | 51/78(65.4) | 0.10 |
| White Race | 197/220(89.6) | 129/142(90.9) | 68/78(87.2) | 0.40 |
| Solid Organ Transplantc | ||||
| Lung | 107(47.1) | 84(56.4) | 23(29.5) | <0.001 |
| Kidney | 47(20.7) | 24(16.1) | 23(29.5) | 0.018 |
| Liver | 42(18.5) | 17(11.4) | 25(32.1) | <0.001 |
| Heart | 23(10.1) | 18(12.1) | 5(6.4) | 0.18 |
| Otherd | 8(3.5) | 6(4.0) | 2(2.6) | 0.72 |
| Fevere | 87(38.3) | 52(34.9) | 35(44.9) | 0.14 |
| Organ Rejectionf | 112(49.3) | 71(47.7) | 41(52.6) | 0.48 |
| CMV Diseaseg | 51(22.5) | 30(20.3) | 21(26.9) | 0.24 |
| Renal Insufficiencyh | 106(46.7) | 57(38.3) | 49(62.8) | <0.001 |
| Liver insufficiencyi | 30(13.2) | 8(5.4) | 22(28.2) | <0.001 |
| Malnutritionj | 49(21.6) | 21(14.1) | 28(35.9) | <0.001 |
| Diabetes | 105(46.3) | 66(44.3) | 39(50.0) | 0.41 |
| Aspergillus fumigatus | 137(60.4) | 84(56.4) | 53(68.0) | 0.091 |
| Proven IA (versus probable) | 96(42.3) | 53(35.6) | 43(55.1) | 0.005 |
| Prednisone usek | 174(76.7) | 125(83.9) | 49(62.8) | <0.001 |
| Methylprednisone usek | 43(18.9) | 20(13.4) | 23(29.5) | 0.003 |
| IA Onset >30 days after transplant | 164/226(72.6) | 108/149(72.5) | 56/77(72.7) | 0.97 |
| Site of IA | ||||
| Pulmonary | 206(90.8) | 134(89.9) | 72(92.3) | 0.56 |
| Disseminatedl | 49(21.6) | 24(16.1) | 25(32.1) | 0.006 |
| Central nervous system | 16(7.0) | 3(2.0) | 13(16.7) | <0.001 |
| Antifungal Therapym | ||||
| Combination Therapyn | 56/202(27.7) | 38/131(29.0) | 18/71(25.4) | 0.58 |
| Voriconazole | 95/202(47.0) | 75/131(57.3) | 20/71(28.2) | <0.001 |
| Caspofungin | 53/202(26.2) | 29/131(22.1) | 24/71(33.8) | 0.072 |
| Amphotericin B Preparation | 82/202(40.6) | 39/131(29.8) | 43/71(60.6) | <0.001 |
| Lipid AMB | 70/202(34.7) | 33/131(25.2) | 37/71(52.1) | <0.001 |
| Amphotericin B | 12/202(5.9) | 6/131(4.6) | 6/71(8.5) | 0.35 |
| Itraconazole | 28/202(13.9) | 25/131(19.1) | 3/71(4.2) | 0.004 |
Mortality defined at 12-weeks post IA diagnosis
P-values computed with Chi-square or Fisher's exact testing for categorical variables and Student's T-test for the variable age.
P value represents nalysis of organ type versus all others
Pancreas, small bowel or multiple organ transplants Defined as absolute neurophil count <500 within 30 days prior to IA diagnosis
Temperature > 100.5 with 7 days of IA diagnosis
acute or chronic rejection at time of IA diagnosis
Positive antigen, culture, or histopathology in association with signs and symptoms consistent with CMV
Defined as serum creatinine >3.0 mg/dl or creatine clearance <30cc/min at diagnosis of IA
Hepatic insufficiency was defined as ascites, stigmata of liver disease, or elevated laboratory values related to the liver (prothrombin time, INR, liver function tests) at time of diagnosis of IA
Serum albumin <2g/dl; or ≤5% ideal body weight if albumin between 2.1-2.5g/dl
Use at time of diagnosis of IA
Defined as extrapulmonary disease, excluding sinuses
Data available for 202 patients, and refers to drug(s) used in initial regimen (primary therapy)
Combination therapy defined as initiation of two antifungal agents within 48 hours as primary therapy SD=standard deviation; HSCT-hematopoietic stem cell transplamnt; GVHD=graft-versus-host disease; CMV=cytomegalovirus
Figure 1.
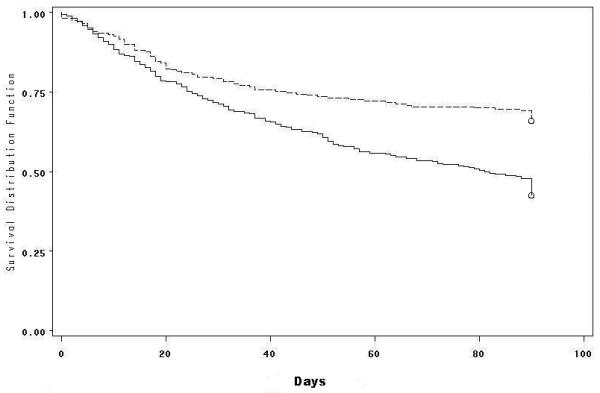
Kaplan-Meier probability of survival after diagnosis of IA according to transplant type. Survival of SOT (top curve, dashed line) is compared with HSCT patients (bottom curve, solid line; p<0.001)
Factors associated with mortality in HSCT patients
Characteristics among the HSCT recipients, including neutropenia (54%), fever (49.9%) and > grade II GHVD (40%) are depicted in Table 1. By univariate analysis, mortality was more likely among patients with the following characteristics: allogeneic SCT (86.1% vs. 74.4%; p=0.002); neutropenia (59% vs. 47.1%; p=0.017); CMV disease (29.3% vs. 19.3%; p=0.02); renal insufficiency (36.4% vs. 17.6%; p<0.001); hepatic insufficiency (31.4% vs. 6.3%; p<0.001); malnutrition (20.9% vs. 10.8%; p=0.006); proven IA (34.7% vs. 17.1%; p<0.001); methylprednisolone use (39.3% vs. 21%; p<0.001); CNS IA (8.8% vs. 2.3%;p=0.006); and disseminated IA (20.9% vs. 9.1%;p=0.001).
Multivariable logistic regression analysis demonstrated that neutropenia (OR 2.3, 95% CI 1.4, 4.1); renal insufficiency (OR 2.2, 95% CI 1.2-3.8); hepatic insufficiency (OR 6.2, 95% CI 2.8-13.5); early onset (<30 days) IA (OR 2.2, 95% CI 1.2-3.8); proven IA (OR 2.4, 95% CI 1.4-4.1); and methylprednisolone use (OR 1.9, 95% CI 1.1-3.2) were independently associated with mortality. White race was associated with decreased mortality risk (OR 0.4, 95% CI 0.2-0.8) (Table 3). After incorporation of an interaction term of GVHD and methylprednisone use into the final model, there was evidence of an interaction between GVHD and methylprednisone (p=0.025). A time-to-death analysis was performed for confirmation of findings. Cox regression modeling showed similar findings to the logistic regression model, with the exception of neutropenia and early-onset IA, which were not poor prognostic indicators (data not shown).
Table 3.
Multivariable logistic regression analysis of factors associated with mortality
| Multivariablea | ||
|---|---|---|
| Variable | OR (95% CI) | P-Value |
| HSCT-specific model (n=415): | ||
| Age | 1.0 (1.0, 1.0) | 0.57 |
| Male sex | 1.1 (0.6, 1.7) | 0.82 |
| White Race | 0.4 (0.2, 0.8) | 0.017 |
| Neutropenia | 2.3 (1.4, 4.1) | 0.002 |
| Renal Insufficiency | 2.2 (1.2, 3.8) | 0.007 |
| Hepatic Insufficiency | 6.2 (2.8, 13.5) | <0.001 |
| Early Onset IA | 2.2 (1.2, 3.8) | 0.007 |
| Proven IA (versus probable) | 2.4 (1.4, 4.1) | 0.002 |
| Methylprednisone Use | 1.9 (1.1, 3.2) | 0.016 |
| SOT-specific model(n=227): | ||
| Age | 1.0 (1.0, 1.0) | 0.11 |
| Male sex | 1.7 (0.9, 3.3) | 0.13 |
| White Race | 0.4 (0.1, 1.2) | 0.11 |
| Hepatic Insufficiency | 3.9 (1.3, 11.8) | 0.015 |
| Malnutrition | 2.3 (1.0, 5.1) | 0.044 |
| Prednisone Use | 0.4 (0.2, 0.8) | 0.011 |
| CNS Disease | 6.6 (1.4, 29.9) | 0.015 |
Logistic regression analysis. Variables with p<0.20 on univariate analysis were included in stepwise regression models in addition to variables for age, race and sex. Only significant (p<0.05) values are shown.
Factors associated with mortality in SOT patients
Among SOT recipients, lung were most frequent (47.1%), followed by kidney (20.7%), liver (18.5%), and heart (10.1%) transplants recipients. Characteristics among the SOT recipients are depicted in Table 2, including prednisone use (76.7%), renal insufficiency (46.7%), organ rejection (49.3%), and CMV disease (22.5%). By univariate analysis, non-survivors were more likely to have the following characteristics: kidney (29.5% vs. 16.1%; p=0.018) or liver (32.1% vs. 11.4%; p<0.001) transplant recipients; renal insufficiency (62.8% vs. 38.3%; p<0.001); hepatic insufficiency (8.2% vs. 5.4%; p<0.001); malnutrition (35.9% vs. 14.1%; p<0.001); proven IA (55.1% vs. 35.6%; p=0.005); use of methylprednisolone (29.5% vs. 13.4%; p=0.003), CNS IA (16.7% vs. 2%;p<0.001) and disseminated IA (32.1% vs. 16.1%;p=0.006). Lung transplantation (29.5% vs. 56.4%; p<0.001) and prednisone use (62.8% vs. 83.9%; p<0.001) were less frequent among non-survivors.
Multivariable logistic regression analysis demonstrated that hepatic insufficiency (OR 3.9, 95% CI 1.3-11.8); malnutrition (OR 2.3, 95% CI 1.0-5.1); and CNS disease (OR 6.6, 95% CI 1.4-29.9) were independently associated with increased risk of death. Prednisone use (OR 0.4, 95% CI 0.2, 0.8) was associated with decreased risk of death (Table 3). After the incorporation of an interaction term of rejection and prednisone in the final model, no statistically significant interaction was observed. Multivariable Cox regression demonstrated findings similar to the logistic regression model. Important exceptions were that kidney transplantation (OR 1.7; 95% CI 1.0-3.0) and white race (OR 0.5, 95% CI 0.2-1.0) were predictors of mortality. Malnutrition was not a poor prognostic indicator.
Antifungal therapy and impact on mortality
A sub-analysis of the impact of antifungal therapy on mortality was performed for 550 patients who received antifungal therapy (Tables 1,2,4). Among 348 HSCT patients, receipt of an amphotericin B (AmB) formulation as part of initial antifungal therapy was more common among non-survivors compared with survivors (47.7% vs 33.1%; p=0.006); receipt of voriconazole was less common among non-survivors (39.1% vs 53.6%; p=0.007). There was no significant association of primary combination antifungal therapy with death. Multivariable logistic regression analysis demonstrated that receipt of an AmB formulation as initial therapy was associated with increased mortality (OR 2.2, 95% CI 1.3, 3.7)(Table 4). A Cox proportional hazards model (not shown) provided similar results.
Table 4.
Multivariable logistic regression analysis of factors associated with mortality among 550 patients who received antifungal therapy
| Multivariablea | ||
|---|---|---|
| Variable | OR (95% CI) | P-Value |
| HSCT-specific model (n=348): | ||
| Age | 1.0 (1.0, 1.0) | 0.56 |
| Male sex | 1.0 (0.6, 1.7) | 0.99 |
| White Race | 0.3 (0.1, 0.8) | 0.020 |
| Allogeneic HSCT (vs. Autologous) | 2.5 (1.2, 5.0) | 0.010 |
| Renal Insufficiency | 2.9 (1.6, 5.4) | <0.001 |
| Hepatic Insufficiency | 5.6 (2.5, 12.9) | <0.001 |
| Proven IA | 1.9 (1.0, 3.4) | 0.035 |
| Amphotericin B formulation | 2.0 (1.2, 3.4) | 0.007 |
| SOT-specific model (n=202): | ||
| Age | 1.0 (1.0, 1.1) | 0.20 |
| Male sex | 2.0 (0.9, 4.4) | 0.076 |
| White Race | 0.6 (0.2, 1.8) | 0.34 |
| Renal Insufficiency | 2.0 (1.0, 4.1) | 0.047 |
| Prednisone Use | 0.3 (0.1, 0.7) | 0.005 |
| CNS Disease | 5.4 (1.2, 24.1) | 0.028 |
| Amphotericin B formulation | 4.4 (2.1, 9.1) | <0.001 |
| Caspofungin | 2.7 (1.1, 6.3) | 0.023 |
Logistic regression analysis. Variables with p<0.20 on univariate analysis were included in stepwise regression models in addition to variables for age, race and sex. Only significant (p<0.05) values are shown.
Among 202 SOT patients, receipt of an AmB formulation as part of initial antifungal therapy was more common among non-survivors (60.6% vs 29.8%; p<0.001). In contrast, receipt of voriconazole (28.2.1% vs 57.3%; p<0.001) or itraconazole (4.2% vs 19.1%; p=0.004) was less common among non-survivors. There was no significant association of primary combination antifungal therapy with death. Multivariable logistic regression analysis showed that receipt of an AmB formulation (OR 4.4, 95% CI 2.1-9.1) or caspofungin (OR 2.7, 05% CI 1.1, 6.3) was associated with increased mortality (Table 4). A Cox proportional hazards model (not shown) confirmed these findings.
Discussion
There has been dramatic improvement in the early diagnosis and treatment of transplant-associated IA over the past decade [10-14]. Identification of important prognostic factors could influence patient outcomes through improved prevention and treatment strategies [1, 4]. To date, there have been several IA outcomes studies among HSCT recipients, but few studies among SOT recipients [6, 7, 15]. This analysis of the TRANSNET database, a large, prospectively collected cohort of HSCT and SOT recipients, for the first time identified important factors associated with mortality in both transplant populations. Using two different analytic methodologies to assess mortality risk, these methods provided similar, but not identical results, highlighting the complexity in analyzing outcomes in these patients.
Overall mortality at 12 weeks among HSCT patients was 57.5%, consistent with the poor outcomes described in previous studies [3-5, 16-18]. Independent prognostic factors included neutropenia, renal insufficiency, hepatic insufficiency, early onset IA, proven IA, and methylprednisolone use. Using a time-to-death analysis, neutropenia and early-onset IA did not predict mortality. The majority of studies to date have recognized neutropenia as an important influence on mortality, underscoring the importance of immune system recovery among HSCT patients with IA [3,4]. That these data do not consistently demonstrate this association may be related to our definition of neutropenia. We did not collect detailed laboratory data as part of this study, but rather reported any episode of neutropenia, defined as an ANC<500 cells/mm3, within 30 days prior to IA diagnosis.
Renal and hepatic insufficiency were predictors of mortality among HSCT patients with IA; both have been associated with poor outcomes in previous studies [4-6]. In our population, hepatic insufficiency was the strongest predictor of mortality (OR 6.2), confirming the findings of one study among HSCT patients with IA [4], possibly explained by the complexity of drug delivery in these patients.
As demonstrated in prior experimental and observational studies, proven IA (as opposed to probable disease) is a predictor of poor outcomes [4,6-10]. This intuitive observation- probable IA cases represent less diagnostic certainty- also suggests that proven IA may represent more advanced disease, even though this association was not affected by adjusting for disseminated IA in our final model.
Numerous studies have documented the impact of steroids on outcome in HSCT patients with IA, with receipt of high-dose (≥2 mg/kg) corticosteroids daily at the time of diagnosis being a precise predictor [2-6, 19]. We did not routinely collect daily corticosteroid doses and could not explore fully the impact of dose or duration on mortality, but among patients this reflects treatment for GVHD. GVHD did not fulfill criteria for stepwise entry into the final model, but we observed a significant interaction between GVHD and methylprednisolone.
We observed that Caucasian patients with IA had lower overall mortality when compared to other races. A clear racial predisposition to severe fungal disease has been best described among patients with coccidioidomycosis [20]. This is a novel observation among patients with IA, and explanations for this apparent predisposition may relate to less optimal donor matching or other confounding factors.
All-cause mortality at twelve weeks was 34.4% among SOT patients with IA, and lowest among lung transplant recipients. Independent predictors of mortality included hepatic insufficiency, malnutrition and CNS disease. Prednisone use was associated with decreased risk of mortality, possibly representing more stable maintenance immunosuppression. Cox regression analysis and the logistic regression model revealed similar results, except that kidney transplantation and non-Caucasian race were poor prognostic indicators.
Several prior studies have linked CNS involvement with poor outcome [1, 5]. Although only 16 (7%) SOT patients had CNS IA, 13 (81%) died. Disseminated IA, defined as any extra-pulmonary disease, was also a significant predictor of death in univariate analysis, similar to what has been previously reported [1, 3, 4, 6]. Timeliness in diagnosis of IA among SOT patients remains an important issue, possibly contributing to the frequency of complicated IA in these patients. Except for routine post-transplant surveillance bronchoscopy in lung recipients, screening methods for IA are not regularly performed in SOT populations.
Antifungal management of IA remains problematic. Despite the availability of newer antifungal drugs, clinical response remains poor and few randomized, comparative treatment trials are available [10, 21-23]. Combination antifungal therapy is attractive, but there are few prospective data to support this approach [7, 24-26]. Current guidelines endorse voriconazole monotherapy as the first-line choice for primary IA [10, 11]. In this study, voriconazole alone was the most common approach to primary therapy, whereas primary combination therapy was used in 28% of patients. We did not demonstrate that combination therapy had an impact on survival. In comparing those who did or did not receive combination therapy, underlying characteristics were similar with few exceptions. For example, HSCT patients who received combination therapy were more likely to be malnourished (p=0.001) and older (p=0.01). These findings alone likely do not explain the lack of an observed difference among the groups; there may be confounding and unmeasured variables which more likely explain this observation.
We observed distinct differences in mortality according to the antifungal agents used. Use of an AmB formulation in HSCT or SOT patients resulted in increased risk of death at 12-weeks, despite adjustment for renal insufficiency. This confirms data from a recent trial associating initial AmB use with increased mortality [10]. Importantly, we observed decreasing mortality during our study period, and a significant decrease in use of AmB over time. This observation has been described in several recent studies, and may be related to differences in patient selection, supportive care, and/or newer antifungal agents [1, 27]. Survivors were more likely to have received voriconazole, and it was used with increasing frequency throughout the study, but it was not an independent predictor of mortality.
SOT patients who received caspofungin as initial therapy had an increased risk of death. Among these patients there was an increased frequency of malnutrition (p=0.05); liver disease (p=0.008); renal insufficiency (p=0.06); and CNS disease (p=0.09), suggesting that SOT patients who received caspofungin were perceived to be more ill. Notably, caspofungin was commonly used as a combination agent with voriconazole or an AmB formulation.
Our study has several limitations, including lack of collection of extensive laboratory data [4, 6]. Only antifungal drug type and start date were collected, thus insights into dose, duration, and combination therapy were limited. We did not analyze attributable mortality, as a standardized definition was not employed for TRANSNET. It is notable that among four recent studies investigating predictors of IA attributable mortality, three different attributable mortality definitions were used [3, 4, 6, 17]. This underscores the difficulty of defining mortality attributable to IA and the necessity of a consensus definition [21]. Our outcome analysis endpoint was 12-weeks after IA diagnosis. Recent reports suggest that early mortality is mostly attributable to IA, whereas later mortality is more likely related to other causes [28]. Our database lacked some variables that may impact overall survival, including risk of underlying disease, relapsed malignancy and transplant incompatibility. Finally, we did not capture dose and duration of corticosteroid or other immunosuppressive use. Despite the limitations, this large and broadly based surveillance study provides greater credence to previous observations, and we are encouraged by the general concordance between prognostic factors described herein and those from previous studies.
Figure 2.
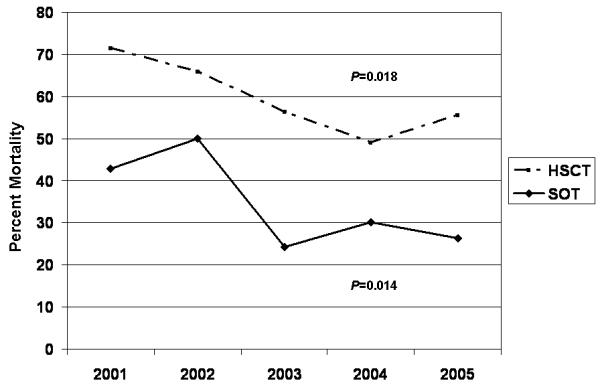
Mortality per year during the study. For both transplant groups there was a significant linear trend in decreased mortality (Cochran-Armitage Trend Test). Data for years 2005 and 2006 are combined, as only 13 patients developed IA in 2006.
Footnotes
This work was presented in part at the 41st Annual Meeting of the Infectious Diseases Society of America, October, 2004, Boston, MA
Potential conflicts of interest. TRANSNET was sponsored by the CDC, Astellas, Pfizer, Merck, and Schering Plough; JWB: Sponsored in part by NIH K23AI064613. Research grant from Pfizer. Speaker's bureau for Enzon; DRA: Research grants from Merck, Astellas and Pfizer. Consultant for Astellas, Novartis, Merck, Schering Plough; KAM: Research grants from Enzon, Merck, Astellas and Pfizer. Advisory board or consulting for Astellas, Basilea, Merck, Pfizer, Schering Plough; DPK: Research support and honoraria from Schering-Plough, Pfizer, Astellas Pharma Inc., Enzon and Merck; BDA: Sponsored in part by NIH NIAID K24 AI072522. Research grants from Astellas, Pfizer and Enzon. Honoraria for CME accredited lectures supported by Astellas and Basilea. Advisory boards for Abbott Diagnostics and Astellas; CAK: Research grants from Merck and Astellas; RAO: None; EAA: None; TAW: None; MGS: None; JRW: Speaker/advisor for Merck, Pfizer, Astellas, Enzon; TFP: Research grants from Basilea, Merck, Pfizer and Sschering-Plough. Advisory boards or consulting for Basilea and Pfizer; JII: Speakers' bureaus for Astellas, Enzon, Pfizer, Schering-Plough; ODW: None; TC: None; PGP: Research support and ad hoc advisor for Merck, Pfizer, Astellas, Schering-Plough, Basilea and Novartis.
REFERENCES
- 1.Parody R, Martino R, Sanchez F, et al. Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplanation : Single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes. Am J Hematol. 2009;84(9):571–8. doi: 10.1002/ajh.21488. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 3.Cordonnier C, Ribaud P, Herbrecht R, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42(7):955–63. doi: 10.1086/500934. [DOI] [PubMed] [Google Scholar]
- 4.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 5.Ribaud P, Chastang C, Latge JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1999;28(2):322–30. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 6.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47(9):1176–84. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Limaye AP, Forrest G, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006;81(3):320–6. doi: 10.1097/01.tp.0000202421.94822.f7. [DOI] [PubMed] [Google Scholar]
- 8.Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl 1):S49–58. doi: 10.1080/13693780400020113. [DOI] [PubMed] [Google Scholar]
- 9.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 10.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New Engl J Med. 2002;347(6):408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 12.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44(3):373–9. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42(10):1417–27. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 14.Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67(11):1567–601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- 15.Iversen M, Burton CM, Vand S, et al. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur J Clin Microbiol Infect Dis. 2007;26(12):879–86. doi: 10.1007/s10096-007-0376-3. [DOI] [PubMed] [Google Scholar]
- 16.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–75. [PubMed] [Google Scholar]
- 17.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45(9):1161–70. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 18.Mihu CN, King E, Yossepovitch O, et al. Risk factors and attributable mortality of late aspergillosis after T-cell depleted hematopoietic stem cell transplantation. Transpl Infect Dis. 2008;10(3):162–7. doi: 10.1111/j.1399-3062.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 19.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. The Journal of infectious diseases. 1997;175(6):1459–66. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 20.Flaherman VJ, Hector R, Rutherford GW. Estimating severe coccidioidomycosis in California. Emerging Infect Dis. 2007;13(7):1087–90. doi: 10.3201/eid1307.061480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44(10):1289–97. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 22.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47(5):674–83. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis DP, Hachem R, Lewis RE, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003;98(2):292–9. doi: 10.1002/cncr.11479. [DOI] [PubMed] [Google Scholar]
- 25.Baddley JW, Pappas PG. Antifungal combination therapy: clinical potential. Drugs. 2005;65(11):1461–80. doi: 10.2165/00003495-200565110-00002. [DOI] [PubMed] [Google Scholar]
- 26.Marr KA, Boeckh M, Carter RA, Kim HW, Corey L. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis. 2004;39(6):797–802. doi: 10.1086/423380. [DOI] [PubMed] [Google Scholar]
- 27.Hahn-Ast C, Glasmacher A, Mückter S, et al. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 1996. J Antimicrob Chemother. 2010 doi: 10.1093/jac/dkp507. Jan 27 epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer. 2008;112(10):2309–12. doi: 10.1002/cncr.23441. [DOI] [PubMed] [Google Scholar]


