Abstract
Importance of the field
Site-specific drug delivery is an important area of research that is anticipated to increase the efficacy of the drug and reduce potential side effects. Due to this, substantial work has been done developing non-invasive and targeted tumor treatment with nano-scale metallic particles.
Areas covered in this review
This review focuses on the work done in the last several years developing gold nanoparticles as cancer therapeutics and diagnostic agents. However, there are challenges in using gold nanoparticles as drug delivery systems such as biodistribution, pharmacokinetics, and possible toxicity. Approaches to limit these issues are proposed.
What the reader will gain
Different approaches from several different disciplines are discussed. Potential clinical applications of these engineered nanoparticles is also presented.
Take home message
Because of their unique size-dependent physico-chemical and optical properties, adaptability, sub-cellular size, and bio-compatibility, these nanosized carriers offer an apt means of transporting small molecules as well as biomacromoleculs to diseased cells/ tissues.
Keywords: gold nanoparticles, cancer, drug delivery, drug targeting, diagnostics, biodistribution, thermal ablation, photodynamic therapy
1. Introduction
Even though our knowledge of cancer biology has significantly increased in the past two decades, cancer is still a major health problem world wide as the second leading cause of death [1]. Each year, there are over 10 million new cases and over 5 million deaths from the disease [2] A diagnosis of cancer was previously considered terminal though, if detected early, the prognosis is favorable. A significant number of cancer patients are asymptomatic until they are in the late stages of the disease. Presently, treatments are limited to chemotherapy, radiation, and surgery. Due to inadequate therapies and clinical procedures for overcoming multi-drug resistant cancer, it is vital that new technologies emerge for accurate early detection and treatment of this disease. The primary goal of cancer treatment should result in enhanced therapeutic efficacy with low to minimal side effects. One possible method is to develop nanotechnology for targeted drug delivery. This review will focus on using gold nanoparticles as cancer therapeutics and diagnostic tools.
2. Synhetic Methodologies of Biological of Gold Nanoparticles
Since ancient times, gold was highly regarded as the “elixir of life” [3]. In 1925 gold complexes were being used in clinical trials to determine its efficacy to help alleviate rheumatoid arthritis [4]. In recent years, significant effort has been devoted to developing nanotechnology for the delivery of low molecular weight drugs, as well as cellular/ tissue delivery of proteins, peptides, or genes [5]. Targeted nanoparticle-mediated drug delivery may be used to direct the particles to specific tissues (minimizing toxicity), improve oral bioavailability and unfavorable pharmacokinetics, sustain a drug/gene effect in the target tissue, solubilize drugs for intravascular delivery, and/or improve the stability of therapeutic agents against decomposition [6]–[7, 8] The relative stability of the ligand-gold bonding outside of the cell combined with its decreased stability within cells, partially due to the high intracellular concentrations of glutathione, also contributes to making gold nanoparticles good candidates for drug delivery and drug release [9].
Nanoparticles are a hybrid material featuring an inorganic core typically surrounded by an organic monolayer. The versatility of nanoparticles as components in nanobiotechnology arises from the ability to tailor their size, shape, and composition. Quantum mechanics exist at the near-atomic scale in which these nanoparticles are synthesized, where the chemical, electrical, and optical characteristics can differ significantly from the bulk solid [10]. Furthermore, slight deviations in nanoparticle size can have radical effects on the nanoparticle properties. The shape of the nanoparticle also influences the particle’s behavior. As the ratio of surface area to volume increases, the behavior of the surface atoms assumes dominance over those in the particle interior [11]. Finally, the organization of the core material determines the physical properties of the nanoparticle.
The organic monolayer of the nanoparticle, while not as influential in determining the physical properties of the core, is fundamentally important in modifying the interactions of the nanoparticle with its environment. The monolayer acts as a barrier between the nanoparticle core and the environment, and effectively protects and stabilizes the core integrity. Furthermore, the reactivity and solubility of the nanoparticles are dictated by the chemical nature of the monolayer periphery. For biological applications, the need for solubility in aqueous environments is addressed by designing a monolayer that incorporates biocompatible ligands [12–19]. The monolayer can further be tailored with more intricate pendant groups to modulate intermolecular interactions of the particle. Most importantly, biologically active components, such as peptides, proteins, and DNA, can be conjugated to the nanoparticle monolayer. In effect, the design of the monolayer dictates the chemical behavior of the nanoparticle in a given environment while the core governs the physical behavior of the nanoparticle.
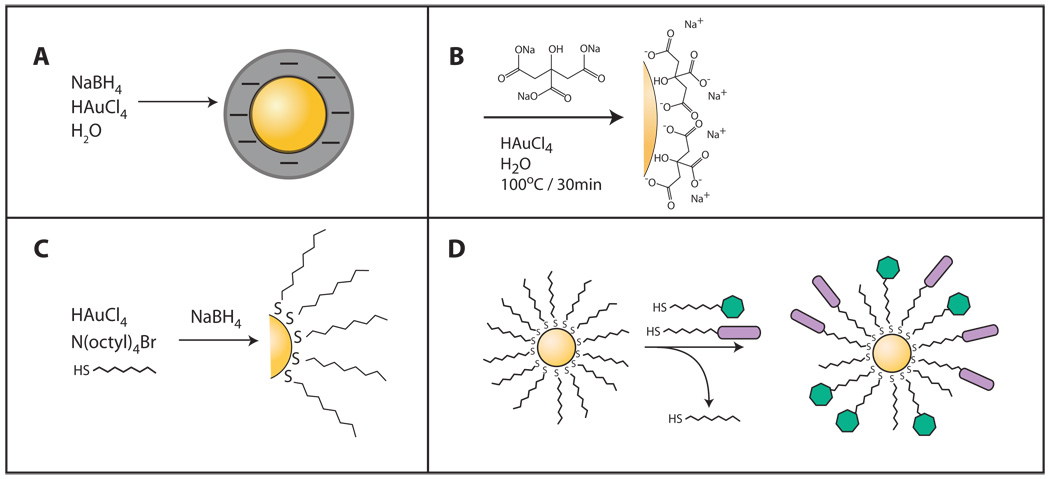
Nanoparticles featuring gold cores have been in use for decades[20]. Numerous approaches exist for nanoparticle fabrication, the most simplistic being the reduction of gold salts in the presence of a reducing agent (Figure 1a). This initiates the nucleation of the gold ions thus forming nanoparticles. To help prevent aggregation, a stabilizing agent is often added during the synthesis process. This method was first reported by Turkevich [21] and later improved on by Frens[22]. In this technique, sodium citrate plays a dual role: (1) as the reducing agent and subsequently, (2) the stabilizer as it is absorbed onto the surface (Figure 1b). Recent advances in nanoparticle production provide a means to synthesize stable particles that are smaller (2–6 nm) and more monodisperse in size and shape. Topmost among these approaches is the Brust-Schiffrin scheme, which produces large quantities of superior quality monolayer protected gold clusters[23]. In short, gold ions are transferred from an aqueous solution of gold salt into toluene using a phase-transfer agent (tetraoctylammonium bromide, TOAB). A nonpolar organic thiol is added (usually a thiol-terminated long-chain alkane), followed by the rapid addition of a reducing agent (see Figure 1c). The thiol-mediated capping of the gold cluster ultimately halts agglomeration of the reduced gold atoms. The thiol ligands form a very stable organic monolayer due to the strong gold-sulfur bonding. The size of the gold clusters can be modulated primarily by altering the gold:ligand stoichiometry.
Figure 1.
One key feature of these monolayer protected gold clusters is that they can be readily modified to incorporate a diverse array of functionalized ligand[24]. Over time, the new ligands are incorporated into the monolayer, whereby the original ligands are displaced into solution (Figure 1d). In this manner, nanoparticles can be endowed with specific attributes such as water solubility, surface charge, or specific recognition. Furthermore, the ligands form a monolayer that is flexible and labile by nature.
3 Targeted delivery in vitro
3.1 Size and surface functionality
As mentioned previously, inorganic core shelled nanoparticles have an increased impact for biomedical applications such as sensing, imaging, and drug delivery[25]. Their success relies on the ability of these particles to target specific cells followed by internalization[26]. It was recently reported that the size and shape of the nanoparticle is important in relation to cellular uptake in vitro. In a study done by Chan et al., spherical shaped nanoparticles had an increased uptake over rod shaped particles[27]. The authors also discovered that the 50 nm spherical gold nanoparticles (AuNPs) had the best internalization of all the nanoparticles studied. Within the last year, the Rotello group has reported using AuNPs with an overall neutral charge to effectively deliver drugs in vitro[28, 29].
Another important factor of mitigating nanoparticle intake is the surface functionality, allowing for either specific or non-specific interactions with the cellular lipid bilayer. Due to the overall anionic surface of the cell exterior, cationic nanoparticles are able to be translocated via favorable electrostatic interactions[30]. However, it has been recently reported that hydrophobicity can also be important in targeting lipophilic domains[31]. In this report, the authors showed that order and structure of the capping ligands played an important role in how the nanoparticle is internalized into the cell. Gold nanoparticles demonstrating an ordered alternate arrangement of the surface ligands or a‘striped’ conformation easily passed through the cellular membrane whereas a random arrangement of the surface ligands or ‘unstriped’ nanoparticles did not internalizes as readily [31]. In addition, there are other reports where proteins in the serum help facilitate nanoparticle uptake into cells[32].
3.2 Using Cell Penetrating Peptides
The protective lipid bilayer of mammalian cells acts as an impermeable barrier to most biomacromolecules. However, certain peptides have been shown to be able to penetrate the membrane and be subsequently internalized. These cell penetrating peptides (CPPs), which are mainly cationic or amphiphilic, have been used in drug delivery systems as well as gene therapies[33, 34]. In one study, Tat protein-derived sequence has been shown to efficiently mediate the nuclear translocation of AuNPs that were less than 30 nm in diameter[35]. A recent publication has provided additional insight on how surface functionality dictates the subcellular localization of AuNPs in vitro [36]. Gold nanoparticles capped with the CCP Tat, a nuclear location signal (NLS), or Antennapedia-Pntn was thoroughly studied using TEM. The CPP-nanoparticle conjugates were transported from the extracellular space through the cell membrane into the cytoplasm, showing their capability to act as a functional unit in drug delivery systems (DDS). Furthermore, researchers have successfully demonstrated that particles could enter cells by a number of different routes. Gold nanoparticles with CCPs appeared to enter the cytoplasm either directly through the cell membrane or via endocytosis followed by endosomal release. These results suggest that a combination of peptides to modify the particle surface allows for their release from endosomes into the cellular matrix. In a separate study, the Feldeim group tagged a citrate gold nanoparticles with a nuclear-targeting peptide that was previously modified with BSA[37]. They tested their system against nuclear-tagged nanoparticles in the absence of a BSA monolayer coating in HepG2 cells. Their results demonstrated the hybrid gold particle to be more efficient in nuclear targeting than the particle with peptide alone.
3.3 Suface Receptor Targeting
The ability of gold nanoparticles to strongly bind with biological molecules is being exploited to target cancer tissues by conjugating the surface of the nanoparticle with suitable biomolecules. These bio-conjugated nanoparticles can be used as vectors[38] for delivering drug moieties. Since it is well known that epidermal growth factor receptor (EGFR) is often overexpressed in cancer cells, it can be used as a targeting agent for drug delivery[39]. Using this concept along with the intrinsic optical properties of AuNPs, researchers have successfully conjugated anti-EGFR to the surface of gold nanoparticles to demonstrate the detection and imaging of malignant cells[40]. Since the conjugated AuNPs have a 6-fold greater absorption in cancer cells than normal human cells, El-Sayed et al. was able to “seek and destroy” cancerous cells using a continuous argon laser at 514 nm. More importantly, they were able to demonstrate that after four minutes, 25 W/cm2 is lethal to cancer cells compared to normal cells 57 W/cm2. These studies showed that effective coupling of targeting agents conjugated to gold nanoparticles could be used as viable cancer therapeutics. Other studies done by Patra et al. have also successfully used a multifunctional gold nanoparticle tagged with C225 and Gemcitabine, a common drug used in the clinics to treat various cancers. This nanoparticle based drug delivery system (DDS) was shown to inhibit the growth of pancreatic cancer cells in vitro and in vivo[41]. Using this strategy, this system can be a viable cancer therapeutic used to target other cancers that overexpress EGFR.
The folic acid receptor is an important receptor for targeted drug delivery due to frequent overexpression on the surface of malignant cells[42]. Based on their previous work[43], Bhattacharya and co-workers used multifunctional gold nanoparticles as a potential therapeutic for ovarian cancer[44]. The authors were able to show that gold nanoparticles conjugated with thiolated polyethylene glycol (HS-PEG), cis-platin, and folic acid had a toxic effect on malignant cells (OV-167, OVCAR 5) versus normal cell lines (HUVECs, OSE). The Baker group also utilized the folic acid receptor as a means for targeted drug delivery using dendrimer encapsulated gold nanoparticles [45, 46]. Using KB cells, they were able to show specific binding and internalization within two hours into lysosomes in vitro. A report by Dreaden et al.[47] targeted the estrogen receptor alpha using gold nanoparticles conjugated with a tamoxifen derivative. The uptake of the nanoparticle was observed to have a 2.7-fold enhanced potency compared to the free drug.
4. Tmor Targeting Efficiency in vivo
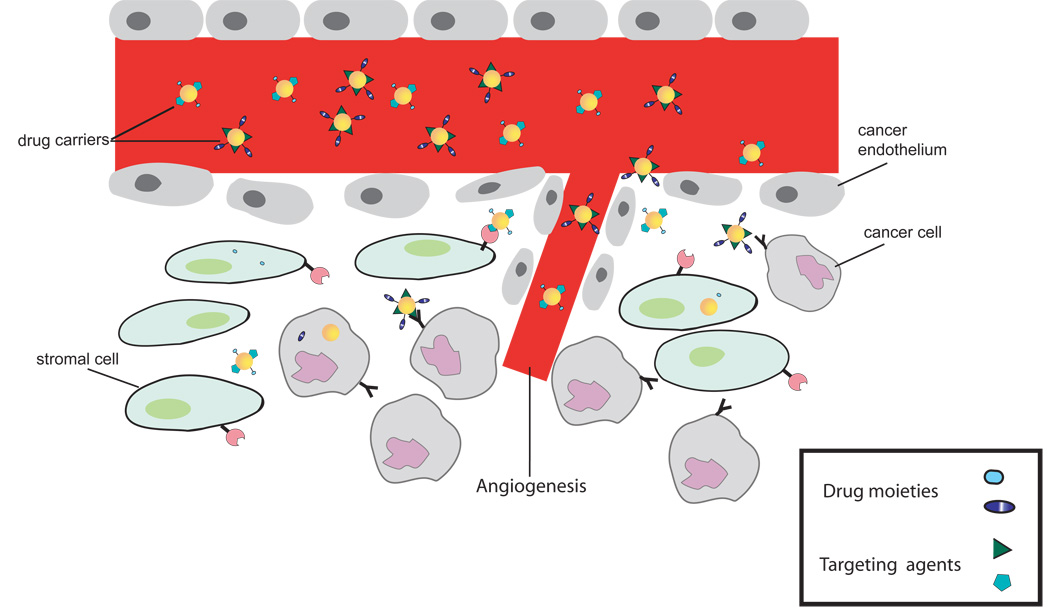
For nanoparticles to advance toward clinical use, it is imperative to have a fundamental understanding of how they behave in the tumor microenvironment in order to optimize their capacity to be efficient therapeutics and diagnostic tools. Due to the large intercapillary distances and variable blood flow typical of solid tumors, penetration and accumulation of cancer therapeutics to the hypoxic center of the tumor is often limited[48–50]. However, the proliferating outer shell of solid tumors is often the site of angiogenesis. This highly vascularized region tends to be “leaky”, inducing the enhanced permeability and retention effect (EPR). One common method in designing nanoparticles for passive uptake takes advantage of the hyperpermeability of solid tumors[51–53]. The angiogenic blood vessels in these tissues can have gaps up to 600 nm between adjacent endothelial cells, allowing carriers to extravasate into the interstitial space. This results in the concentration of the carrier at the tumor to be 10-fold or higher than the relative levels of same administered dosage of free drug[54, 55]. (Figure 2)
Figure 2.
Despite the inherent low toxicity of gold nanoparticles[56–59], there have been to date only a handful of studies have investigated the interaction of gold nanoparticle with tumor tissue. Recently, Perrault et al. studied the pharmacokinetics and passive uptake of gold nanoparticles (d = 20, 40, 60, 80, 100 nm) conjugated with mPEG (m.w. = 2, 5, and 10 kDA) into tumor tissue[60]. Using CDI mice (n=4), it was determined that the half-life of the particle is eight times greater when smaller particles (d=20 nm, 51 h) were conjugated with 10 kDA mPEG compared to the large particle (d=80 nm, 6.6 h). Conversely, nanoparticle accumulation into tumors is core size dependent, with the larger particles having the best amassing. Their results correlate nicely with previous work with fluorescently labeled dextrans done by Dreher et al.[61] showing that small particles are not apt for accumulation into the tumor matrix due to their high diffusion rate. Furthermore, it is important to note that accumulation of the smaller core sized particles into tumors depends on size and their respective half-life compared to the larger particles that are solely dependent on half-life circulation.
In a recent groundbreaking study, in vivo delivery of gold nanoparticles loaded with tumor necrosis factor (TNF) to a colon tumor after intravenous injection was demonstrated[62]. Nanoparticle-bound TNF was found to be less toxic and more effective than native TNF, demonstrating the potential of this delivery technique. In these applications, the nanoparticles role is twofold: a targeting agent as well as a therapeutic. Firstly, the packaging of the desired cargo using nonspecific interactions with the nanoparticle surface confers a certain degree of stability by protecting the cargo from enzymatic degradation[26]. Consequently, the nanoparticles can be targeted at the cellular, tissue, or organ level by integrating molecules into the monolayer such as antibodies (and their fragments), lectins, proteins, hormones, or charged molecules[63].
5. Photothermal/Radioactive Properties of Gold Nanoparticles
Some of the pioneering work to design methods for selective cell targeting based on the use of lasers and light-absorbing nanoparticles was done by Pitsillides et al. The optical properties of gold nanoparticles are based on the surface plasmon resonance and can be employed to provide photodynamic therapy (PDT) to diseased tissues. Using short laser pulses focused on monoclonal antibodies conjugated with light-absorbing microparticles and nanoparticles, the researchers developed a novel methodology for selective cell targeting and providing highly localized cell damage by photodynamic therapy[64].
Dr. Katti and co-workers have developed hybrid gold-198/199 nanoparticles tagged with prostate cancer specific bombesin peptide analogs in pursuit of this idea[65]. These radioactive gold nanoparticles can deliver effective therapeutic response for treating prostate cancer. By using gold 198/199 nanoparticles, which emits beta-radiation, a higher therapeutic dose is ensured. This design could also be applied to breast and small cell lung cancer cells since the peptide analog also has a high affinity towards receptors on these cell surfaces.
Surgical removal of tumors becomes difficult once they reach a certain size. However the work of Dr. Katti and Dr. Kannan have recently reported therapeutic efficacy of gum-arabic glycoprotein (GA) functionalized gold nanoparticles[66]. In this study, they administrated a single dose of radioactive GA-198 gold nanoparticles in SCID mice. After three weeks, the researches saw an extraordinary tumor reduction of 82% between the treatment and control groups. Furthermore, there was minimal radioactive leakage (2%) to various non-target organs and the blood levels of the treatment group returned to a normal baseline, showing high tolerance of GA-198 AuNPs.
By changing the shape of AuNPs from spheres to rods, the frequency of the surface plasmon band (SPR) of the nanoparticle is modified[11]. One of the advantages of gold nanorods is the duality of the observed plasmon band that is tunable through its aspect ratio. Secondly, the nanorod can be further tailored due to the distinctive surface chemistries along their crystal faces. Moreover the shift of the SPR allows for near infrared (NIR) absorption at the cross sections permitting a deeper penetration into living tissues. Utilizing these properties, Bhatia et al. has developed nanorods that target and reduce tumors[67]. In this study, PEG coated nanorods were injected into the tail veins of tumor-bearing mice. The treated mice were then exposed to NIR and after 15 days showed a significant reduction in tumor size. Expanding on their work, the authors tagged the nanorods with SERS reporters and demonstrated their effectiveness to image and ablate tumors in vivo[68].
Another method used to help reduce tumor size is through radio frequency. Since the early 1990’s, it has been used as a treatment for destroying liver tumors [69]. However, this method does have its drawbacks such as accuracy, invasive needle placement, and damage to non-targeted surrounding tissues. Due to these issues, Curley and his research team has recently designed a method using the Kanzius device[70] combined with gold nanoparticles functionalized with the EGFR inhibitor cetuximab (C225) [71, 72]. The researchers tested the C225 conjugated gold nanoparticles in Panc-1 (pancreatic adenocarcinoma) and Difi (colorectal adenocarcinoma) cells that express high levels of EGFR. As a control, they used a breast cancer cell line, CAMA-1, that does not express the growth factor receptor. Each cell line was then incubated with C225-AuNPs for two hours and then exposed to 600 W for two minutes from the Kanzius' device. This method proved to be cytotoxic to nearly 100 percent of the pancreatic and colorectal cells, but hardly any of the cells from the control group were damaged. This technique may establish an accurate and effective treatment for malignant tumors.
A significant attribute of gold nanoparticles is their stability against oxidation and degradation in vivo. These attractive properties play a considerable role in the advancement of nanomaterials for use as clinical therapeutics and diagnostic tools[73]. Kattumuri et al. has shown that gold nanoparticles functionalized with gum-arabic vectors can be used as a contrast agent in X-ray CT[74]. In addition, the Eck group fabricated pegylated gold nanoparticles conjugated with F19 antibodies to label human pancreatic adenocarnicoma cells that was resected from patients[75]. By using the strong optical properties of gold nanoparticles, they were able to image the actual spatial distribution of the tumor and its subsequent stromal tissue using darkfield microscopy near the nanoparticle resonance scattering maximum (560 nm). This novel technique may provide clinicians a facile identification of malignant tissue.
Other reports mention using gold nanoparticles in X-ray radiotherapy enhancement. In one study, mice-bearing EMT-6 mammary carcinomas received a single intravenous injection of gold nanoparticles (7 mg Au/g in tumor) and were further subjected to several minutes of X-ray therapy at 250 kVp[76]. After one year, the survival rate was an astonishing 86% compared to the control groups (20% X-ray only, 0% gold only).
6. Using Gold Nanoparticles to Inhibit Angiogenesis
As described earlier, angiogenesis is believed to play an important role in the growth and spread of cancerous tissue. This complex process is tightly regulated between a balance of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) and anti-angiogenic factors such as thrombospondin-1 (TSP-1) [77, 78]. However, during tumerogenisis, pro-angiogenic growth factors are over secreted to interact with their respective surface receptors creating the signaling cascade involved in angiogenesis. One method to inhibit angiogenesis in vivo is to block the interaction of VEGF with its receptor. Recently, our group has reported that ”naked” gold nanoparticles inhibit VEGF165 induced proliferation of endothelial cells in a dose dependent manner [79]. Furthermore, it was shown that the nanoparticles also bound to other heparin-binding growth factors such as basic fibroblast growth factor (bFGF). It has been suggested that the mechanism of inhibition is through the cysteine residues of the heparin-binding domain.
The effect of the “naked” gold nanoparticles to inhibit angiogenesis was tested in vivo using a nude mouse ear model. VEGF 165 was introduced into the ears of nude mice through an adenoviral expression vector (Ad-VEGF). A decrease in edema formation was seen in mice treated with the “naked” gold versus the sham treated mice. These results were further paralleled in experiments using C3H mice injected with mouse ovarian tumor (MOT) cells. This mouse model has been well characterized and confirmed that VEGF 165 is responsible for ascites formation in human epithelial ovarian cancer [80–82]. After a week of daily intraperitoneal injections, the ascites volume had reduced in the nanoparticle treated mice compared to the non-treated tumor bearing mice. Taken together, “naked” gold nanoparticles inhibit VEGF, reducing angiogenesis and ascites accumulation.
7. Biodistribution
For gold nanoparticles to be effective as a pharmaceutical, it is essential to have a firm understanding of their biodistribution/accumulation in living systems. To achieve this, it is necessary to have proper characterization of the nanomaterials and a good animal model with an appropriate sample size and robust statistical analyses. The biodistribution of drug carriers is often affected by the route of administration [83]. Nanoparticles used as drug carriers tend to have a longer retention time, generally in the local lymph node, compared to the free drug when administered subcutaneously, intramuscularly, or topically [84, 85]. However, the biodistribution of the nanoparticles largely depends on its surface charge and hydrodynamic radius [86, 87]. In work done by the Makino group, particle size was deemed to be important for in vivo permeation. Citrate capped gold nanoparticles of various sizes (d = 15 nm, 100 nm, 200 nm) were prepared using previously published methods[88]. Their studies showed that the 15 nm particle had the highest permeation coefficient. The larger particles showed a lag time of three hours to six hours, respectively. It was also noted that the smaller particles permeated into the deep regions of the skin whereas the 100 nm and 200 nm particles remained on the surface. Furthering their work in this area, they injected mice with gold nanoparticles with diameters of 15, 50, 100 and 200 nm [89]. After ICP analysis of the various organs and blood, it was revealed that the majority of the gold, regardless of size, was present in the liver, lung, and spleen. The 15 nm particle seemed to have accumulated the most in all the tissues including blood, liver, lung, spleen, kidney, brain, heart, and stomach. Also it was discovered that the 15 nm and 50 nm were able to cross the blood brain barrier whereas the 200 nm particle showed a very minute presence in the organs including blood, brain, stomach and pancreas. According to another study done by Geertsma et al, they illustrated that 10 nm gold nanoparticles were widely dispersed in various organs while the larger particles were detected only in the liver, blood, and spleen [90].
In a study done by Khan and coworkers [91], five types of dendrimer encapsulated gold nanoparticles (d = 5–22 nm) bearing positive, negative or neural surface charges were injected into mice. After sacrifice, the various organs, blood, and excrements were analyzed for gold content. The researchers concluded that size and surface charge of the nanoparticles affect biodistribution with the smallest positive particles accumulating in the kidneys and larger ones accumulating in the spleen, liver, lungs and heart. Work done in the Brandau lab systematically analyzed the effect of size and ionic ligands of monodisperse 1.4 nm and 18 nm gold nanoparticles after two routes of administration: 1) intratracheal instillation into the lungs (IT) and 2) intravenous injection into the tail vein of rats [92]. Their results indicated that the 1.4 nm particle translocated through the respiratory tract compared to the 18 nm particle. However after i.v. injection, the accumulation pattern in other organs revealed a different pattern. The authors hypothesized that proteins and cells modify the gold nanoparticles during the translocation process.
To design a “biocompatible” nanoparticle monolayer, it is necessary to incorporate a chemical functionality that masks the hydrophobic nature of the monolayer core, such as poly(ethylene glycol) (PEG). PEG has been extensively used to passivate surfaces, as it fundamentally resists nonspecific interaction, especially of biomolecules [93–95]. Work done by Zhang et al. [86] investigated the pharmacokinetics and biodistribution of various PEG-AuNPs in nude mice. Their studies revealed that thioctic PEG produced a more stable nanoparticle compared to HS-PEG in the presence of dithiothreitol. Based on these observations, the researchers synthesized AuNPs with high (5000 kDa) and low molecular weight (2000 kDa) thioctic terminated PEG. From this array, it was observed that the 20 nm AuNPs tagged with PEG5000 exhibited the lowest uptake in the RES and the longest half-life. Compared to the 40 nm and 80 nm PEG5000 AuNPs, the 20 nm particles also showed significantly higher tumor extravasation.
8. Conclusion
Nanotechnology can play an intimate role in individualized medicine with potential enhancements increasing the affinity of the nanoparticle for cancerous cells and amplifying the uptake of diagnostic and therapeutic moieties into diseased cells with greater efficiency. Although more research is necessary, it has had an impact on our understanding of biology by introducing unique approaches to numerous fields of research, ranging from bioanalysis to imaging. The term nanobiotechnology encompasses a myriad of interdisciplinary technologies based on the integration of molecular-scale synthetic materials with biological building blocks, as well as utilizing nanoscale materials to influence biological components. Since systems at atomic, molecular, and supramolecular scales (1–100 nm) [97] exhibit fundamentally new properties resulting from their small structure, novel molecular architectures can be developed with a high degree of precision and flexibility. Utilizing these principles, nanoparicles can be tuned to enhance its affinity for different cancer/diseased cells and effectively facilitate the advancement of diagnostic and treatment methods.
9. Expert Opinion
Gold nanoparticles have materialized as promising candidates for drug delivery systems and cancer therapeutics. The active application of AuNPs center primarily on two key aspects that make them apt for use in biological systems: 1) the intrinsic properties of the gold core and 2) the ability to tailor the functionality of the surface. In general, current applications of nanoparticles have focused primarily on the detection of biological molecules or events. This has been accomplished with great success, as illustrated above and highlighted in recent reviews [11], and continues to be a major aim of nanoparticle research and funding. However, more research needs to be focused on optimizing the design of nanoparticles as multifaceted vectors to treat diseases such as cancer. For example, the nanoparticle scaffold can be decorated with a pro-drug, a targeting moiety, and an imaging agent. Thus, this type of nanoparticle will have multifunctional activities for a more effective cancer treatment that could be easily translated into the clinics. Furthermore, fundamental studies to understand the molecular interactions of nanoparticles with their target cells (normal as well as malignant) remain largely unexplored. Such a fundamental understanding of nanoparticle-cell interactions is important in nanotechnology for cancer therapeutics as well as cancer detection and diagnosis.
There are certain critical questions in nanoparticle design and application that need to be addressed to prior to further use in the clinics. One important problem in potential therapy is that toxicity must be carefully examined. Even though it has been reported that gold nanoparticles are inherently non-toxic, it is important to discern the toxicity of the nanoparticle core and that of its capping ligands. Some toxicity may be specific to certain ligands. For example, cationic ligands clearly cause moderate toxicity in vitro [96]. Equally important is considering how the conjugated ligands may change the pharmacokinetics, biodistribution, and eventual side effects. Similarly, packaging technology needs to be optimized if we are to overcome the obstacles of immunogenicity and tumor penetration.
Due to chemoresistance and the heterogeneity among cancer cells, certain therapeutics may not be successful for every patient. One strategy to overcome tumor heterogeneity is probably to target stromal cells (Table 1). By tagging nanoparticles with stromal antagonists, it is possible to greatly increase the effectiveness of anti-cancer therapeutics. Further investigation is warranted to reveal novel molecular targets that are only expressed in the tumor microenvironment to aid the targeting of nanoparticle based therapy. Cancer stem cells (CSC) or cancer initiating cells (CICs) are also important candidates for drug targeting. By eradicating CSCs/CICs chemoresistance and tumor reoccurrence may be eliminated.
Table 1.
Possible Targets for Nanoparticles [98]
| Target | Function |
|---|---|
| VEGF | Angiogenic factor |
| Flt-1, Flk-1 | VEGF receptors |
| CD105 | TGF-β superfamily receptor |
| PSMA | Glutamate carboxypeptidase |
| TEM8 | Adhesion molecule |
| CTGF | Growth factor |
| ανβ3 integrin | Adhesion molecule |
| MMPs | Endopeptidases |
| uPa | Serine protease |
| uPAR | uPa receptor |
| Tenascin-C | Mitogenic and adhesive effects |
| FAPα | Serine protease |
| CAIX | Carbonic anhydrase |
Currently, there are several candidates at different states of preclinical development (Table 2). However, characterization of systemic performance of gold nanoparticles in vivo is essential to in order to advance the future of nanomedicine. Each multifaceted nanoparticle system is unique and must be evaluated individually. At this point, wide inferences should not made as to how various nanoparticles behave and interact in biological systems. Systematic studies on toxicity, pharmacokinetics, and efficacy need to be carried out under precise conditions prior to going to clinical trials.
Table 2.
| Product | Structure | Target/action | Phase |
|---|---|---|---|
| HuJ591 | Nanoparticle aptamer bioconjugate | PMSA | Precinical |
| Aurimmune | PEGylated colloidal gold particles | Solid tumors | Phase I |
| DF1 | Dendritic fullerene | Chemoprotection | Preclinical |
| AuroLase | Gold nanoshell | Head and neck cancer |
Preclinical |
Article highlights
Major benefits of gold nanoparticles as drug delivery agents include i) ease of synthesis; ii) surface easily modified to incorporate an array of ligands for multifuntionality such as targeted delivery.
Physio-chemical properties of the gold core is ideal for photodynamic therapies, contrast imaging, and thermal ablation.
Engineered nanoparticles encompass the capability for early disease detection.
Advances in the field require a multidisciplinary approach to realize the therapeutic potential of gold nanoparticles
Acknowledgments
Declaration of interest:
P Mukherjee’s work is partly supported by NIH CA135011 and CA136494 grants.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BW, Coates AS. Cancer Prevention: A Global Perspective. J Clin Oncol. 2005;23(2):392–403. doi: 10.1200/JCO.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 3.Edwards PP, Thomas JM. Gold in a Metallic Divided State†-†From Faraday to Present-Day Nanoscience13. Angew. Chem. Int. Ed. 2007;46(29):5480–5486. doi: 10.1002/anie.200700428. [DOI] [PubMed] [Google Scholar]
- 4.Aaseth J, Haugen M, Forre O. Rheumatoid arthritis and metal compounds--perspectives on the role of oxygen radical detoxification. Analyst. 1998 Jan;123(1):3–6. doi: 10.1039/a704840h. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005 Mar;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 6.Xu P, Van†Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted Charge-Reversal Nanoparticles for Nuclear Drug Delivery. Angew. Chem. Int. Ed. 2007;46(26):4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 8.Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 9.Chompoosor A, Han G, Rotello VM. Charge Dependence of Ligand Release and Monolayer Stability of Gold Nanoparticles by Biogenic Thiols. Bioconj. Chem. 2008;19(7):1342–1345. doi: 10.1021/bc8000694. [DOI] [PubMed] [Google Scholar]
- 10.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105(4):1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 11.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. of Chem. Res. 2008;41(12):1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 12.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science. 1998;281(5385):2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 13.Chan WCnW, Nie S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science. 1998;281(5385):2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 14.Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. Synthesis of Compact Multidentate Ligands to Prepare Stable Hydrophilic Quantum Dot Fluorophores. J. Amer. Chem. Soc. 2005;127(11):3870–3878. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- 15.Fischer NO, McIntosh CM, Simard JM, Rotello VM. Inhibition of chymotrypsin through surface binding using nanoparticle-based receptors. Proc Natl Acad Sci U S A. 2002;99(8):5018–5023. doi: 10.1073/pnas.082644099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong R, Fischer NO, Verma A, Goodman CM, Emrick T, Rotello VM. Control of Protein Structure and Function through Surface Recognition by Tailored Nanoparticle Scaffolds. J. Amer. Chem. Soc. 2004;126(3):739–743. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]
- 17.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science. 2002;298(5599):1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotech. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 19.Mattoussi HMJ, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Dawendi MG. Self-Assembly of CdSe-Zns Quantum Dot Bioconjugates Using an Engineered Recombinant Protein. J Amer Chem Soc. 2000;122(49):12142–12150. [Google Scholar]
- 20.Hayat M. Colloidal Gold: Principles. San Diego, CA: Methods and Applications: Academic; 1989. [Google Scholar]
- 21.Turkevitch J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 22.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions Nature. Phys Sci. 1973;241:20–22. [Google Scholar]
- 23.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman RJ. Synthesis of Thiol-derivatised Gold Nanoparticles in a Two-phase Liquid-Liquid System. J Chem Soc, Chem Commun. 1994:801–802. [Google Scholar]
- 24.Hostetler MJ, Templeton AC, Murray RW. Dynamics of Place-Exchange Reactions on Monolayer-Protected Gold Cluster Molecules. Langmuir. 1999;15(11):3782–3789. [Google Scholar]
- 25.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 26.Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 27.Chithrani BD, Ghazani AA, Chan WCW. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Letters. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 28.Agasti SS, Chompoosor A, You C-C, Ghosh P, Kim CK, Rotello VM. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Amer.Chem. Soc. 2009;131(16):5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CK, Ghosh P, Pagliuca C, Zhu Z-J, Menichetti S, Rotello VM. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Amer.Chem. Soc. 2009;131(4):1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 31.Verma A, Stellacci F. Effect of Surface Properties on Nanoparticle-Cell Interactions. Small. 2010;6(1):12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 32.Kam NWS, Liu Z, Dai H. Carbon Nanotubes as Intracellular Transporters for Proteins and DNA: An Investigation of the Uptake Mechanism and Pathway13. Angew. Chem. Int. Ed. 2006;45(4):577–581. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]
- 33.Thoren PE, Persson D, Lincoln P, Norden B. Membrane destabilizing properties of cell-penetrating peptides. Biophys Chem. 2005 Apr 22;114(2–3):169–179. doi: 10.1016/j.bpc.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Kichler A, Mason AJ, Bechinger B. Cationic amphipathic histidine-rich peptides for gene delivery. Biochim Biophys Acta. 2006;1758(3):301–307. doi: 10.1016/j.bbamem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 35.de la Fuente JM, Berry CC. Tat Peptide as an Efficient Molecule To Translocate Gold Nanoparticles into the Cell Nucleus. Bioconj. Chem. 2005;16(5):1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 36.Nativo P, Prior IA, Brust M. Uptake and Intracellular Fate of Surface-Modified Gold Nanoparticles. ACS Nano. 2008;2(8):1639–1644. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 37.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, et al. Multifunctional Gold Nanoparticle,àíPeptide Complexes for Nuclear Targeting. J. Amer.Chem. Soc. 2003;125(16):4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 38.Cheng YC, Samia A, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer. J. Amer.Chem. Soc. 2008;130(32):10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, et al. Targeted Killing of Cancer Cells in Vivo and in Vitro with EGF-Directed Carbon Nanotube-Based Drug Delivery. ACS Nano. 2009;3(2):307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer letters. 2006;239(1):129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, et al. Blockade of Epidermal Growth Factor Receptor Signaling on Tumor Cells and Tumor-Associated Endothelial Cells for Therapy of Human Carcinomas. Am J Pathol. 2002;161(3):929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Exp. Opin. Drug Del. 2008;5(3):309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharya R, Patra CR, Earl A, Wang S, Katarya A, Lu L, et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. 2007;3:224–238. [Google Scholar]
- 44.Patra CR, Bhattacharya R, Mukherjee P. Fabrication and functional characterization of goldnanoconjugates for potential application in ovarian cancer. J. Mat. Chem. 2010;20(3):547–554. doi: 10.1039/b913224d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X, Wang S, Meshinchi S, Van Antwerp ME, Bi X, Lee I, et al. Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small. 2007;3(7):1245–1252. doi: 10.1002/smll.200700054. [DOI] [PubMed] [Google Scholar]
- 46.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57(15):2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA. Tamoxifen àíPoly(ethylene glycol),àíThiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconj.Chem. 2009;20(12):2247–2253. doi: 10.1021/bc9002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannock IF. Tumor physiology and drug resistance. Cancer Metastasis Rev. 2001;20(1–2):123–132. doi: 10.1023/a:1013125027697. [DOI] [PubMed] [Google Scholar]
- 49.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 50.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9(3):253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 51.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46(12_Part_1):6387–6392. [PubMed] [Google Scholar]
- 52.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. J. of Drug Tar. 2007;15(7–8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 53.Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003 Mar;3(3):319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 54.Northfelt D, Martin F, Working P, Volberding P, Russell J, Newman M, et al. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi's sarcoma. J Clin Pharmacol. 1996;36(1):55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 55.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003 Sep;20(9):1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 56.Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir. 2006 Jan 3;22(1):300–305. doi: 10.1021/la051982u. [DOI] [PubMed] [Google Scholar]
- 57.Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chemical Engineering Science. 2006;61(3):1027–1040. [Google Scholar]
- 58.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir. 2005;21(23):10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 59.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 60.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Letters. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 61.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J Natl Cancer Inst. 2006;98(5):335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 62.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 63.Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41(2):147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 64.Pitsillides C, Joe E, Wei X, Anderson R, Lin C. Selective cell targeting with lightabsorbing microparticles and nanoparticles. Biophysical J. 2003;84:4023–4032. doi: 10.1016/S0006-3495(03)75128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hede S, Huilgol N. "Nano": The new nemesis of cancer. Journal of Cancer Research and Therapeutics. 2006;2(4):186–195. doi: 10.4103/0973-1482.29829. [DOI] [PubMed] [Google Scholar]
- 66.Chanda N, Kan P, Watkinson LD, Shukla R, Zambre A, Carmack TL, et al. Radioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of 198AuNP-GA nanoconstruct in prostate tumor-bearing mice. Nanomedicine: Nanotechnology, Biology and Medicine. doi: 10.1016/j.nano.2009.11.001. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- 67.von Maltzahn G, Park J-H, Agrawal A, Bandaru NK, Das SK, Sailor MJ, et al. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009;69(9):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maltzahn Gv, Centrone A, Park J-H, Ramanathan R, Sailor MJ, Hatton TA, et al. SERS-Coded Gold Nanorods as a Multifunctional Platform for Densely Multiplexed Near-Infrared Imaging and Photothermal Heating. Adv. Mater. 2009;21(31):3175–3180. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood BJRJ, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;94(2):443–451. doi: 10.1002/cncr.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt C. The Kanzius Machine: A New Cancer Treatment Idea From an Unexpected Source. J Natl Cancer Inst. 2008;100(14):985–986. doi: 10.1093/jnci/djn246. [DOI] [PubMed] [Google Scholar]
- 71.Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotech. 2008;6:2. doi: 10.1186/1477-3155-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curley SACP, Briggs K, Patra CR, Upton M, Dolson E, Mukherjee P. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Exp Ther Oncol. 2008;7(4):313–326. [PubMed] [Google Scholar]
- 73.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006 Mar;79(939):248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 74.Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, et al. Gum Arabic as a Phytochemical Construct for the Stabilization of Gold Nanoparticles: In†Vivo Pharmacokinetics and X-ray-Contrast-Imaging Studies. Small. 2007;3(2):333–341. doi: 10.1002/smll.200600427. [DOI] [PubMed] [Google Scholar]
- 75.Eck W, Craig G, Sigdel A, Ritter G, Old LJ, Tang L, et al. PEGylated Gold Nanoparticles Conjugated to Monoclonal F19 Antibodies as Targeted Labeling Agents for Human Pancreatic Carcinoma Tissue. ACS Nano. 2008;2(11):2263–2272. doi: 10.1021/nn800429d. [DOI] [PubMed] [Google Scholar]
- 76.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004 Sep 21;49(18):N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 77.Volpert OV. Modulation of Endothelial Cell Survival by an Inhibitor of Angiogenesis Thrombospondin-1: a Dynamic Balance. Cancer and Metastasis Reviews. 2000;19(1):87–92. doi: 10.1023/a:1026560618302. [DOI] [PubMed] [Google Scholar]
- 78.Hanahan D, Folkman JP. atterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 79.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11(9):3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Research and Treatment. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 81.Duyndam MCA, Hilhorst MCGW, Schluper HMMMW, Verheul H, van Diest PJ, Kraal G, et al. Vascular Endothelial Growth Factor-165 Overexpression Stimulates Angiogenesis and Induces Cyst Formation and Macrophage Infiltration in Human Ovarian Cancer Xenografts. Am J Pathol. 2002;160(2):537–548. doi: 10.1016/s0002-9440(10)64873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schumacher JJ, Dings RPM, Cosin J, Subramanian, Auersperg N, Ramakrishnan S. Modulation of Angiogenic Phenotype Alters Tumorigenicity in Rat Ovarian Epithelial Cells. Cancer Res. 2007;67(8):3683–3690. doi: 10.1158/0008-5472.CAN-06-3608. [DOI] [PubMed] [Google Scholar]
- 83.Hillyer J, Albrecht R. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 84.Kreuter J. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189(Pt 3):503–505. [PMC free article] [PubMed] [Google Scholar]
- 85.Florence AT. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res. 1997;14(3):259–266. doi: 10.1023/a:1012029517394. [DOI] [PubMed] [Google Scholar]
- 86.Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomat. 2009;30(10):1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst. 1987;3(3):233–261. [PubMed] [Google Scholar]
- 88.Sonavane G, Tomoda K, Sano A, Ohshima H, Terada H, Makino K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Coll. and Surf B: Biointerfaces. 2008;65(1):1–10. doi: 10.1016/j.colsurfb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Coll. and Surf B: Biointerfaces. 2008;66(2):274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 90.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomat. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 91.Balogh L, Nigavekar SS, Nair BM, Lesniak W, Zhang C, Sung LY, et al. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomedicine. 2007;3(4):281–296. doi: 10.1016/j.nano.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, et al. Biodistribution of 1.4- and 18-nm Gold Particles in Rats. Small. 2008;4(12):2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 93.S Sanchez-ferrer AB, Garcia-carmona R. Phase Separation of Biomolecules in Polyoxyethylene Glycol Nonionic Detergents. Crit. Rev. Biochem. Mol. Bio. 1994;29(4):275–313. doi: 10.3109/10409239409083483. [DOI] [PubMed] [Google Scholar]
- 94.Harris J. Poly(ethlyene glycol): Chemistry and Biological Applications. American Chemical Society. 1997 [Google Scholar]
- 95.Kingshott PGH. Surfaces that resist bioadhesion. Curr Opin Solid State Mat Sci. 1999;4:403–412. [Google Scholar]
- 96.Goodman C, McCusker C, Yilmaz T, Rotello V. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconj.Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 97.Roco MC. Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol. 2003 Jun;14(3):337–346. doi: 10.1016/s0958-1669(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 98.Hofmeister V, Schrama D, Becker Anti-cancer therapies targeting the tumor stroma. Can. Immunology, Immunotherapy. 2008;57(1):1–17. doi: 10.1007/s00262-007-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]