Abstract
Urinary levoglucosan was investigated as a potential biomarker for wood smoke exposure in two different controlled experimental settings. Nine subjects were exposed to smoke from a campfire in a controlled setting and four were exposed to smoke from an older model wood stove. All subjects were asked to provide urine samples before and after exposure, and to wear personal PM2.5 monitors during the exposure. Urinary levoglucosan measurements from both studies showed no consistent response to the smoke exposure. A third experiment was conducted to assess the contribution of dietary factors to urinary levoglucosan levels. Nine subjects were asked to consume caramel and provide urine samples before and after consumption. Urinary levoglucosan levels increased within 2 hours of caramel consumption and returned to pre-exposure levels within 24 hours. These studies suggest that diet is a major factor in determining urinary levoglucosan levels and recent dietary history needs to be taken into account for future work involving levoglucosan as a biomarker of wood smoke exposure.
Keywords: woodsmoke, particulate matter, levoglucosan, urine, exposure assessment
Introduction
Airborne particulate matter (PM) can be formed during combustion reactions and industrial processes. PM with a diameter of 2.5 microns or less (PM2.5) can travel farther into the lungs than larger particles and is thus believed to have a greater effect on human health. PM2.5 has been tied to increases in the number of asthma attacks (Slaughter et al. 2003) and has been indicated to increase the number of hospitalizations for upper respiratory effects (Jaffe et al. 2003). Long term exposure to PM2.5 has been associated with increased acute and chronic mortality rates (Laden et al. 2006). The United States Environmental Protection Agency's (USEPA) National Ambient Air Quality Standards (NAAQS) include an annual PM2.5 standard (15 μg/m3, based on a 3-year average) as well as a 24-hour standard (35 μg /m3). An area is designated as nonattainment for the fine fraction if it exceeds either the daily or annual PM2.5 standard, or if relevant information indicates that it contributes to violations in a nearby area (USEPA 2006).
One common source for human PM exposure is through biomass burning, primarily from wildfires or stoves used for heating or cooking in the home. Exposure to wood smoke can occur outdoors through ambient air or indoors through cooking and heating devices, leakage from boilers and stoves, or from infiltration of outdoor sources (Boman et al. 2003). Some occupations, such as fire fighting or charcoal production, can result in high biomass PM2.5 exposures as well (Boman et al. 2003). In situations such as chronic or occupational exposure, it is often difficult to measure the actual amount of smoke exposure. Personal PM2.5 monitors are inconvenient and impractical in these settings. Estimating exposures can also be difficult because of variable PM2.5 production depending on the fuel and burn conditions (Hinwood et al. 2008).
A biomarker of wood smoke exposure would be a useful tool to assess individual exposures. A key aspect of such a biomarker would be the ability to account for variables in exposure and individual metabolism. In addition to its ease of use as non-invasive, a urinary biomarker gives a more accurate measurement of actual smoke exposure, as it takes into account individual variations such as breathing rate (Needham et al. 2007). A non-invasive biomarker would also be more practical than personal environmental monitoring for measuring occupational exposures (i.e. fire-fighters) or for chronic exposures (Neitzel et al. 2009).
Only a few compounds have thus far been investigated as potential urinary biomarkers for wood smoke exposure. Dills et al. evaluated several methoxyphenols as biomarkers for wood smoke exposure (Dills et al. 2001; Dills et al. 2006). Subjects were exposed to campfire smoke for 2 hours and personal PM2.5 exposure was measured. Propylguaiacol, syringol, methylsyringol, ethylsyringol, and propylsyringol all had peak concentrations in the urine approximately 6 hours after wood smoke exposure. A 12-hour average of these 5 compounds was found to be the most practical metric for the biomarker of wood smoke exposure to reduce the influence of diet (Dills et al. 2006). The sum of urinary concentrations of these five methoxyphenols was shown to have a good correlation to levoglucosan in airborne PM2.5; however urinary levoglucosan was not measured. Another study found that four low molecular weight methoxyphenols (syringol, methylsyringol, ethylsyringol, and propylsyringol) were each moderately correlated with personal exposures of smoke from an indoor cook stove in Guatemala (Clark et al. 2007). One drawback to using methoxyphenols as tracers for wood smoke exposure is that they are widely found in foods and can be released into the air by industrial processes. An increase in urinary methoxyphenols after smoke exposure has also not been observed in all settings (Hinwood et al. 2008).
Levoglucosan has been suggested as another potential urinary biomarker for wood smoke exposure. Levoglucosan (1,6-anhydro-B-d-glucopyranose) is a pyrolysis product of cellulose and is one of the major organic components in biomass combustion PM. Levoglucosan is frequently used as a environmental tracer for biomass burning because it is produced at relatively high levels and is stable in the atmosphere (Simoneit et al. 1998; Fraser et al. 2000). Levoglucosan represented 2.8–3.8% of PM2.5 mass from open burning of foliar fuels (Hays et al. 2002) and 5.7% of PM2.5 mass emissions from prescribed burns of forests in Georgia (Lee et al. 2005). During the Montana forest fire season of 2003, levoglucosan concentrations ranged from 900–6000 ng/m3 in the Missoula valley, and were highly correlated with PM2.5 mass (r=0.935) (Ward et al. 2006). Daily levoglucosan has also been shown to have a positive correlation to daily PM2.5 levels in a community where residential wood stove usage is a major contributing factor to both ambient and indoor PM2.5 levels, with an average of 3040 ng levoglucosan/m3 air over the winter (Bergauff et al. 2008). A similar correlation (r2=0.80, p=0.05) was noted for levoglucosan versus the estimated PM2.5 contribution from wood burning in residential wood boilers (Hedberg et al. 2006).
In previously published work, we have demonstrated that levoglucosan can be detected in mouse urine (using GC-MS) after multiple instillations and/or exposures that included pure levoglucosan, concentrated wood smoke particulates, and wood smoke inhalation (Migliaccio et al. 2009). These studies confirmed that levoglucosan is not metabolized, and that it has a short residence time, as about 40% of the pure levoglucosan instilled intranasally in the mice was recovered within 4 hours. Controlled exposure to wood smoke within an exposure chamber also resulted in an increase in levoglucosan detected in mouse urine following the individual trials (Migliaccio et al. 2009). Levoglucosan was not present in the urine of unexposed mice.
The objective of this study was to evaluate the potential of levoglucosan as a biomarker in human urine after exposure to wood smoke in two different controlled settings (campfire exposure study and wood stove exposure study). The potential confounding effects of levoglucosan in the diet (using caramel) were also investigated.
Experimental Methods
Materials
Levoglucosan (99+%) was purchased from Acros Organics (Geel, Belgium). Ethyl acetate (reagent grade), ethanol (95%) and triethylamine (reagent grade) were purchased from Fisher Scientific (Hampton, NH). N-O-bis(trimethylsilyl)trifluoroacetamide (derivatization grade, 99+ %), trimethylchlorosilane (97%), trimethylsilylimidazole (derivatization grade), and urease (type C-3 from Canavalia ensiformis) were purchased from Sigma (St. Louis, MO). All chemicals were used as received.
Standards
Deuterated levoglucosan was employed as an internal standard in the procedure to eliminate possible matrix effects and other variations throughout the analysis period. D-levoglucosan (D7) 98% was purchased from Cambridge Isotope Laboratories (Andover, MA). The solution containing D-levoglucosan was prepared in distilled water and stored in the refrigerator.
Subject selection-wood stove smoke exposure and caramel study
For both the wood stove exposure study and the caramel study, subjects were healthy, non-smoking adults between the ages of 18 and 65. Beginning 24 hours before the exposure and continuing until the completion of the study, subjects were asked to avoid exposure to smoke of any type. Previous studies with mice have demonstrated that 86% of levoglucosan instilled intranasally in mice is recovered within 4 hours of exposure, so 24 hours should be sufficient to avoid any effects from prior wood smoke or levoglucosan exposure (Migliaccio et al. 2009). Subjects were also asked to avoid consuming a variety of foods, including smoked or grilled foods, bacon, foods containing artificial wood smoke flavoring, and foods containing caramel that could potentially interfere with study results. People with asthma or other lung diseases were excluded from the smoke exposure study, but were able to participate in the caramel study. People with diabetes were excluded from the caramel study only. All procedures were approved by the University of Montana Institutional Review Board.
Campfire smoke exposure
Samples were obtained from a previous campfire smoke exposure study published by Dills et al. (Dills et al. 2006), designed to measure urinary methoxyphenols before and after exposure to wood smoke. Briefly, subjects were exposed to wood smoke from a continuous open fire for 2 hours, and all urine voided by the subjects was collected (as separate voids) beginning 24 hours prior to the study up until 48 hours after the exposure (Table 1). Samples were stored at −80°C and remained frozen during shipment. One personal PM2.5 sample was collected for each subject using the Harvard Personal Environmental Monitor for PM2.5 and analyzed as previously reported for various chemicals in wood smoke, including levoglucosan. (Dills et al. 2006).
Table 1.
Overview of exposures and sample collection for each study.
| Type of exposure | Subjects | Time Point Number | Sample collection time (hours post exposure)a | Length of exposure | Nondetects/total number of samples |
|---|---|---|---|---|---|
| campfire smoke | 9 | 1–13b | samples collected ad libitum over 72 hours, beginning 24 hours before exposure | 2 hours | 26/117 |
| wood stove smoke, trial #1 | 4 | 1 | 0 | 2 hours | 0/20 |
| 2 | 3.2 (0.2) | ||||
| 3 | 6.3 (0.6) | ||||
| 4 | 12.3 (1.3) | ||||
| 5 | 20.9 (0.8) | ||||
| wood stove smoke, trial #2 | 5 | 1 | 0 | 2 hours | 0/30 |
| 2 | 2.6 (0.1) | ||||
| 3 | 7.9 (1.5) | ||||
| 4 | 12.5 (0.8) | ||||
| 5 | 21.4 (0.8) | ||||
| 6 | 25.7 (1.1) | ||||
| caramel | 9 | 1 | 0 | N/A | 0/45 |
| 2 | 2.3 (0.4) | ||||
| 3 | 6.1 (0.2) | ||||
| 4 | 13.3 (2.8) | ||||
| 5 | 23.6 (1.3) |
Average sample collection time for each time point (standard deviation)
Subjects each had 3–4 pre exposure samples and 9–10 post exposure samples
Wood stove smoke exposure trials
Two separate exposure trials were conducted using smoke generated from an older-model wood stove. In the first trial, four non-smoking male subjects between the ages of 18 and 65 participated in the wood stove smoke exposure study. Subjects were asked to collect spot urine samples immediately pre-exposure, and at 4 time points post exposure (Table 1). In the second trial, the same male subjects plus one female subject participated. Subjects were asked to collect spot urine samples immediately pre-exposure, and at various time points post-exposure. In the first trial, 4 post-exposure samples were collected from each subject, and a fifth time point was added for the second trial so that 2 samples were collected the morning after exposure (Table 1). Smoke was generated within an enclosed laboratory using an older model, non-EPA-certified wood stove. Locally available softwood species (Douglas fir, larch, and Ponderosa pine) were used for the exposure. Fires were started with 4 g of paper and 20 g of kindling, and maintained by the addition of pre-weighed wood batches (50.00–54.99 g) approximately every 5 min. Within-room PM2.5 concentrations were monitored continuously using a TSI DustTrak (TSI, Inc., Minneapolis, MN). In addition, each subject wore a DustTrak to determine personal PM2.5 exposures during the two trials. It is important to note that the DustTrak is not a Federal Reference Method (FRM) sampler. DustTrak measurements have been shown to be reasonably precise (R2 = 0.859) when compared with an FRM sampler (Yanosky et al. 2002). However, the results presented here were not validated using a co-located FRM sampler from which a correction factor (i.e. wood smoke PM correction factor) could be developed.
Personal breathing zone monitoring for the study subjects began approximately 1 hour before the exposure, throughout the approximately 2 hour exposure trials, and for 6 hours after smoke exposure to monitor any other potential sources of PM2.5. For both the in-room and personal breathing zone sampling, 60-second intervals were recorded. Levoglucosan in the air was not measured during the wood stove smoke exposure trials.
Caramel exposure study
Nine non-smoking subjects between the ages of 18 and 65 (6 female, 3 male) participated in the caramel study. Subjects were asked to consume cubes of caramel in a short period of time (no more than 30 minutes). Subjects each consumed five cubes of caramel, for an average of 42.2 g consumed per person (sd=0.49). Urine samples were collected immediately before exposure, and 2, 6, 12, and 24 hours after exposure. Subjects were asked to avoid smoke exposure and consuming smoked foods or foods containing caramel beginning 24 hours before exposure until completion of the study.
Sample preparation
Urine samples from all three studies were analyzed using a method optimized in our lab based on a previously published method (Migliaccio et al. 2009). Briefly, 100 μL of human urine was placed in an eppendorf tube. Approximately 30 Units of urease was added and the samples were placed in an oil bath at 37°C for 30 minutes. To precipitate out the protein, 900 μL of ethanol was added and the samples were centrifuged for 8 minutes. The supernatant was then transferred to a clean eppendorf tube and the remaining solids were discarded. The sample was then dried in a vacuum manifold for 6+ hours to evaporate the ethanol. To ensure the samples were completely dry, 100 μL of distilled water as added and then the samples were lyophilized until dry (minimum of 4 hours). The remaining solids were derivatized with 75 μL of BSTFA, 10 μL of TMCS, and 10 μL of TMSI in an oil bath at 70°C for 1 hour. After derivatization, the samples were diluted with ethyl acetate containing 3.6 mM TEA and were transferred to GCMS vials for analysis.
GCMS analysis conditions
Analysis was performed on an Agilent 6890N Gas Chromatograph with an Agilent 5973 Mass Spectrometer. An HP-5MS column ((5%-Phenyl)-methylpolysiloxane) was used with dimensions of 0.25 mm ID × 30 m length × 0.25 μm film thickness. A volume of 2 μL was injected for each analysis into a Split/Splitless FocusLiner™ for HP, single taper liner packed with quartz wool. Split injection was used to analyze for levoglucosan with a split ratio of 50:1. Helium was used as the carrier gas at an initial flow rate of 1mL/min through the column. The inlet temperature was set to 250°C and the auxiliary transfer line temperature was set at 280°C. The temperature program was started at 40°C for 1.5 minutes, ramped at 30°C/min to 175°C, 20°C/min to 220°C, held for 2 minutes at 220°C, and then ramped at 50°C/min to a final temperature of 300°C, which was held for 1.5 minutes for a total run time of 13.95 minutes. The mass spectrometer was operated with a solvent delay of 4.00 minutes and the mass range from 40–450 was scanned. For all compounds, highly selective quantitation was performed using the signal for representative ions extracted from the total ion chromatogram. Levoglucosan was analyzed using an m/z of 217, while m/z 220 was used for D7-levoglucosan. These two ions were selected for analysis because they are predominant ions in the mass spectra that are semi-unique to the compounds of interest and represent the same fragment in the normal and deuterated levoglucosan.
Creatinine analysis
Samples from the wood stove smoke exposure trials and caramel studies were analyzed for creatinine using a creatinine ELISA kit purchased from Cayman Chemical Company (Ann Arbor, MI). Creatinine analysis was performed in the same week as analysis for levoglucosan. Standards and samples were analyzed in duplicate. Values were used to normalize levoglucosan measurements to account for dilution. Creatinine analysis for the campfire smoke exposure was performed as previously reported as part of the original study and was not repeated at the time of levoglucosan analysis (Dills et al. 2006).
Calibration and recovery
Calibration standards were prepared containing variable concentrations of levoglucosan and a fixed concentration of D-levoglucosan in distilled water. The standards were evaporated to dryness, derivatized and analysed with GC-MS. The ratio of the peak area of each compound to the peak area of the corresponding deuterated standard was found for each calibration standard. A calibration curve was prepared by plotting the ratio of the two peak areas versus the concentration of the tracer (R2 > 0.99). The concentration of extracted analytes was determined by measuring the ratio of the peak area for the analyte to that of the corresponding deuterated standard, and reading the concentration from the calibration curve. Recoveries were calculated for distilled water blanks spiked with levoglucosan at known amounts.
Method Validation
Blanks of distilled water were analyzed daily with the samples (no less than 1 blank per 10 samples) to monitor for contamination. Levoglucosan was not detected on any of the blanks analyzed (n=21), confirming there is no contamination during analysis. A blank of distilled water was spiked with levoglucosan and D-levoglucosan daily and analyzed with the samples to monitor instrument performance and solution composition (no less than 1 spike per 10 samples analyzed). Average recovery was 104±4.1% (n=21).
Detection limits for the method were defined as the concentration of analyte that gives an instrument response that is three times the standard deviation of the instrumental baseline signal. The detection limit for levoglucosan in the final ethyl acetate extract was determined to be 0.92 μg/ml (1.8 ng injected, 37 pg on-column), which equates to a detection limit of 0.23 μg in 100 μl of urine sample with the dilutions used in our method. Samples below the detection limit were assigned a value of ½ the detection limit for calculations (Nehls et al. 1973; Hornung et al. 1990; Helsel 2005).
Results
Campfire smoke exposure
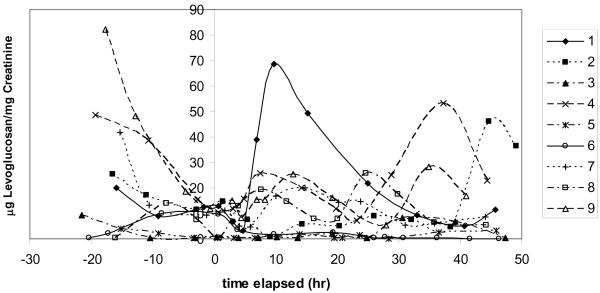
Nine subjects were exposed to PM2.5 generated from a campfire for 2 hours in a controlled setting. Individual exposures ranged from 0.84–2.99 mg/m3 PM2.5, and from 76–256 μg/m3 levoglucosan (Dills et al. 2006). Urinary levoglucosan levels from the subjects in the campfire wood smoke exposure showed no consistent response to the exposure (FIGURE 1). Seven of the nine subjects had measurable levels of urinary levoglucosan at the zero time point. Several of the subjects showed only low levels throughout the entire study. Others showed peaks of urinary levoglucosan before or more than 24 hours after the exposure. Only 1 of the subjects (#1 at 9.75 h post exposure) showed a maximum urinary levoglucosan level within 24 hours of the exposure, while three had a maximum before the exposure, and five had a maximum more than 24 hours post-exposure. Several subjects also showed multiple levoglucosan peaks. The initial intent of this campfire exposure was to evaluate urinary methoxyphenols, so subjects were asked to avoid smoked or grilled foods and other sources of smoke. Foods containing caramel as a potential source of levoglucosan were not monitored or restricted as the initial intention of this study was to measure methoxyphenols.
Figure 1.
Urinary levoglucosan for each subject after controlled smoke exposure from a campfire. Smoke exposure occurred between time 0 and 2 hours.
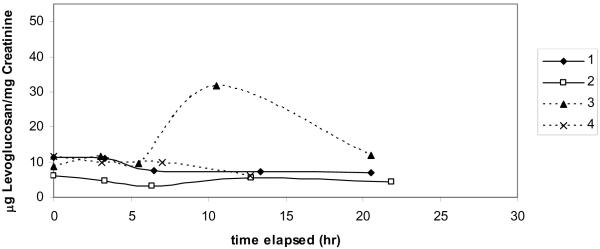
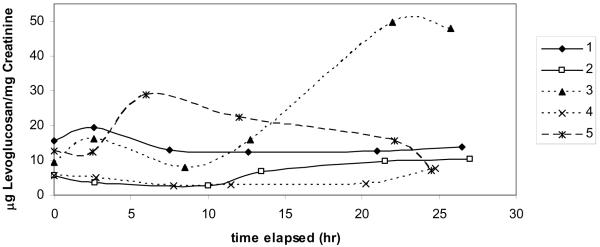
Wood stove smoke exposure trials
Subjects were exposed to wood smoke generated with an older model, non-EPA certified wood stove in a controlled setting. Individual exposures ranged from 1.15–1.97 mg/m3 PM2.5. Urinary levoglucosan measurements from the subjects in the controlled wood stove smoke exposure showed no consistent response to the exposure (FIGURE 2a and 2b). In exposure trial #1, one subject showed an increase in urinary levoglucosan 10 hours post exposure, while the other three subjects showed no change (FIGURE 2a). Because of this inconclusive result, a second exposure was carried out using the same subjects plus one additional subject. In exposure trial #2, subjects also showed a variable response of either no change in urinary levoglucosan or multiple peaks within 24 hours post exposure (FIGURE 2b). One subject showed an elevated level of urinary levoglucosan beginning 12.75 h post exposure and for the remainder of the monitoring time (24 h post exposure). Another subject showed a small increase in urinary levoglucosan 8.5 h post exposure, but all other points were the same as pre-exposure. All subjects showed a low level of urinary levoglucosan pre-exposure, suggesting that levoglucosan is present in the diet or from other airborne sources.
Figure 2.
Urinary levoglucosan for each subject after 2 controlled smoke exposures from an older model wood stove. Smoke exposure occurred between time 0 and 2 hours. (a) first exposure trial (b) second exposure trial.
Caramel study
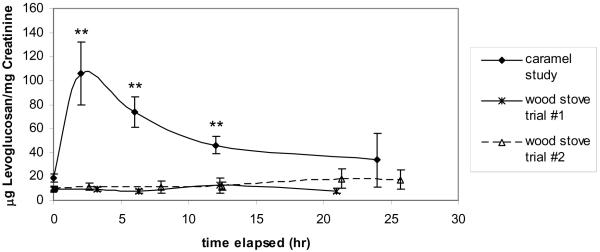
Subjects each consumed five cubes of caramel, for an average of 42.2 g consumed per person (sd=0.49). The caramel was found to have approximately 5.3 mg of levoglucosan per cube, giving an average individual exposure of 26.5 mg of levoglucosan, compared to individual levoglucosan exposures for the campfire study of 0.076–0.256 mg. Pre-consumption urine samples showed an average of 18.3 (±10.2) μg of levoglucosan per mg of creatinine. All nine subjects showed an increase in levoglucosan levels of at least 2x the pre-consumption value beginning two hours post exposure. Eight of the subjects had the highest levoglucosan readings two hours post-exposure, while one was highest in the six hour post-exposure sample, suggesting that levoglucosan has a short residence time in the human body when consumed in the diet. Average levoglucosan levels decreased 12 hours post consumption and returned to pre-consumption values for seven of the nine subjects (FIGURE 3). The other two subjects showed initial decreases in levoglucosan six and 12 hours post consumption, but then showed an increased levoglucosan level again 24 hours post-consumption. In both the pre-consumption and 24 hour post-consumption samples, all of the subjects showed a low level of levoglucosan, suggesting again that there are likely other sources for levoglucosan in the diet or elsewhere. The average level of urinary levoglucosan measured after caramel consumption was more than five times higher than the average urinary levoglucosan measured after either of the two wood stove smoke exposure trials (FIGURE 3).
Figure 3.
Average levoglucosan values at each time point during the caramel consumption study (** difference from pre-exposure is significant at p<0.01). The average values at each time point for the 2 wood stove smoke exposure trials are also included for comparison.
Discussion
Levoglucosan in human urine was first reported in 1986 by Dorland et al. using one-dimensional thin-layer chromatography (Dorland et al. 1986). In this study, levoglucosan was observed in approximately 20% of all urine samples screened at levels ranging from 0 up to 0.85 mg/mL. There was no apparent correlation with age or disease and levoglucosan levels, however it is suggested that the source was likely dietary polysaccharides that have been heated (Dorland et al. 1986). Such dietary exposures are a concern for the use of levoglucosan as an exposure marker since levoglucosan has also been reported in several types of caramel (Ratsimba et al. 1999).
Levels of urinary levoglucosan and methoxyphenols have been measured in subjects after wood smoke exposure from a fire training exercise (Hinwood et al. 2008). The authors reported no significant increase in levoglucosan or methoxyphenols after smoke exposure. This study did not report personal PM2.5 or levoglucosan exposure and samples were collected at only one time point after smoke exposure.
Some subjects from our studies showed elevated urinary levoglucosan in response to smoke exposure, but this was not consistently observed for all subjects. For the campfire smoke exposure study, both PM2.5 and levoglucosan personal breathing zone exposures were measured for each subject. No overall associations were observed when comparing average personal breathing zone exposures with average urinary levoglucosan concentrations from zero to 12 hours post-exposure (r=0.26 (p=0.49) for PM2.5 and r=0.22 (p=0.56) for levoglucosan). For the two wood stove exposure trials, personal breathing zone concentrations of PM2.5 did not show a correlation to 12-hour urinary levoglucosan measurements, with r=0.27 (p=0.48). Twelve hour averages were chosen because previous studies with mice (levoglucosan instillation, PM2.5 instillation, and wood smoke exposure) suggest that this is sufficient time to observe any changes in urinary levoglucosan levels (Migliaccio et al. 2009). Given the small sample sizes for each of these studies and the high degree of inter-individual variability we had limited power to detect patterns of response to smoke exposure.
During these exposure studies, subjects were exposed to elevated levels of wood smoke particulate matter representative of high exposure (acute) scenarios. In the two controlled wood stove exposure studies, individual exposures ranged from 1.15–1.97 mg/m3, while in the campfire study individual PM2.5 exposures ranged from 0.84–2.99 mg/m3. For reference, these levels are 24 to 85 times higher than the EPA's 24 hour standard for PM2.5 of 0.035 mg/m3. Levels of PM2.5 for wildland firefighters have been reported at 1.054 ± 0.415 mg/m3, which is comparable to the levels in our two exposure studies (Neitzel et al. 2009). Particulate exposure from wood burning cook stoves in developing countries have been measured from 0.097–3.50 mg/m3 (Naeher et al. 2007).
In previously reported results, an increase in urinary levoglucosan was observed in mice after exposure to wood smoke (Migliaccio et al. 2009), which is contradictory to the results reported here for humans following controlled wood smoke exposures. The mouse studies were conducted at higher PM2.5 concentrations than the human exposure studies (average PM2.5 exposure of 3.14–3.75 mg/m3 for the mouse studies compared to 1.3–1.5 mg/m3 for the human studies). In the mouse exposure studies, levoglucosan was detected in only one pre-exposure sample (n=14), while it was detected at low levels in every pre-exposure sample for all three human studies. This is likely due to differences in diet between mice and humans. The diet was much easier to control in the mouse studies. The food provided to the mice was analyzed for levoglucosan and none was detected, whereas the extent of levoglucosan from the human diet is unknown. It is also possible that there is a difference in metabolism or uptake of levoglucosan between mice and humans, as little is known about these mechanisms.
In a human pilot study previously reported, levoglucosan was measured in fourteen school children in Libby, MT, some of which had wood stoves located in their homes (Migliaccio et al. 2009). There was no statistical difference in urinary levoglucosan measured in children with or without a wood stove in their home. The average PM2.5 inside and outside of the school at the time of sample collection was 41.1 μg/m3 and 5.9 μg/m3, respectively. The average levoglucosan in the particulates inside the school was only 98.5 ng/m3 on the sample collection day. The PM2.5 levels used in the controlled exposure studies reported in this manuscript are more than 30 times higher than the ambient levels measured in Libby (Bergauff et al. 2009), and more than 200 times higher than the PM2.5 levels inside the school in Libby (Ward 2008). The levoglucosan measured in the campfire exposure studies reported here is 1000 times higher than the level measured inside the school. Based on the low exposures in the Libby school study previously reported and the strong influence of diet, we speculate that the results observed in that study were caused by dietary influences and are not likely correlated to wood smoke exposure.
There are additional potential limitations with the use of levoglucosan as a quantitative biomarker of exposure to wood smoke. Previously reported values of the ratio of levoglucosan to PM in fireplace emissions span a wide range between 0.8% and 26% (Fine et al. 2001; Fine et al. 2002; Fine et al. 2004). This ratio is dependent upon the type of wood burned, fuel moisture, combustion conditions and the type of combustion device. However, measurements of the ratio of levoglucosan to PM2.5 based upon ambient samples collected from wood smoke-dominated airsheds frequently exhibit less variability than the data from laboratory based studies. Ward et el reported a ratio of 4.2±0.5% from samples collected in Missoula during the 2003 fire season (Ward et al. 2006), whilst Neitzel et al reported a ratio of 8±4% in smoke from controlled burning of forests in Savannah, Georgia (Neitzel et al. 2009). In a community in Montana where wood smoke represented 81% of the wintertime PM2.5 mass, the ratio of levoglucosan to PM2.5 was 11.2±1.5% (Bergauff et al. 2009). After a wood stove changeout program in the same community, the ratio of levoglucosan to PM2.5 was 6.9±0.6% (Bergauff et al. 2009). Nevertheless, the use of urinary levoglucosan as a quantitative marker of exposure to wood smoke would be affected by variability in the levoglucosan emission factor, and would benefit from the simultaneous characterization of the levoglucosan content of the specific wood smoke. Additionally, levoglucosan is a component of tobacco smoke (Schumacher et al. 1977; Saint-Jalm 1981), so exposure to tobacco smoke would either need to be eliminated or corrected for through the determination of secondary biomarkers (e.g. cotinine).
Conclusion
These results suggest that there is not a consistent increase in urinary levoglucosan in humans following an exposure to wood smoke. In both of our controlled wood smoke studies, some subjects had an increase in urinary levoglucosan after smoke exposure, while other subjects exhibited higher levoglucosan levels before exposure. None of the urinary levoglucosan levels measured showed a correlation to PM2.5 or
levoglucosan exposure. Both studies also further confirm that there is a background level of levoglucosan present in all urine samples. Since most occupational or chronic PM2.5 exposures are at levels similar to or lower than those used in this study, detectable increases in urinary levoglucosan after biomass smoke exposures are unlikely.
The caramel study suggests that levoglucosan levels are subject to a strong short-term dietary influence. The average level of urinary levoglucosan measured after caramel consumption was more than five times higher than the average urinary levoglucosan measured after either of the two wood stove smoke exposure trials, suggesting that even a small amount of dietary levoglucosan will likely have a greater influence on urinary levoglucosan levels than exposure to wood smoke, even at high levels. While these complicating factors diminish the potential use of levoglucosan as a biomarker of biomass smoke exposure in community-wide studies, carefully controlled studies may prove to be useful in developing levoglucosan as a tool in controlled laboratory studies. Urinary levoglucosan has been shown to increase in mice after wood smoke exposure (Migliaccio et al. 2009), so it also could still be useful in studies with mice and potentially other animals where the diet is easily controlled or does not contain levoglucosan. After the dietary influence of levoglucosan is more completely characterized, it may be possible to carefully monitor the diet in the hours before sampling occurs to minimize or eliminate interference.
Acknowledgements
This research was supported by the Health Effects Institute (#4743-RFA04-4/06-4), NCRR (COBRE P20RR017670), the National Science Foundation Research Experience for Undergraduates (REU) program (#0649306), the National Institute of Environmental Health Sciences (1R01ES016336-01), and the National Institute of Occupational Safety and Health (R03-OH007656).
References
- Bergauff M, Ward T, Noonan C, Palmer CP. Determination and evaluation of selected organic chemical tracers for wood smoke in airborne particulate matter. International Journal of Environmental Analytical Chemistry. 2008;88(7):473–486. [Google Scholar]
- Bergauff MA, Ward TJ, Noonan CW, Palmer CP. The effect of a woodstove changeout on ambient levels of PM2.5 and chemical tracers for woodsmoke in Libby, Montana. Atmospheric Environment. 2009 doi:10.1016/j.atmosenv.2009.02.055. [Google Scholar]
- Boman BC, Forsberg AB, Jaervholm BG. Adverse health effects from ambient air pollution in relation to residential wood combustion in modern society. Scandinavian Journal of Work, Environment & Health. 2003;29(4):251–260. doi: 10.5271/sjweh.729. [DOI] [PubMed] [Google Scholar]
- Clark M, Paulsen M, Smith KR, Canuz E, Simpson CD. Urinary Methoxyphenol Biomarkers and Woodsmoke Exposure: Comparisons in Rural Guatemala with Personal CO and Kitchen CO, Levoglucosan, and PM2.5. Environmental Science & Technology. 2007;41(10):3481–3487. doi: 10.1021/es061524n. [DOI] [PubMed] [Google Scholar]
- Dills RL, Paulsen M, Ahmad J, Kalman DA, Elias FN, Simpson CD. Evaluation of Urinary Methoxyphenols as Biomarkers of Woodsmoke Exposure. Environmental Science & Technology. 2006;40(7):2163–2170. doi: 10.1021/es051886f. [DOI] [PubMed] [Google Scholar]
- Dills RL, Zhu X, Kalman DA. Measurement of Urinary Methoxyphenols and Their Use for Biological Monitoring of Wood Smoke Exposure. Environmental Research. 2001;85(2):145–158. doi: 10.1006/enrs.2000.4107. [DOI] [PubMed] [Google Scholar]
- Dorland L, Wadman SK, Fabery de Jonge H, Ketting D. 1,6-Anhydro-b-D-glucopyranose (b-glucosan), a constituent of human urine. Clinica Chimica Acta. 1986;159(1):11–16. doi: 10.1016/0009-8981(86)90161-0. [DOI] [PubMed] [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical Characterization of Fine Particle Emissions from Fireplace Combustion of Woods Grown in the Northeastern United States. Environmental Science and Technology. 2001;35(13):2665–2675. doi: 10.1021/es001466k. [DOI] [PubMed] [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical Characterization of Fine Particle Emissions from the Fireplace Combustion of Woods Grown in the Southern United States. Environmental Science and Technology. 2002;36(7):1442–1451. doi: 10.1021/es0108988. [DOI] [PubMed] [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical Characterization of Fine Particle Emissions from the Wood Stove Combustion of Prevalent United States Tree Species. Environmental Engineering Science. 2004;21(6):705–721. [Google Scholar]
- Fraser MP, Lakshmanan K. Using Levoglucosan as a Molecular Marker for the Long-Range Transport of Biomass Combustion Aerosols. Environmental Science and Technology. 2000;34(21):4560–4564. [Google Scholar]
- Hays MD, Geron CD, Linna KJ, Smith ND, Schauer JJ. Speciation of Gas-Phase and Fine Particle Emissions from Burning of Foliar Fuels. Environmental Science and Technology. 2002;36(11):2281–2295. doi: 10.1021/es0111683. [DOI] [PubMed] [Google Scholar]
- Hedberg E, Johansson C, Johansson L, Swietlicki E, Brorstrom-Lunden E. Is levoglucosan a suitable quantitative tracer for wood burning? Comparison with receptor modeling on trace elements in Lycksele, Sweden. Journal of the Air & Waste Management Association. 2006;56(12):1669–78. doi: 10.1080/10473289.2006.10464572. [DOI] [PubMed] [Google Scholar]
- Helsel DR. More than obvious: better methods for interpreting nondetect data. Environ. Sci. Technol. FIELD Full Journal Title:Environmental Science and Technology. 2005;39(20):419A–423A. doi: 10.1021/es053368a. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, Trout M, Murby J, Barton C, Symons B. Assessing urinary levoglucosan and methoxyphenols as biomarkers for use in woodsmoke exposure studies. Science of the Total Environment. 2008;402(1):139–146. doi: 10.1016/j.scitotenv.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. FIELD Full Journal Title:Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- Jaffe DH, Singer ME, Rimm AA. Air pollution and emergency department visits for asthma among Ohio Medicaid recipients, 1991–1996. Environmental Research. 2003;91(1):21–28. doi: 10.1016/s0013-9351(02)00004-x. [DOI] [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities Study. American Journal of Respiratory and Critical Care Medicine. 2006;173(6):667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Baumann K, Schauer James J, Sheesley Rebecca J, Naeher Luke P, Meinardi S, Blake Donald R, Edgerton Eric S, Russell Armistead G, Clements M. Gaseous and particulate emissions from prescribed burning in Georgia. Environ Sci Technol FIELD Full Journal Title:Environmental science & technology. 2005;39(23):9049–56. doi: 10.1021/es051583l. [DOI] [PubMed] [Google Scholar]
- Migliaccio CT, Bergauff MA, Palmer CP, Jessop F, Noonan CW, Ward TJ. Urinary Levoglucosan as a Biomarker of Wood Smoke Exposure: Observations in a Mouse Model and in Children. Environmental Health Perspectives. 2009;117(1):74–79. doi: 10.1289/ehp.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhalation Toxicology. 2007;19(1):67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Needham LL, Calafat AM, Barr DB. Uses and issues of biomonitoring. International Journal of Hygiene and Environmental Health. 2007;210(3–4):229–238. doi: 10.1016/j.ijheh.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Nehls GJ, Akland GG. Procedures for handling aerometric data. Journal of the Air Pollution Control Association. 1973;23:180. [Google Scholar]
- Neitzel R, Naeher L, Paulsen M, Dunn K, Stock A, Simpson C. Biological monitoring of smoke exposure among wildland firefighters: A pilot study comparing urinary methoxyphenols with personal exposures to carbon monoxide, particulate matter, and levoglucosan. Journal of Exposure Science and Environmental Epidemiology. 2009;19:349–358. doi: 10.1038/jes.2008.21. [DOI] [PubMed] [Google Scholar]
- Ratsimba V, Fernandez JMG, Defaye J, Nigay H, Voilley A. Qualitative and quantitative evaluation of mono- and disaccharides in D-fructose, D-glucose and sucrose caramels by gas-liquid chromatography-mass spectrometry Di-D-fructose dianhydrides as tracers of caramel authenticity. Journal of Chromatography, A. 1999;844(1–2):283–293. doi: 10.1016/s0021-9673(99)00322-2. [DOI] [PubMed] [Google Scholar]
- Saint-Jalm Y. Qualitative analysis of the hydroxyl fraction of cigarette smoke. Annales du Tabac Sect. 1. 1981;18:41–48. [Google Scholar]
- Schumacher JN, Green CR, Best FW, Newell MP. Smoke composition. An extensive investigation of the water-soluble portion of cigarette smoke. Journal of Agricultural and Food Chemistry. 1977;25(2):310–20. doi: 10.1021/jf60210a003. [DOI] [PubMed] [Google Scholar]
- Simoneit BRT, Schauer JJ, Nolte CG, Oros DR, Elias VO, Fraser MP, Rogge WF, Cass GR. Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmospheric Environment. 1998;33(2):173–182. [Google Scholar]
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Annals of Allergy, Asthma, & Immunology. 2003;91(4):346–353. doi: 10.1016/S1081-1206(10)61681-X. [DOI] [PubMed] [Google Scholar]
- USEPA National ambient air quality standards for particulate matter: Final Rule. Federal Register. 2006;71(200):61143–61233. [Google Scholar]
- Ward TJ, Hamilton RF, Dixon RW, Paulsen M, Simpson CD. Characterization and evaluation of smoke tracers in PM: results from the 2003 Montana wildfire season. Atmospheric Environment. 2006;40(36):7005–7017. [Google Scholar]
- Ward TJ, Palmer C, Bergauff M, Hooper K, Noonan C. Results of a Residential Indoor PM2.5 Sampling Program Before and After a Woodstove Changeout. Indoor Air. 2008;18:408–415. doi: 10.1111/j.1600-0668.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Williams PL, MacIntosh DL. A comparison of two direct-reading aerosol monitors with the federal reference method for PM2.5 in indoor air. Atmospheric Environment. 2002;36:107–113. [Google Scholar]