Abstract
De novo lipogenesis (DNL) is a complex yet highly regulated metabolic pathway, and transcription factors such as liver X receptor (LXR), sterol regulatory element-binding protein-1c (SREBP-1c), and carbohydrate response element binding protein (ChREBP) exert significant control over the de novo synthesis of fatty acids. An increase in de novo lipogenesis (DNL) is an important contributor to increased fat mass, while a reduction in lipogenesis may be protective against the development of obesity. In this review, we explore fatty acid synthesis in the context of new insights gleaned from global and tissue-specific gene knockout mouse models of enzymes involved in fatty acid synthesis, namely acetyl-CoA carboxylase, fatty acid synthase, fatty acid elongase 6, and stearoyl-CoA desaturase 1. A disruption in fatty acid synthesis, induced by the deficiency of any one of these enzymes, affects lipid metabolism and in some cases may protect against obesity in a tissue and gene-specific manner, as discussed in detail in this review.
Keywords: de novo lipogenesis, LXR, SREBP-1c, ChREBP, acetyl-CoA carboxylase, fatty acid synthase, elongase 6, stearoyl-CoA desaturase
Introduction
The prevalence of obesity is alarmingly high, and the proportion of adults who are overweight and obese in the United States and elsewhere continues to increase. Recent estimates indicate that, with only one exception, every state in the United States has an estimated prevalence of obesity of at least 20% (CDC, 2008a). The obesity burden is also increasing in developing nations, leading to a particularly complex public health problem, as undernutrition may often coexist. Obesity and diabetes have emerged as major chronic diseases afflicting adults, and more recently, children in increasing proportions. It is well known that genetic (O’Rahilly, 2009; Lusis et al., 2008) and lifestyle factors (Magkos et al., 2009) and importantly, interactions among such factors (Bouchard, 2008; Sampath and Ntambi, 2004; Qi and Cho, 2008), contribute to the susceptibility of the development of metabolic diseases. The complexity of interactions among dietary and genetic factors is becoming more appreciated, and much research has been undertaken in recent years to probe various interactions between diets and genes, which has allowed for a greater understanding of the intricacy of this field of research.
With the current obesity epidemic, it is of immediate importance to understand the contribution of key cellular components as well as specific tissues involved in the development of impaired metabolic regulation. This is important not only in order to understand more fully the complex metabolic relationships that exist among cellular factors in a number of tissues, but also because the development of pharmacological agents to treat or to prevent obesity and other related metabolic diseases is an active area of research (Shi and Burn, 2004; Powell, 2006). Many studies have unmasked important roles that proteins expressed in specific tissues play in the development of or protection from metabolic diseases such as obesity and diabetes. Although metabolic tissues such as liver, muscle, and adipose are most commonly implicated as primary tissues involved in the development of insulin resistance, dyslipidemia, and obesity, other tissues have more recently come to light as possessing important roles in metabolism and in the maintenance of metabolic homeostasis.
Many recent studies have been undertaken in an effort to understand better the importance of specific tissues and genes involved in metabolic regulation, and this research thrust has lead to the development of several tissue-specific knockout or tissue-specific overexpressing mouse models of genes that encode proteins involved in lipogenesis. The development of tissue-specific knockout mouse models is commonly achieved via use of the Cre-loxP system in which Cre recombinase, an enzyme endogenously expressed in bacteriophage P1, is transgenically expressed under the control of a tissue-specific promoter (see reviews by Sauer, 1998; Kos, 2004). Antisense oligonucleotide (ASO) technology is another method that allows for transient, selective reduction of gene expression. This method takes advantage of RNase H, an enzyme that recognizes and degrades single-stranded mRNA bound to single-stranded DNA (Scherer and Rossi, 2003). Recent advancements in our understanding of lipid metabolism and susceptibility to the development of obesity have been largely gleaned from biochemical and nutritional studies carried out in mouse models that have been genetically modified by these mechanisms. Thus, technological advancements in recent decades have enabled genetic design of mouse models that have subsequently allowed for an impressive array of metabolic, nutritional, and diet-gene interaction experimental studies to be conducted while studying specific genes of interest.
A number of reviews describe various metabolic diseases, namely obesity, diabetes, and the clustering of metabolic disturbances known as metabolic syndrome, and their related complications (Hotamisligil, 2006; Lusis et al., 2008; Postic and Girard, 2008; Muoio and Newgard, 2006). In this review, we seek to highlight recent discoveries that have allowed for a more refined understanding regarding the roles of gene products within specific tissues in the regulation of mammalian metabolic processes, with a focus on diet-induced obesity. First, we review de novo lipogenesis (DNL) and a number of primary regulators of DNL including insulin and lipogenic transcription factors including liver X receptors (LXR), sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate response element binding protein (ChREBP). We then discuss specific genes and results of studies in which global or tissue-specific gene expression was assessed in the context of diet-induced obesity (Table 1). Specifically, we focus on genes/gene products that are involved in the synthesis of fatty acids and begin with ATP-citrate lyase, which catalyzes a reaction that yields acetyl-CoA and oxaloacetate, and continue through the pathway of fatty acid biosynthesis to the point of fatty acid desaturation by stearoyl-CoA desaturase. However, it is important to note that DNL indeed requires a number of additional proteins, including enzymes that catalyze NADPH-producing reactions.
Table 1.
Knockout mouse models of enzymes involved in synthesis of fatty acids and triglycerides
| Protein | Knockout mouse model | Reference |
|---|---|---|
| ATP-citrate lyase (ACL) | Global | Beigneux et al., 2004 |
|
| ||
| Acetyl-CoA carboxylase-1 (ACC1) |
Global | Abu-Elheiga et al., 2005 |
| Liver |
Mao et al., 2006; Harada et al., 2007 |
|
| Adipose, macrophages, bone marrow |
Mao et al., 2009 | |
|
| ||
| Acetyl-CoA carboxylase-2 (ACC2) |
Global |
Abu-Elheiga et al., 2001; Abu-Elheiga et al., 2003; Oh et al., 2005; Choi et al., 2007 |
|
| ||
| Fatty acid synthase (FAS) | Global | Chirala et al., 2003 |
| Liver | Chakravarthy et al., 2005 | |
| Hypothalamus, β-cells | Chakravarthy et al., 2007; 2009 |
|
|
| ||
| Elongase 6 (ELOVL6) | Global | Matsuzaka et al., 2007 |
|
| ||
| Stearoyl-CoA desaturase-1 (SCD1) |
Global |
Miyazaki et al., 2001; Ntambi et al., 2004 |
| Liver | Miyazaki et al., 2007 | |
| Skin | Sampath et al., 2009 | |
|
| ||
| Acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) |
Global | Smith et al., 2000; Shih et al., 2009 |
|
| ||
| Acyl-CoA:diacylglycerol acyltransferase-2 (DGAT2) |
Global | Stone et al., 2004 |
|
| ||
| Acyl-CoA:glycerol-3- phosphate acyltransferase- 1 (GPAT1) |
Global |
Hammond et al., 2002; Hammond et al., 2005; Neschen et al., 2005 |
|
| ||
| Acyl-CoA:glycerol-3- phosphate acyltransferase- 4 (GPAT4) |
Global | Vergnes et al., 2006; Nagle et al., 2008 |
|
| ||
| 1-acylglycerol-3-phosphate acyltransferase-2 (AGPAT2) |
Global | Cortes et al., 2009 |
Lipogenesis and diet-induced obesity
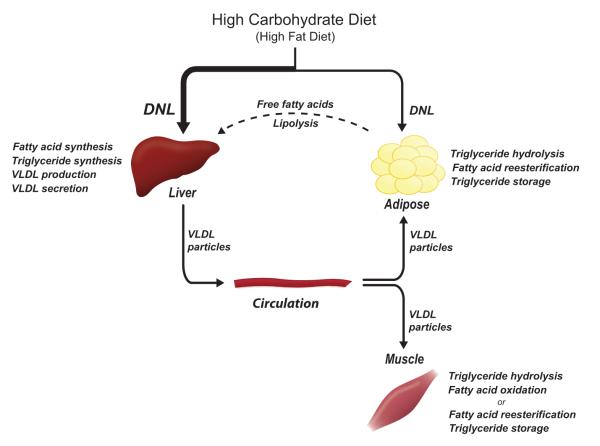
Carbohydrates consumed in excess of caloric requirements and in excess of the hepatic glycogen storage capacity must be converted into lipids for subsequent storage; white adipose tissue (WAT) is the primary lipid-storing tissue. De novo lipogenesis is the metabolic pathway that synthesizes fatty acids from excess carbohydrates; these fatty acids can then be incorporated into triglycerides for energy storage. Importantly, hepatic DNL and triglyceride synthesis following a high carbohydrate load can lead to an increase in the synthesis and secretion of very low density lipoproteins (VLDL) particles (Figure 1). VLDL particles enter the circulation, and the triglycerides can undergo hydrolysis within capillaries of extrahepatic tissues, of which adipose and muscle are primary tissues. The free fatty acids may be reesterified and stored in triglycerides or can be oxidized for energy. During fasting, hydrolysis of adipose tissue triglycerides occurs, liberating fatty acids that can be taken up by the liver. Such adipose tissue-derived free fatty acids can be reincorporated into triglycerides and secreted in VLDL particles (Figure 1). Barrows and Park (2006) demonstrated that adiposederived free fatty acids contribute significantly to triglyceride and VLDL formation in both the fed and fasted states.
Figure 1.
De novo lipogenesis occurs in both liver and adipose tissue and can be stimulated by high carbohydrate and high fat diets. Dietary, de novo synthesized, and adipose tissue-derived fatty acids are incorporated into triacylglycerol, packaged into VLDL particles and secreted by the liver. Fatty acids are hydrolyzed from triacylglycerol molecules in peripheral tissues such as adipose and muscle. DNL, de novo lipogenesis; VLDL, very low density lipoprotein. Color version of figure is available online.
In addition to high-carbohydrate diets, high-fat diet has been shown to activate a lipogenic response in liver tissue. Recent work has shown that PGC-1β, a member of the PGC-1 family of transcriptional coactivators, is important in mediating the metabolic effects of dietary fat (Lin et al., 2005a; Lin et al., 2005b) as well as high-fructose containing diets (Nagai et al., 2009). Importantly, PGC-1β over-expression has been shown to induce the hepatic expression of several lipogenic enzymes involved in the de novo synthesis of fatty acids and other lipids (Lin et al., 2005a) while PGC-1β knockdown reduced the expression of lipogenic genes (Nagai et al., 2009). High-fat diets contribute to obesity, as the caloric excess must be stored; adipose tissue expands to accommodate this increase in exogenous lipids and endogenous lipid synthesis. The regulation of lipogenesis is a highly regulated process and important regulators of this pathway will be reviewed here.
Role of liver X receptors
Liver X receptors (LXR) are members of the nuclear receptor superfamily that heterodimerize with retinoid X receptor (RXR) (see review by Chawla et al., 2001). Two isoforms of LXR have been identified, LXRα and LXRβ (Willy et al., 1995; Janowski et al., 1996; Song et al., 1994). Oxysterols are the endogenous ligands for activation of LXRs (Janowski et al., 1996), and activated LXRs have been shown to regulate expression of genes important in cholesterol metabolism as well as fatty acid synthesis. LXRs have also been shown to respond to fatty acid treatment (Tobin et al., 2000). LXRα, but not LXRβ, has been shown to positively regulate itself transcriptionally (Laffitte et al., 2001).
Repa and colleagues (2000) demonstrated an important role for LXRs in regulation of fatty acid synthesis. Their work showed that in the absence of LXRα and LXRβ (LXRα/β double knockout mice), a blunted increase in SREBP-1c expression occurs when stimulated with dietary cholesterol alone or in addition to RXR ligand or LXR agonist (Repa et al., 2000). This was a significant discovery because SREBP-1c regulates the expression of many genes that code for enzymes involved in fatty acid and lipid biosynthesis (see below). Additionally, LXRs have also been shown to directly activate lipogenic genes in a distinct manner from their ability to activate SREBP-1c. Fatty acid synthase (FAS) was one of the first such genes shown to be transcriptionally regulated by both LXRα and LXRβ (Joseph et al., 2002). Microarray analysis of gene expression in liver, WAT and brown adipose tissue (BAT) following LXR agonist treatment in mice revealed several additional genes important for de novo fatty acid that are likely direct targets of the LXRs (Figure 2) (Stuling et al., 2002). The functions and effects of LXRs are now recognized to be wide-ranging in terms of gene regulation and tissue specificity (see review by Steffensen and Gustafsson, 2004).
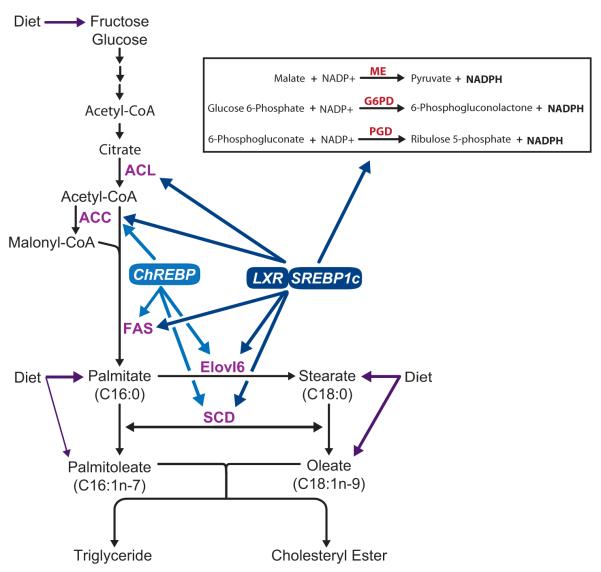
Figure 2.
Pathway of de novo synthesis of saturated and monounsaturated fatty acids and regulation of lipogenic genes by transcription factors (LXR, SREBP-1c and ChREBP). NADPH is required for fatty acid synthesis and three chemical reactions yield NADPH (inset). PGC-1β has been shown to coactivate LXR and SREBP-1c, as discussed in the text. ACL, ATP-citrate lyase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; ELOVL6, elongation of long-chain fatty acids family member 6 (elongase 6); SCD, stearoyl-CoA desaturase; LXR, liver X receptor; SREBP-1c, sterol regulatory element-binding protein 1c; ChREBP, carbohydrate response element binding protein; ME, malic enzyme; G6PD, glucose 6-phosphate dehydrogenase; PGD, Phosphogluconate dehydrogenase. Color version of figure is available online.
Role of sterol regulatory element-binding proteins
In addition to LXRs, insulin and glucagon are also intimately involved in the regulation of DNL (Horton et al., 2002). Their control of the de novo lipogenic pathway was discovered to occur largely through their effects on the family of sterol regulatory element-binding proteins (SREBP). The SREBPs are a group of three transcription factors (SREBP-1a, SREBP-1c and SREBP-2) that are all members of the basic helix-loop-helix-leucine zipper protein family; SREBPs are subjected to both transcriptional and post-transcriptional regulation. SREBP-1a and SREBP-1c are expressed from the same gene, but they possess different promoters and transcriptional start sites, which result in different forms of exon 1; SREBP-2 is expressed from a separate gene (Horton et al., 2002). SREBP-1c is the primary form expressed in human and mouse liver, while cell culture models tend to predominantly express the SREBP-1a form (Shimomura et al., 1997). SREBP-2 preferentially regulates expression of enzymes involved in cholesterol biosynthesis (Horton et al., 1998; 2003), and SREBP-1c is primarily involved in regulation of genes related to fatty acid and triglyceride synthesis including, but not limited to, ATP-citrate lyase (ACL), acetyl-CoA Carboxylase (ACC), FAS, stearoyl-CoA desaturase (SCD), and glyceraldehyde-3-phosphate acyltransferase (GPAT) (Figure 2) (Shimomura et al., 1998; Horton et al., 1998; 2002). SREBP-1a, however, has been shown to activate the expression of genes in both pathways, although it is typically present at low levels in vivo (Shimomura et al., 1998; Horton et al., 2002). In addition, all SREBP isoforms increase the expression of genes that encode enzymes that catalyze NADPH-yielding reactions, which is important as NADPH supplies the requisite reducing equivalents for cholesterol and fatty acid synthesis (Shimomura et al., 1998).
The SREBPs function as transcription factors, but they are initially produced as large (~125 kDa) precursors that reside in the endoplasmic reticulum (ER) (see review by Goldstein et al., 2006). SREBP cleavage-activating protein (SCAP) also resides in the ER and functions as an escort protein in the translocation of SREBP from the ER to the Golgi for processing. SCAP binds sterols when they are abundant in the ER membrane. Upon binding sterol, SCAP undergoes a conformational change and subsequently binds yet another important protein involved in SREBP regulation, an Insig (insulin-induced gene) protein. When SCAP binds to Insig, it cannot bind to COPII proteins and therefore cannot escort SREBP to the Golgi for processing. However, in the absence or low abundance of sterols, SCAP does not bind sterols and thus its conformational shape allows it to bind COPII proteins. The binding of SCAP to COPII proteins allows the SCAP-SREBP complex to travel to the Golgi where SREBP undergoes proteolytic cleavage at two sites. The Site-1 protease and Site-2 protease proteins cleave SREBPs thereby processing them into their active N-terminal fragment transcription factors. It is not known if SCAP or other proteins involved in SREBP processing contain fatty acid binding domains that would sense cellular levels in a similar manner as with sterols.
High-carbohydrate diets induce DNL, as a majority of excess carbohydrates that are not oxidized for energy are used as substrates to synthesize fatty acids and are then incorporated into triglycerides for storage. Furthermore, it has been shown in humans that an increase in carbohydrate consumption increases fatty acid synthesis even when the caloric content of the diet is maintained (Hudgins et al., 1996). In addition to the post-transcriptional regulation mediated through SCAP, hormonal regulation of SREBP-1c can occur through insulin and glucagon. A fasting-refeeding (FRF) program, in which a period of fasting (typically 24 hours in many experimental designs) is followed by a period of refeeding a low-fat high-carbohydrate diet, induces very high fatty acid biosynthetic gene expression (Horton et al., 1998b; Shimomura et al., 2000; Sampath et al., 2007). This dietary program also alters the insulin blood profile, suggestive of hormonal regulation of SREBP-1c, as it is a major transcriptional regulator of many genes induced by the FRF program. Primary hepatocyte experiments demonstrated that a dominant active form of SREBP-1c induced glucokinase expression to a similar extent as insulin treatment (Foretz et al., 1999). Furthermore, rats treated with streptozotocin, a chemical that destroys pancreatic β-cells, have dramatically reduced levels of SREBP-1c mRNA in addition to both precursor and nuclear forms of SREBP-1 protein, and insulin treatment restores both mRNA and protein levels (Shimomura et al., 1999). Interestingly, mice that become insulin resistant continue to be sensitive to this hormone’s positive regulation of lipogenic gene expression and thus fatty acid and triglyceride synthesis can proceed during a state of insulin resistance (Shimomura et al., 2000).
Since the work describing the effect of insulin on SREBP-1c expression, it has been demonstrated that LXR is important in mediating this powerful effect of insulin (Chen et al., 2004). Reporter constructs were used to show that mutation of the LXREs in the promoter significantly reduced insulin’s ability to activate SREBP-1c transcription (Chen et al., 2004). Regulation of SREBP-1c at the transcriptional level also occurs through LXRs, which stimulate SREBP-1 gene expression through an LXRE located in the promoter of SREBP-1c (Horton et al., 2002). SREBP-1c expression is significantly reduced in mice deficient in both LXRα and LXRβ; however, deficiency of only LXRα slightly reduces SREBP-1c mRNA while there is no effect when only LXRβ is lacking (Repa et al., 2000). Additionally, high-fat diets induce lipogenesis, with an increase in SREBP-1 activity with concomitant increased expression of SREBP downstream targets (Lin et al., 2005; Sampath et al., 2007). The transcriptional coactivator PGC-1β has been shown to play a significant role in mediating such lipogenic effects of fatty acids (Lin et al., 2005). High-fat diet induces SREBP-1c expression, and it is likely that LXR may be involved in this phenomenon (Lin et al., 2005). Thus several studies have shown a role for SREBP-1 as a key mediator in the induction of lipogenesis during experimental caloric excess, regardless of whether the calories are derived largely from dietary carbohydrates or dietary fats.
Role of carbohydrate responsive element binding protein
The role of SREBPs in mediating the regulation of lipogenic gene expression was conclusively demonstrated in a number of studies. However, there was also evidence to suggest that SREBP-1c was not the sole transcription factor involved in the regulation of the expression of these genes induced by carbohydrates. In fasted and refed SREBP-1c deficient mice, expression of SREBP-1c target genes was reduced compared to wild-type (WT) mice but expression was not completely eliminated (Shimano et al., 1999). Furthermore, studies conducted in the early 1990’s provided evidence that a specific, glucose-responsive element was present in the promoter region of lipogenic genes (Bergot et al., 1992; Cuif et al., 1993; Doiron et al., 1996), but it was not until 2001 that the glucose-responsive transcription factor that binds specifically to the glucose responsive element was reported (Yamashita et al., 2001). Similar to the SREBPs, ChREBP is also a basic helix-loop-helix leucine zipper transcription factor that is expressed in a number of tissues including liver, WAT, BAT, kidney, and small intestine (Yamashita et al., 2001; Iizuka et al., 2004). Subsequently, Max-like protein X (Mlx) was identified as the protein partner that ChREBP heterodimerizes with and binds to E boxes with in promoters of target genes (Stoeckman et al., 2004). It was also shown that mice deficient in ChREBP have reduced expression of a number of lipogenic genes. Importantly, however, the lack of ChREBP does not affect the expression of any members of the SREBP family (Iizuka et al., 2004). The ability to synthesize fatty acids in liver is also significantly impaired in ChREBP deficient mice (Iizuka et al., 2004).
ChREBP has been shown to be transcriptionally regulated by LXRs, as chromatin immunoprecipitation and luciferase reporter-based experiments demonstrated that LXRs bind to LXR elements in the promoter of ChREBP (Cha and Repa, 2007). However, a more recent report demonstrated that an appropriate lipogenic response to glucose/high-carbohydrate diet occurs even in LXRα/β-deficient mice (Denechaud et al., 2008). Specifically, nuclear ChREBP levels increased as did the expression of several ChREBP target genes including liver-pyruvate kinase, ACC, and FAS (Figure 2). SCD and GPAT, however, increased in WT mice but not in LXRα/β knockout mice in response to the high-carbohydrate diet (Denechaud et al., 2008). These data suggest that LXRs may not be required for the ability of ChREBP to induce all of its target genes in response to glucose metabolism.
In addition to transcriptional regulation, the activation and deactivation of ChREBP requires posttranslational regulation. One hypothesis proposes that the activation of ChREBP into a nuclear-localized, functional transcription factor occurs via sequential dephosphorylation steps at residues that have been phosphorylated by protein kinase A. This hypothesis suggests that xylulose 5-phosphate, a glucose metabolite, activates protein phosphatase 2A (PP2A), which dephosphorylates ChREBP on its serine 196 residue; ChREBP can then enter the nucleus upon this dephosphorylation-dependent activation. PP2A then dephosphorylates the threonine 666 residue of nuclear ChREBP, thus activating ChREBP (see review by Postic et al., 2007). In contrast to this hypothesis, data from Towle and colleagues have shown in primary rat hepatocytes that high glucose treatment does not reduce the phosphorylation state of ChREBP (Tsatsos and Towle, 2008).
Tissue-specific regulation of diet-induced obesity
As compared to global knockout models, the development of tissue-specific knockout mouse models has allowed for more finely tuned research to explore the relationship between diet and metabolic disease in a tissue-specific manner in order to understand complex metabolic regulation more thoroughly. Tissue-specific models are especially important in metabolic studies, which allow for the contribution of single tissues in the maintenance of metabolic homeostasis to be realized. Protection from obesity can typically be attributed to increased nutrient oxidation, increased thermogenesis, increased activity, and reduced lipogenesis. While liver, adipose, and skeletal muscle tissues are commonly recognized as the key metabolic tissues, recent studies have uncovered roles for other tissues in metabolic homeostasis, as will be discussed in more detail below. We specifically focus on reviewing global and tissue-specific gene knockout mouse models of enzymes involved in fatty acid synthesis but also refer the reader to references of knockout mouse models of enzymes important for triglyceride synthesis (Table 1).
Acetyl-CoA Carboxylase
The cytosolic synthesis of acetyl-CoA from citrate, a reaction catalyzed by ATP-citrate lyase (ACL) is essential during embryogenesis, as an attempt to make mice deficient in this enzyme did not yield any homozygous knockout embryos or pups (Beigneux et al., 2004). However, heterozygous mice were viable and did not differ from WT littermates with respect to body weight, plasma and hepatic lipids, and lipogenic gene expression (Beigneux et al., 2004). Acetyl-CoA is a crucial component in lipid metabolism, as it is a requisite carbon donor in the de novo synthesis of fatty acids and supplies the first acyl group in a growing fatty acid chain and it is the substrate used by acetyl-CoA carboxylase (ACC) to catalyze the synthesis of malonyl-CoA (Figure 2). Malonyl-CoA is essential for fatty acid synthesis, because it donates two carbon units during each sequential round of condensation in the synthesis of sixteen-carbon fatty acid chains. However, the function of malonyl-CoA in lipid metabolism is two-fold: it also serves to inhibit the mitochondrial oxidation of fatty acids by binding to and blocking carnitine palmitoyltransferase (CPT1) (Saggerson, 2008). CPT1 is required for the synthesis of long chain fatty acyl carnitine molecules, the fatty acid form required for fatty acid transport across mitochondrial membrane to be utilized for β-oxidation. Two isoforms of ACC exist in animals, ACC1 and ACC2, and different genes encode for them (Brownsey et al., 2006). It has been demonstrated that ACC1 is located in the cytosol and the malonyl-CoA produced by ACC1 is important for the de novo synthesis of palmitate, whereas ACC2 is localized to mitochondria and the malonyl-CoA it produces is important in fatty acid oxidation (Abu-Elheiga et al., 2000; Mao et al., 2006). Thus, as expected, the ACC enzymes are highly regulated because the product of the reaction catalyzed by ACC is a crucial molecule involved in the determination of whether fatty acids are synthesized or oxidized (see reviews by Brownsey et al., 2006; Kim, 1997). Therefore, the control of ACC and production of malonyl-CoA is a crucial step in lipid metabolism that may be important in the development of metabolic disorders (Wakil and Abu-Elhegia, 2009).
Global knockout (GKO) mouse models of the two ACC isoforms have provided strong evidence for the hypothesis that ACC1 and ACC2 perform independent functions and largely do not compensate for each other when one isoform is absent. Whole body deficiency of ACC1 is lethal during early embryogenesis (Abu-Elheiga et al., 2005). In striking contrast to the deletion of ACC1, ACC2 null and heterozygous mice are born at expected frequency (Abu-Elheiga et al., 2001). Hepatic lipogenesis is not impaired in ACC2 GKO yet lipids do not accumulate in the liver, suggestive of increased fatty acid oxidation (Abu-Elheiga et al., 2001). Subsequent studies demonstrated that the oxidation of both fat and glucose is increased in these mice (Oh et al., 2005; Choi et al., 2007). Furthermore, ACC2 GKO mice are protected from high-fat/high-carbohydrate diet-induced obesity as well as insulin resistance (Abu-Elheiga et al., 2003; Choi et al., 2007). This suggests that the lack of malonyl-CoA produced by ACC2 confers the protection against obesity, as the synthesis of the molecule responsible for inhibition of CPT1 is itself inhibited, allowing fatty acid oxidation to occur. Although these data suggest that the malonyl-CoA produced from ACC1 and ACC2 are functionally distinct, recent evidence from ASO experiments is largely inconsistent with this hypothesis. Fatty acid oxidation is significantly increased when either isoform is selectively reduced or when both isoforms are simultaneously reduced (Savage et al., 2006). However, triglyceride synthesis was reduced only when ACC1 alone or ACC1 and ACC2 together were decreased but not when ACC2 alone was reduced. These results suggest that the malonyl-CoA produced from either isoform can regulate fatty acid oxidation but only malonyl-CoA produced by ACC1 is used for fatty acid synthesis (Savage et al., 2006).
More recently, studies have emerged on the tissue-specific importance of the ACC1 isoform in DNL. Liver-specific deletion of ACC1 (ACC1 LKO) resulted in reduced accumulation of lipids in liver tissue during chow-diet or short-term fat-free diet conditions. Additionally, the synthesis of cholesterol and fatty acids was significantly impaired in primary hepatocytes from these mice (Mao et al., 2006). Surprisingly, in ACC1 LKO, the hepatic expression of several genes that encode proteins involved in DNL including ACL, FAS, long chain fatty acid elongase, and PPARγ were all increased. In addition, ACC2 was increased and CPT1 was decreased (Mao et al., 2006). The gene expression and nuclear protein level of SREBP-1 was not reported but may be an important factor accounting for the increased expression of the lipogenic genes. It was hypothesized that this gene expression profile may be a metabolic response of the liver in an effort to increase the synthesis of fatty acids when they are limiting (Mao et al., 2006). Despite impaired hepatic DNL, total fat pad weights did not differ by genotype following short-term very low-fat diet feeding, which suggests that liver-specific ACC1 deficiency might not be protective against the development of obesity during a more chronic exposure to adverse dietary conditions. As expected, ACC1 LKO mice fed a high-fat/high-carbohydrate diet for five months were susceptible to diet-induced obesity and gained weight to a similar extent as the WT mice (Figure 3) (Mao et al., 2006). It should also be noted that under this chronic dietary treatment, hepatic steatosis was not prevented in ACC1 LKO. Overall, these data suggest that ACC1 is required for an appropriate hepatic lipogenic response to short-term feeding of high-carbohydrate diets, and the deficiency of ACC1 only in liver tissue is not sufficient to reduce fatty acid and triglyceride synthesis to protect against the obesigenic consequences of long-term consumption of a high-fat/high-carbohydrate diet.
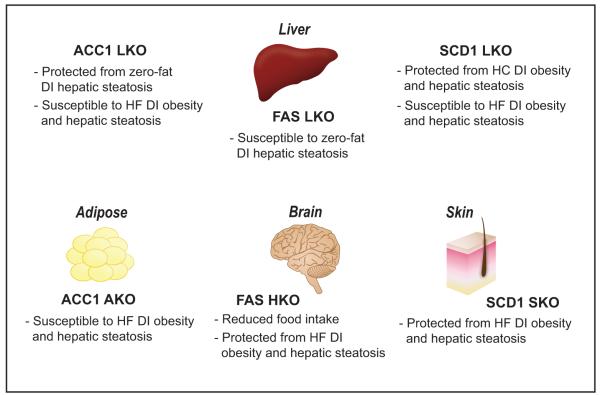
Figure 3.
Summary of metabolic phenotypes of tissue-specific gene knockout mouse models discussed in detail in text: ACC1, FAS, and SCD1 liver knockout (LKO) models, ACC1 adipose tissue knockout (AKO), FAS hypothalamus knockout (HKO), and SCD1 skin knockout (SKO). ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; SCD, stearoyl-CoA desaturase; HC, high carbohydrate; HF, high-fat; DI, diet-induced. Color version of figure is available online.
Harada et al. (2007) also explored the metabolic effects of hepatic ACC1 deficiency. Chow-fed ACC1 LKO mice did not exhibit altered hepatic lipid accumulation or impaired hepatic fatty acid synthesis relative to WT animals. However, after short-term high-sucrose feeding, liver triglycerides were dramatically reduced and hepatic DNL was slightly reduced in ACC1 LKO mice relative to WT mice (Harada et al., 2007). These results provide additional evidence to demonstrate that the lack of hepatic ACC1 may protect against the development of hepatic triglyceride accumulation induced by a short-term high-carbohydrate diet while the results by Mao et al. (2006) demonstrate that ACC1 LKO mice are not protected from high-fat diet-induced weight gain.
A recent report describes the metabolic effects of aP2-cre-mediated deficiency of ACC1 (ACC1 AKO) in a panel of tissues including WAT, BAT, peritoneal macrophages, and bone marrow (Mao et al., 2009). Although it will not be discussed in significant detail, it should be noted that profound growth and bone development defects are evident in ACC1 AKO mice, demonstrating that in addition to lipogenesis, ACC1 plays a role in other pathways, such as skeletal development (Mao et al., 2009). ACC1 AKO mice exhibited reduced cellular differentiation at growth plate chondrocytes as well as significantly reduced IGF-1 levels, both of which were likely to have had negative effects on the bone growth (Mao et al., 2009). Knockout mice (ACC1 AKO) weigh significantly less than the WT counterparts, however this reduction in body weight is not solely due to reduced fat mass, per se. Rather, aP2-cre positive mice exhibited an overall reduction in body size with significantly shorter bones, although they followed a post-weaning growth trajectory similar to WT mice (Mao et al., 2009). Despite reduced ACC1 mRNA and activity levels in adipose tissue, lipogenesis was not negatively impacted in ACC1 AKO mice under control or insulin-induced conditions. This lack of reduced lipogenesis may be due to inefficient Cre recombinase mediated recombination at loxP sites in the ACC1 gene (Mao et al., 2009). Although lipogenesis itself was not impaired, adipocytes in ACC1 AKO mice accumulated fewer lipids following a FRF dietary program. However, long-term consumption of a high-fat/high-carbohydrate diet induced weight gain, obesity, and hepatic lipid accumulation to a similar extent in ACC1 AKO and WT mice (Figure 3) (Mao et al., 2009).
Fatty Acid Synthase
Fatty acid synthase (FAS) is a large multi-subunit protein that synthesizes the sixteen-carbon saturated fatty acid palmitate through the sequential addition of two carbon units at a time, with the carbons donated by malonyl-CoA (Figure 2). The absolute requirement for FAS during embryonic development was first demonstrated during an effort undertaken to produce FAS GKO mice (Chirala et al., 2003). However, global FAS deficiency was discovered to be embryonically lethal and mice with a complete deficiency in the enzyme were not born. FAS heterozygotes displayed a high degree of lethality as well, with a reduction in expected frequency by approximately 70%. The requirement for de novo fatty acids to support embryonic development was demonstrated further by a study in which male and female FAS heterozygote breeders were fed a high-fat, lard based diet. Despite this dietary supplement of saturated fatty acids, only a minor increase in the number of heterozygote FAS mice born was observed (Chirala et al., 2003). These data imply that the fatty acids absorbed by the parents during the breeding period and the fatty acids absorbed by the mother during gestation were largely insufficient to support the development and growth of pups. These data may therefore suggest that fatty acids synthesized de novo and fatty acids provided through the diet are functionally distinct in vivo.
Chirala and coworkers (2003) clearly demonstrated that FAS is required for normal embryonic development; however, much remained to be understood regarding the specific role of FAS in maintenance of lipid metabolism homeostasis in the liver as well as in extrahepatic tissues. A liver-specific FAS knockout model (FAS LKO) has been produced and the metabolic effects described (Chakravarthy et al., 2005). FAS LKO mice have increased level of malonyl-CoA and decreased level of palmitate in the liver, as might be expected in the absence of the enzyme that utilizes malonyl-CoA to ultimately synthesize palmitate. Long-term (four weeks) zero-fat, high-carbohydrate diet feeding induced hypoglycemia with both low insulin and high glucagon levels. Fat pad weight was significantly reduced in FAS LKO mice compared to WT mice, which may be explained by reduced expression of SREBP-1c. Additionally, the lack of FAS in the liver caused hepatic steatosis when the zero-fat diet was fed (Figure 3). The increased accumulation of lipids in the liver may be due to the increase in malonyl-CoA levels and its negative regulation of fatty acid oxidation. The authors noted that the phenotype of FAS LKO mice is strikingly similar to that of whole-body PPARα deficient mice (Kersten et al., 1999).
New fat, that is, fat synthesized de novo or provided via dietary sources, was hypothesized to be the endogenous ligand for PPARα, as PPARα-regulated genes and metabolic pathways were blunted in the absence of hepatic FAS but restored following PPARα activation with a known PPARα agonist, Wy14,643. Furthermore, fatty acids liberated from adipose tissue largely failed to activate the receptor to induce gluconeogenic and fatty acid oxidative pathways. The mechanism by which new fatty acids, but not old fatty acids, would have access to PPARα is unknown but it was suggested that perhaps PPARα moves back and forth between the nucleus and the cytoplasm and subsequently binds “new fat” available in the cytoplasm (Chakravarthy et al., 2005). Palmitate is the primary product of the reaction catalyzed by FAS, and the hypothesis posed by this group therefore suggested that palmitate could be the specific fatty acid that serves as the endogenous PPARα ligand. However, more recent work, in which sophisticated mass spectrometry-based analyses were conducted, demonstrated that a more complex molecule, 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC) may be an important physiological ligand for PPARα (Chakravarthy et al., 2009). The identification of a molecule that incorporates one molecule of 16:0 and one of 18:1 is interesting in that these are the main products of FAS and SCD1, respectively, enzymes that catalyze rate-limiting steps in the synthesis of saturated and monounsaturated fatty acids, respectively, that are commonly incorporated into other complex lipid species as well.
In addition to its direct role in de novo fatty acid synthesis, FAS has also been shown to be important in food intake, originally demonstrated through the use of FAS chemical inhibitors (Loftus et al., 2000). Following intraperitoneal injection, radiolabeled FAS inhibitor was detected in the brain, and a significant reduction in food intake and body weight occurred. The physiological mechanisms by which reduced FAS activity leads to reduced food intake was determined to be through the accumulation of malonyl-CoA in the brain with subsequent protection conferred against the development of obesity (Loftus et al., 2000). The role of the central nervous system in regulation of food intake and metabolism is a rapidly expanding area of research (see reviews by Dowell et al., 2005; Wolfgang and Lane, 2006).
Additional insights regarding the mechanism of inhibition of FAS activity on metabolism have been obtained from a genetic mouse model in which FAS expression is disrupted in the hypothalamus and pancreatic β-cells (FAS HKO) (Chakravarthy et al., 2007; 2009). While there was no apparent phenotype specifically related to β-cell function, chow-fed FAS HKO mice gained significantly less weight than their WT counterparts (Figure 3). This reduction in body weight was largely determined to be due to their hypophagic feeding behavior but also perhaps also partially due to increased physical activity. Similar to the study by Loftus et al., hypothalamic malonyl-CoA increased in FAS HKO mice, and was lowered upon PPARα agonist treatment (Chakravarthy et al., 2007). As expected, treatment with PPARα agonist also induced expression of PPARα target genes in the hypothalamus, which were expressed to a higher degree than in WT mice, but food intake and body weight normalized to WT levels. These data suggest that a relationship exists among FAS, PPARα activation, and feeding behavior. Additionally, the results from this study suggested that the reduction of FAS activity in the hypothalamus may be a useful method to reduce food consumption and prevent diet-induced obesity, although not tested in this study (Chakravarthy et al., 2007).
Follow-up work conducted in the FAS HKO model did, however, test whether FAS deficiency in the hypothalamus protects against the development of obesity due to consumption of a high-fat diet (Chakravarthy et al., 2009). High-fat fed FAS HKO mice gained significantly less weight and were protected against obesity as compared to WT mice (Figure 3). Not only was fat mass significantly reduced, but lean body mass was increased. Similar to the response in chow-fed FAS HKO mice, high-fat fed mice lacking hypothalamic FAS consumed less food when it was provided ad libitum and had increased energy expenditure (Chakravarthy et al., 2009). Notably, FAS HKO mice were protected from hepatic steatosis, with significantly reduced accumulation of both triglycerides and cholesterol following long-term HFD feeding (Figure 3); hepatic expression of the gene encoding SREBP-1c was also dramatically reduced. It is striking that inactivation of a single enzyme in a specific region of the brain not only modulates behavior, but also imparts dramatic metabolic effects in peripheral tissues and protect against the development of various metabolic diseases.
Elongation of long-chain fatty acids family member 6
Palmitate is the final product of the series of reactions catalyzed by FAS. However, the major fatty acids that are used to synthesize other lipid compounds commonly include 18-carbon fatty acids as well as the 16-carbon chains; thus, 16-carbon chains must be elongated. Elongation of long-chain fatty acids family member 6 (ELOVL6) is an elongase that catalyzes the addition of two-carbon units primarily to 12, 14, and 16-carbon fatty acid chains (Figure 2) (see review by Matsuzaka and Shimano, 2009). ELOVL6 is highly expressed in several tissues including liver, BAT, WAT, brain, skin and kidney, among others (Moon et al., 2001; Matsuzaka et al., 2002). Numerous studies have demonstrated that ELOVL6, like many of the other fatty acid biosynthetic enzymes, is a target gene of the SREBP family of transcription factors (Moon et al., 2001; Matsuzaka et al., 2002; Kumadaki et al., 2008). Consistent with regulation by SREBPs, the expression of hepatic ELOVL6 is dramatically induced upon refeeding of a high-sucrose diet following a period of fasting (Matsuzaka et al., 2002). However, ELOVL6 expression induced by FRF is not completely eliminated in livers of SREBP-1 knockout mice, suggesting that other factors in addition to SREBP-1 are involved in the regulation of ELOVL6 expression (Matsuzaka et al., 2002).
Although the development of tissue-specific mouse models of ELOVL6 deficiency has not been reported, a global knockout mouse model of ELOVL6 (ELOVL6 GKO) has been produced and recently described (Matsuzaka et al., 2007). ELOVL6 null mice display partial embryonic lethality; however, metabolic characterization of surviving ELOVL6 GKO mice was conducted. Relative to WT mice, ELOVL6 GKO mice maintained slightly lower body weights when fed either chow or high-fat/high-sucrose diets over the course of long-term feeding studies, with a body weight reduction of approximately 10%. The expression of several lipogenic as well as oxidative genes was dramatically blunted in livers of ELOVL6 GKO mice (Matsuzaka et al., 2007). Primary hepatocyte experiments further demonstrated that both fatty acid synthesis and fatty acid oxidation was impaired in ELOVL6 GKO mice. Nuclear ChREBP protein was not assessed, but the mature, nuclear form of SREBP-1 was determined to be lower in livers of ELOVL6 GKO, which would at least partially explain reduced expression of lipogenic genes. Surprisingly, lipids tended to accumulate more in ELOVL6 GKO livers as compared to WT.
Fatty acid analyses revealed significant accumulation of 16-carbon fatty acids, including 16:0 and 16:1n-7, concomitantly with reductions in 18:0 and 18:1n-9 in both plasma and liver tissue in ELOVL6 GKO mice relative to WT mice (Matsuzaka et al., 2007). The significant enrichment of 16:1n-7 in liver and plasma of Elovl6 GKO mice is reminiscent of the fatty acid profiles of adipose tissue and plasma of mice lacking fatty acid binding proteins (FABP GKO) 4 and 5 (Cao et al., 2008). FABP GKO mice are less susceptible to diet-induced obesity and hepatic lipid accumulation and are more insulin sensitive as compared to WT mice (Maeda et al., 2005), which has subsequently been hypothesized to be due to the effects of increased 16:1n-7 levels in adipose tissue and plasma (Cao et al., 2008). Interestingly, despite ELOVL6 GKO mice not being protected from diet-induced obesity, they are more insulin sensitive than WT mice (Matsuzaka et al., 2007). Perhaps the increased level of circulating palmitoleate can explain the similarity in improved insulin sensitivity in these two models, although this has not been directly tested in the ELOVL6 GKO model. It should be noted, however, that while FABP GKO mice are also resistant to diet-induced obesity and fatty liver disease, these two specific metabolic improvements are in contrast to those observed in ELOVL6 GKO mice. Perhaps other factors that regulate lipogenesis and fatty acid oxidation are be responsible for these divergent effects or possibly the level of 16:1n-7 is not sufficiently elevated in ELOVL6 GKO mice to protect against these additional diet-induced metabolic consequences. Perhaps future studies in ELOVL6 GKO or tissue-specific knockout mice will provide additional insight into metabolic regulation during ELOVL6 deficiency.
Stearoyl-CoA Desaturase
Stearoyl-CoA desaturase (SCD) is a delta-9 desaturase that catalyzes the conversion of saturated fatty acids, with preference for stearate and palmitate, to their monounsaturated fatty acid (MUFA) counterparts, oleate and palmitoleate, respectively (Figure 2). This is the rate-limiting enzymatic reaction in the synthesis of MUFAs. The mouse SCD1 isoform is expressed in a wide panel of tissues and at relatively high levels in skin, WAT, and BAT (Miyazaki et al., 2001; Zheng et al., 2001). In addition, lipogenic, high-carbohydrate diets induce SCD1 gene expression to a very high level in liver (Miyazaki et al., 2004) which may be explained by increased nuclear levels of the active SREBP-1 and ChREBP transcription factors, which are known to be induced by this feeding pattern (Horton et al., 1998b; Denechaud et al., 2008). Mice with a naturally occurring mutation in the SCD1 gene (referred to as asebia mice) and those with a whole-body targeted disruption of SCD1 (SCD1 GKO) have been studied extensively over the past several years (Miyazaki et al., 2001; Miyazaki et al., 2004; Ntambi et al., 2002).
SCD1 asebia mice exhibit reduced liver triglyceride levels when fed either chow or high-carbohydrate diets and reduced plasma triglycerides when fed a lipogenic high-carbohydrate diet (Miyazaki et al., 2001; Miyazaki et al., 2004). SCD1 GKO mice are protected from diet-induced obesity after long-term feeding of either high-fat or high-carbohydrate diets (Ntambi et al., 2002; Miyazaki et al., 2007). The protection from diet-induced obesity appears to be multi-factorial with increased energy expenditure and fatty acid oxidation as well as a concomitant reduction in the lipogenic program regulated by SREBP-1c (Ntambi et al., 2002). The blunted lipogenic response includes a reduction in expression of SREBP-1c as well as several target genes including ACC, FAS, and GPAT (Ntambi et al., 2002). In addition, convincing evidence exists to support a role for AMP-activated protein kinase (AMPK) in mediating some of these beneficial metabolic effects due to SCD1 deficiency (Dobrzyn et al., 2004). In SCD1 GKO mice fed a standard chow diet, hepatic AMPK activity was increased. The increased AMPK phosphorylation and activation was associated with increased phosphorylation of ACC and a significant reduction in its activity. Malonyl-CoA, the product of the reaction catalyzed by ACC, inhibits CPT1 and thereby reduces fatty acid oxidation (McGarry et al., 1978). Thus, in SCD1 GKO mice, it is perhaps not surprising that an increase in CPT1 activity and fatty acid oxidation accompanies the reduction in hepatic malonyl-CoA (Dobrzyn et al., 2004).
Although much information regarding the role of SCD1 in metabolism was gleaned from studying mice with complete deficiency of functional SCD1, it has been of interest to identify key tissues that contribute to the improved metabolic profile when the mice are challenged with high-fat or high-carbohydrate diets. Furthermore, the absence of SCD1 throughout the body results in undesirable side effects including dry skin and alopecia (Zheng et al., 1999; Miyazaki et al., 2001). Two distinct technologies have been used in order to probe the role of liver SCD1 in metabolism and the susceptibility to obesity: the use of ASO-mediated inhibition of SCD1 (Jiang et al., 2005) and the use of the Cre-loxP system to generate liver-specific SCD1 knockout mice (Miyazaki et al., 2007).
The development of a mouse model that is deficient in SCD1 in the liver only (SCD1 LKO) and its altered metabolism has been described recently (Miyazaki et al., 2007). Under control dietary conditions, SCD1 LKO mice lack the dry skin and thin hair phenotypes of SCD1 GKO mice and are indistinguishable from their SCD1 flox/flox (Lox) littermates. One of the most striking metabolic effects of the SCD1 LKO mice is their resistance to high-carbohydrate diet-induced obesity. When fed a high-carbohydrate diet for 18 weeks, SCD1 LKO mice gained significantly less weight than the Lox controls, and they are protected from the development of hepatic steatosis (Figure 3) (Miyazaki et al., 2007). The reduced weight gain in SCD1 LKO mice is likely be due to impaired lipogenesis in response to carbohydrate feeding, as a reduction in expression of SREBP-1c and its downstream target genes involved in DNL including ACC, FAS, and ELOVL6 was observed. However, palmitoleate (16:1n-7), a product of the reaction catalyzed by SCD1, has recently been proposed to be a lipokine that acts as both an insulin sensitizing lipid and a regulator of hepatic lipid synthesis (Cao et al., 2008). While mice infused with triglyceride:palmitate had a significant induction of the expression of several hepatic lipogenic genes including SCD1, FAS and Elovl6, infusion of triglyceride:palmitoleate reduced the expression of these genes (Cao et al., 2008). Thus, the complexity of the metabolic effects of specific fatty acids remains to be fully understood.
Despite the significant increase in the hepatic expression of PGC-1α in SCD1 LKO mice, there was no significant increase in expression of oxidative genes (Miyazaki et al., 2007). Thus, increased oxidation was not convincingly demonstrated and may not be a likely explanation for the high-carbohydrate diet-induced obesity protection in SCD1 LKO, although additional experiments are necessary to conclusively determine a lack of increased fatty acid oxidation. However, consistent with increased PGC-1α expression there was evidence for increased thermogenesis, as the expression of several uncoupling proteins (UCPs) was significantly increased. Additionally, SCD1 LKO mice are hypoglycemic when fed a very low-fat diet, which is surprising given that PGC-1α, an important coactivator of a number of genes involved in gluconeogenesis, is up-regulated (Miyazaki et al., 2007). Evidence for hepatic inflammation has also been demonstrated when SCD1 LKO mice consume a very low-fat diet. These two metabolic phenotypes are in striking contrast to those described in the liver-specific ACC1 null mice, in which blood glucose did not differ from control mice and there was no evidence of liver inflammation or damage (Mao et al., 2006). The hypoglycemia is, however, consistent with that in FAS LKO, as discussed previously in this review. In contrast to FAS LKO mice, however, PPARα activation could not rescue the hypoglycemic response of SCD1 LKO mice (Miyazaki et al., 2007). Another noteworthy difference is that FAS LKO mice develop hepatic steatosis while SCD1 LKO mice do not. A surprising result from the SCD1 LKO study is that these mice gained weight to a similar extent as their lox littermates when fed a lard-based high-fat diet during an 18-week long-term study and thus were quite susceptible to high-fat diet-induced obesity (Miyazaki et al., 2007). These data from the SCD1 LKO study demonstrate that liver SCD1 is important in maintaining normal lipogenesis in response to consumption of a high-carbohydrate load. In addition, these data also suggest that the increase in fatty acid oxidation and energy expenditure and protection from a high-fat diet-induced obesity observed in SCD1 GKO mice is apparently due to the lack of SCD1 in extrahepatic tissues, as discussed in greater detail below.
In contrast to the results with SCD1 LKO mice, C57/B6 mice treated with twice weekly injections of ASOs against SCD1 were largely resistant to high-fat diet-induced weight gain and hepatic steatosis (Jiang et al., 2005). Similar to the SCD1 LKO mice, however, was a reduction in expression of lipogenic genes including SREBP-1c, FAS, and ACC after ten weeks of ASO treatment. The resistance to obesity during treatment with ASO may be at least partially due to increased energy expenditure and activity. Although there was an increase in CPT1 expression, which conflicts with the data from the SCD1 LKO study, other genes involved in fatty acid oxidation were not simultaneously increased, which is in agreement with the study by Miyazaki and colleagues (2007). An explanation for the conflicting results regarding high-fat diet-induced susceptibility is currently unknown, but may be due to the lack of strict specificity of the ASOs for the liver. For example, a reduction in WAT and BAT SCD1 expression occurred following ASO treatment (Jiang et al., 2005). Expression of thermogenic genes, such as UCPs was increased in BAT. If SCD1 deficiency is required in extrahepatic tissues in order for high-fat diet-induced obesity resistance, then a lack of ASO specificity for liver tissue could potentially confound results and explain the apparent discrepancy between the two studies. A separate study demonstrated that short-term SCD1 ASO treatment improves hepatic insulin sensitivity and levels in rats (Gutierrez-Juarez et al., 2006). In addition, short-term SCD1 ASO treatment reduced hepatic ACC but not FAS, and an impact of ASO treatment on lipogenesis was not conclusively demonstrated although liver triglycerides significantly increased (Gutierrez-Juarez et al., 2006).
Results from the dietary studies conducted in SCD1 LKO demonstrated that although the liver is an important tissue in mediating the metabolic effects of SCD1 during high-carbohydrate feeding, SCD1 deficiency in extrahepatic tissues must be involved in mediating protection from high-fat diet-induced obesity. Additionally, the strong skin phenotype that presents in SCD1 GKO mice is suggestive for an important role for SCD1 in the maintenance of healthy skin. It is well known that the lack of SCD1 in the skin imparts a negative impact on sebaceous glands and hair follicles (Zheng et al., 1999; Miyazaki et al., 2001). Interestingly, mice lacking acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1 GKO) share a number of skin-specific characteristics in common with SCD1 GKO mice including hair loss, atrophy of sebaceous glands, and impaired thermoregulation (Chen et al., 2002). It has recently been discovered that DGAT1 activity is important in retinol esterification (Shih et al., 2009). Interestingly, dietary retinol deficiency prevents hair loss in DGAT1 GKO mice, which suggests that retinol toxicity develops in skin of DGAT1 GKO and leads to the alopecia (Shih et al., 2009).
SCD1 is typically expressed at a high level in skin tissue, and as with the generation of SCD1 LKO, Cre-loxP technology was used to produce mice with a skin-specific SCD1 deletion (SCD1 SKO) using Cre recombinase expression driven by the Keratin-14 promoter (Sampath et al., 2009). Skin-specific SCD1 deficiency resulted in a dramatic loss of wax di-esters but increase in free cholesterol in skin. Similar to SCD1 GKO mice, energy expenditure is increased in SCD1 SKO mice during both light and dark periods. Surprisingly, SCD1 SKO mice were also resistant to high-fat diet-induced obesity during an 8-week feeding study (Figure 3) (Sampath et al., 2009). However, although hepatic SREBP-1c expression was reduced in SCD1 SKO, other lipogenic genes including ACC and FAS were expressed at comparable levels to Lox controls. Furthermore, hepatic nuclear SREBP-1 protein levels were comparable to controls, yet SCD1 SKO mice had reduced fat pad weights and reduced hepatic lipid accumulation (Sampath et al., 2009). These molecular results suggest that reduced lipogenesis is not the mechanism responsible for the protection from high-fat diet-induced obesity during skin-specific SCD1 deficiency. An alternative explanation for the protection is that fatty acid oxidation is increased sufficiently to prevent the accumulation of the excess dietary fat. Indeed, during chow and high-fat dietary treatments, a number of oxidative genes are significantly increased in several tissues including liver, muscle and adipose tissue of SCD1 SKO mice including CPT1, acyl-CoA oxidase and the transcriptional coactivator PGC-1α (Sampath et al., 2009). In addition, increased thermogenesis is also a likely contributor to reduced fat storage in SCD1 SKO mice, and several UCPs, which are also regulated by PGC-1α, were consistently up-regulated (Sampath et al., 2009). The mechanism by which deficiency of SCD1 in skin tissue affects systemic metabolism remains unknown at this time. However, we can speculate, based on the current results, that perhaps a skin-derived signaling molecule enters the circulatory system and subsequently affects metabolism in peripheral tissues. Future work will likely aim to identify the link that connects skin tissue with peripheral tissues and confers the beneficial metabolic profile.
Fatty acid-mediated regulation of SREBP-1 and ChREBP
The regulation of lipogenesis mediated by SREBP-1 and ChREBP are apparently involved in the down-regulation of carbohydrate-stimulated fatty acid and triglyceride synthesis in the SCD1 LKO model. The mature, nuclear fractions of both lipogenic transcription factors were reduced in the livers of SCD1 LKO mice, but not Lox mice, after short-term feeding of a high-sucrose diet (Miyazaki et al., 2007). Interestingly, supplementation of the high-sucrose diet with triolein increased the nuclear levels of both SREBP-1 and ChREBP and restored the expression of lipogenic genes to levels observed in the Lox controls (Miyazaki et al., 2007). These data suggest that MUFAs, and more specifically the current data strongly support an in vivo role for oleic acid, are sufficient to restore the maturation and activation of these transcription factors during SCD1 deficiency.
Potential mechanism(s) by which MUFAs or the endogenous products of the reaction catalyzed by SCD1 may regulate lipogenesis via the SREBP-1 and ChREBP transcription factors is unknown, yet several studies have demonstrated that fatty acids are involved in the regulation of SREBP-1 and ChREBP. One study has shown that polyunsaturated fatty acids (PUFA) negatively regulate ChREBP (Dentin et al., 2005). While the consumption of a high-carbohydrate diet supplemented with PUFA was shown to reduce hepatic ChREBP mRNA and nuclear protein levels in mice, triolein supplementation did not have an effect (Dentin et al., 2005). Primary hepatocyte experiments provided additional evidence that various PUFAs down-regulate ChREBP level but neither stearate nor oleate exert such an effect (Dentin et al., 2005).
Additionally, results from in vitro experiments and animal feeding studies are largely inconsistent with observations from SCD1 KO mice that suggest oleic acid positively regulates SREBP-1 maturation. Worgall et al. (1998) demonstrated that oleate and PUFA reduced the expression of reporter gene that contained an SRE in the promoter while saturated fatty acids had no effect. In addition, treatment of CV-1 cells with oleate reduced the nuclear fraction of SREBP-1 (Worgall et al., 1998). Hannah et al. (2001) also demonstrated that palmitoleate, oleate and a number of PUFAs, but not saturated fatty acids, negatively regulate SREBP-1c and SREBP-1a mRNA levels, and that these same fatty acids reduced nuclear SREBP-1 protein levels in HEK-293 cells (Hannah et al., 2001). In contrast, oleate did not have an effect on SREBP-1 expression in HepG2 cells although arachidonic acid drastically reduced SREBP-1 mRNA (Xu et al., 1999). Animal feeding studies have consistently reported that fish oil, which contains high levels of PUFA, reduces hepatic SREBP-1 mRNA and nuclear protein levels (Xu et al., 1999, 2002; Kim et al., 1999). However, feeding triolein as the sole dietary fat source did not have an effect on SREBP-1 mRNA, nuclear protein, or SREBP-1 regulated genes (Xu et al., 1999, 2002). The results from these studies demonstrated that fatty acids regulate SREBP-1 at the level of mRNA as well as protein maturation.
More recent work has provided insight into the mechanism by which fatty acids down-regulate SREBP-1 maturation (Lee et al., 2008). The treatment of CHO cells with oleate or PUFA increased Insig-1, a protein that retains SREBP in the ER membrane and thus prevents it maturation to its nuclear form. Lee et al. further demonstrated that unsaturated fatty acids block the association between ubiquitinated Insig-1 and UBX domain-containing protein 8, an interaction that is required for the Insig-1 protein to be removed from the ER membrane and subsequently degraded in the proteasome (Lee et al., 2008). Consistent with the studies described above, there was no effect of saturated fatty acids on level of Insig-1 protein. While these studies describe the effect of one mechanism by which mono- and polyunsaturated fatty acids reduce SREBP-1 processing and thus presumably affect lipogenesis, they are in stark contrast to the results observed in the SCD1 KO mice in which MUFA are limiting but saturated fatty acids are not, yet nuclear SREBP-1 is reduced and lipogenesis is severely impaired. This suggests a unique mechanism may be involved in the reduction of SREBP-1 processing in the context of SCD1 deficiency.
Conclusion
De novo lipogenesis is a complex and highly regulated metabolic pathway that can lead to adverse metabolic consequences when dysregulated. Transcriptional regulation of many of the genes encoding enzymes involved in DNL oftentimes occurs via more than one transcription factor, of which LXRs, SREBPs, and ChREBP are crucial cellular molecules that mediate adaptive metabolic responses to changing dietary exposures (Figure 2). The ability to manipulate the genome of mice has vastly expanded our knowledge of how the modification of a single enzyme in one metabolic pathway can impart extensive effects on other pathways, thus demonstrating that these metabolic pathways are inextricably connected. Moreover, the ability to create mouse models that lack metabolically important enzymes in a tissue-specific manner has also greatly expanded our current understanding of the array of tissues that contribute to whole body metabolic homeostasis and that signaling among tissues may occur such that metabolic regulation is also affected. While several gene knockout mouse models of enzymes required for fatty acid synthesis have been generated including ACC, FAS, ELOVL6, and SCD1, future experiments will likely yield many additional insights into the regulation of metabolism, lipogenesis and diet-induced obesity and how these enzymes mediate these metabolic processes.
Acknowledgments
We thank Mary Cantu for her assistance with design and production of Figures 1 and 3. We thank Laura Vanderploeg of the Media Center in the Department of Biochemistry, University of Wisconsin for assistance with design and production of Figure 2. We thank Dr. Matthew Flowers for critically reviewing this manuscript. This work was supported by NIH grant R01DK-62388.
Footnotes
Declaration of Interest The authors report no declarations of interest.
References
- Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ. The subcellular localization of acetyl-CoA Carboxylase 2. Proc Natl Acad Sci USA. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA Carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA Carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci USA. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA Carboxylase 1 are embryonically lethal. Proc Natl Acad Sci USA. 2005;201:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- Beligneux AP, Kosinski C, Gavino B, Horton JD, Skarnes WC, Young SG. ATP-citrate lyase deficiency in the mouse. J Biol Chem. 2004;279:9557–9564. doi: 10.1074/jbc.M310512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergot MO, Diaz-Guerra MJ, Puzenat N, Raymondjean M, Kahn A. Cis-regulation of the L-type pryruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nuc Acids Res. 1992;20:1871–1878. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity. 2008;16:S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA Carboxylase. Biochem Soc Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: [Accessed on 23 July 2009]. 2008a. 2008. Available at: http://apps.nccd.cdc.gov/brfss/ [Google Scholar]
- Centers for Disease Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: [Accessed on 29 Nov 2009]. 2008b. 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. [Google Scholar]
- Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RRV, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy MV, Zhu Y, Yin L, Coleman T, Pappan KL, Marshall CA, McDaniel ML, Semenkovich CF. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res. 2009;50:630–640. doi: 10.1194/jlr.M800379-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors andlipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–181. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA Carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuif MH, Porteu A, Kahn A, Vaulont S. Exploration of a liver-specific, glucose/insulin-responsive promoter in transgenic mice. J Biol Chem. 1993;268:13769–13772. [PubMed] [Google Scholar]
- Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–964. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- Dowell P, Hu Z, Lane MD. Monitoring energy balance: metabolites of fatty acid synthesis as hypothalamic sensors. Annu Rev Biochem. 2005;74(5):515–534. doi: 10.1146/annurev.biochem.73.011303.074027. [DOI] [PubMed] [Google Scholar]
- Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22:8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J Biol Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001;276:4365–4372. doi: 10.1074/jbc.M007273200. [DOI] [PubMed] [Google Scholar]
- Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998a;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. 1998b;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signaling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr. 1997;17:77–99. doi: 10.1146/annurev.nutr.17.1.77. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. J Biol Chem. 1999;274:25892–25898. doi: 10.1074/jbc.274.36.25892. [DOI] [PubMed] [Google Scholar]
- Kos CH. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev. 2004;62:243–246. doi: 10.1301/nr2004.jun243-246. [DOI] [PubMed] [Google Scholar]
- Kumadaki S, Matsuzaka T, Kato T, Yahagi N, Yamamoto T, Okada S, Kobayashi K, Takahashi A, Yatoh S, Suzuki H, et al. Mouse Elovl-6 is an SREBP target. Biochem Biophys Res Commun. 2008;368:261–266. doi: 10.1016/j.bbrc.2008.01.075. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Joseph SB, Walczak R, Pei L, Wilpitz DC, Colins JL, Tontonoz P. Autoregulation of the human liver X receptor α promoter. Mol Cell Biol. 2001;21:7558–7568. doi: 10.1128/MCB.21.22.7558-7568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JN, Zhang X, Feramisco JD, Gong Y, Ye J. Unsaturated fatty acids inhibit proteasomal degradation of Insig-1 at a postubiquitination step. J Biol Chem. 2008;283:33772–33783. doi: 10.1074/jbc.M806108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu P, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1b coactivation of SREBP. Cell. 2005a;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005b;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9:819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Magkos F, Yannakoulia M, Chan JL, Mantzoros CS. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Ann Rev Nutr. 2009;29:223–256. doi: 10.1146/annurev-nutr-080508-141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, DeMayo FJ, Li H, Abul-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA Carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Yang T, Gu Z, Heird WC, Finegold MJ, Lee B, Wakil SJ. aP2-Cre-mediated inactivation of acetyl-CoA carboxylase 1 causes growth retardation and reduced lipid accumulation in adipose tissues. Proc Natl Acad Sci USA. 2009;106:17576–17581. doi: 10.1073/pnas.0909055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Yoshikawa T, Amemiya-Kudo M, Hasty AH, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, et al. Cloning and characterization of mammalian fatty acyl-CoA elongase as a lipogenic enzyme regulated by SREBPs. J Lipid Res. 2002;43:911–920. [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H. Elovl6: a new player in fatty acid metabolism and insulin sensitivity. J Mol Med. 2009;87:379–384. doi: 10.1007/s00109-009-0449-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and –independent mechanism. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- Nagle CA, Vergnes L, DeJong H, Wang S, Lewin TM, Reue K, Coleman RA. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6-/- mice. J Lipid Res. 2008;49:823–831. doi: 10.1194/jlr.M700592-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, Chirala SS, Wakil SJ. Glucose and fat metabolism in adipose tissue of acetyl-CoA Carboxylase 2 in knockout mice. Proc Natl Acad Sci USA. 2005;102:1384–1389. doi: 10.1073/pnas.0409451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462:307–314. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007;27:179–192. doi: 10.1146/annurev.nutr.27.061406.093618. [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DR. Obesity drugs and their targets: correlation of mouse knockout phenotypes with drug effects in vivo. Obesity Rev. 2006;7:89–108. doi: 10.1111/j.1467-789X.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–694. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JA, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptor, LXRα and LXRβ. Genes & Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev. 2004;62:333–339. doi: 10.1111/j.1753-4887.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high-fat diet-induced obesity. J Biol Chem. 2009;284:19961–19973. doi: 10.1074/jbc.M109.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Savage DB, Choii CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nat Biotech. 2003;21:1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov. 2004;3:695–710. doi: 10.1038/nrd1469. [DOI] [PubMed] [Google Scholar]
- Shih MYS, Kane MA, Zhou P, Yen CL, Streeper RS, Napoli JL, Farese RV., Jr. Retinol esterificiation by DGAT1 is essential for retinoid homeostasis in murine skin. J Biol Chem. 2009;284:4292–4299. doi: 10.1074/jbc.M807503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Korn BS, Basmakov Y, Horton JD. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem. 1998;273:35299–35306. doi: 10.1074/jbc.273.52.35299. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;19:3760–3768. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- Song C, Kokontis JM, Hiipakka RA, Liao S. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc Natl Acad Sci USA. 1994;91:10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen KR, Gustafsson J. Putative metabolic effects of the liver X receptor. Diabetes. 2004;53:S36–S42. doi: 10.2337/diabetes.53.2007.s36. [DOI] [PubMed] [Google Scholar]
- Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Steffensen KR, Gao H, Reimers M, Dahlman-Wright K, Schuster GU, Gustafsson J. Novel roles of liver X receptors exposed by gene expression profiling in liver and adipose tissue. Mol Pharmacol. 2002;62:1299–1305. doi: 10.1124/mol.62.6.1299. [DOI] [PubMed] [Google Scholar]
- Tobin KAR, Steineger HH, Alberti S, Spydevold O, Auwerx J, Gustafsson JA, Nebb HI. Cross-talk between fatty acid and cholesterol metabolism mediated by liver X receptor-α. Mol Endocrinol. 2000;14:741–752. doi: 10.1210/mend.14.5.0459. [DOI] [PubMed] [Google Scholar]
- Tsatsos NG, Towle Glucose activation of ChREBP in hepatocytes occurs via a two-step mechanism. Biochem Biophys Res Commun. 2006;340:449–456. doi: 10.1016/j.bbrc.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Beigneux AP, Davis R, Watkins SM, Young SG, Reue K. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J Lipid Res. 2006;47:745–754. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50:S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willy PJ, b Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes & Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Lane MD. Control of energy homeostasis: role of enzymes and intermediates of fatty acid metabolism in the central nervous system. Annu Rev Nutr. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- Xu J, Nakamura MT, Cho HP, Clarke SD. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- Xu J, Cho H, O’Malley S, Park JHY, Clarke SD. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanisms. J Nutr. 2002;132:3333–3339. doi: 10.1093/jn/132.11.3333. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3—A novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. doi: 10.1006/geno.2000.6429. [DOI] [PubMed] [Google Scholar]