Introduction
This monograph presents a broad overview of human trophoblast differentiation, emphasizing the hormonal factors and transcription factors involved in the differentiation process. Particular focus is given to the role for syncytin in the fusion of mononuclear cytotrophoblast (CTB) cells and the roles for the transcription factor TFAP2A in terminal syncytiotrophoblast (STB) differentiation. Also presented are some studies relating to villous trophoblast differentiation in pre-eclampsia, choriocarcinoma and other pathologic conditions of pregnancy associated with abnormal placentation.
Overview of of human trophoblast differentiation
A schematic representation of human placental development is shown in Figure 1. One of the cells resulting from the first cell division of the fertilized egg is the precursor cell for the inner cell mass, which develops into the fetus. The other cell becomes a placental stem cell that divides to form a villous cytotrophoblast cell, which is the precursor of the syncytiotrophoblast cell, and an extravillous cytotrophoblast cell, which is the precursor of the invasive trophoblast cell. During villous trophoblast morphogenesis, groups of CTB proliferate rapidly and grow distally, forming the primary villi. The primary villi are then invaded by fetal mesenchyme, converting them to secondary villi. Capillaries form in the mesenchyme, converting the secondary villi to tertiary villi that undergo progressive branching and thinning. Extravillous trophoblast cells, which do not become directly involved in the development of placental villi, invade the underlying endometrium and maternal vascular bed, migrating up the uterine spiral arteries that supply the implantation site and placenta. The epithelium-like trophoblastic layer of the placental villus, which is composed of CTB and STB, separates maternal blood from the villus interior (for review see Castellucci et al., 1990; Benirschke and Kaufmann, 1995b). The STB form a continuous, uninterrupted, multinucleated layer that regulates the exchange of substrates, gases and other factors between the maternal and fetal circulations and synthesizes and secretes many protein and steroid hormones and growth factors, including placental lactogen and chorionic gonadotropin. The underlying mononuclear CTB (Langhans’ cells) are located between the STB layer and its basement membrane and are the progenitor cells of the STB layer. A schematic representation of the morphology of placental villi is depicted in Figure 2.
Figure 1.
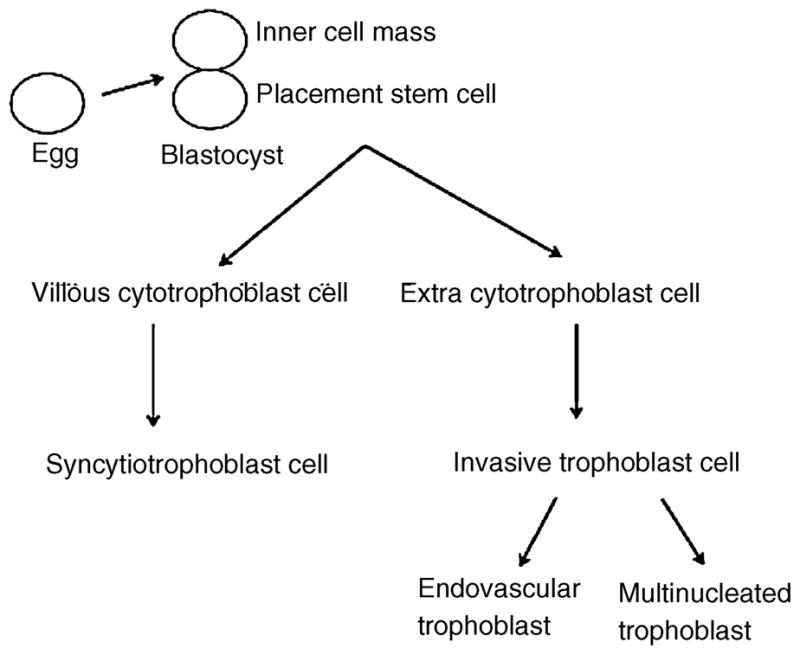
Schematic representation of human placental development. The placenta stem cell divides to form a villous cytotrophoblast cell and an extravillous trophoblast cell. The villous cytotrophoblast cell in turn differentiates to a syncytiotrophoblast cell, while the extravillous cytotrophoblast cell differentiates to an invasive trophoblast cell.
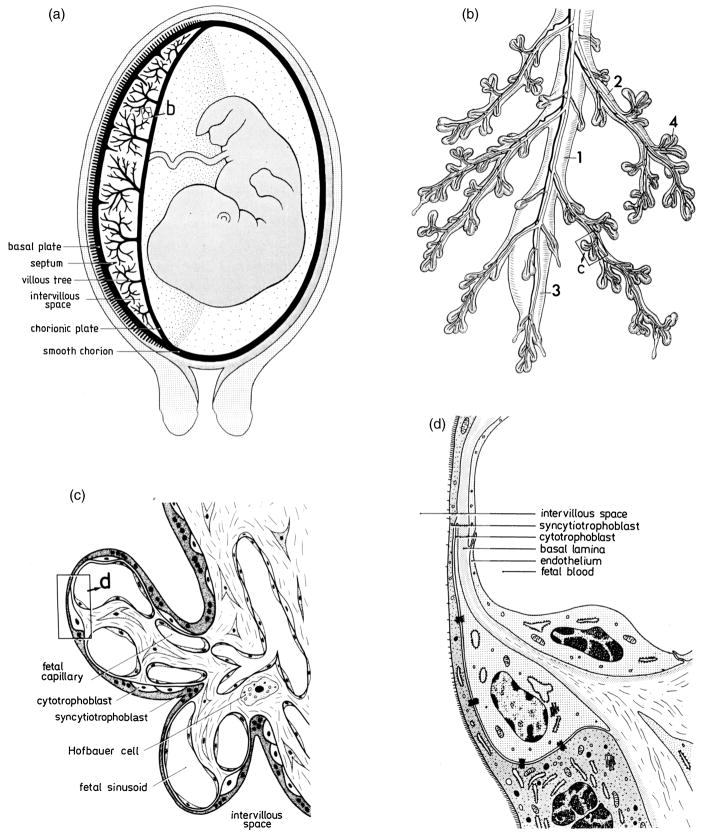
Figure 2.
Basic morphology of human placental villi. (a) Simplified longitudinal section across the uterus, placenta, and membranes in the human. The chorionic sac, consisting of placenta (left half) and membranes (right half), is thick black. (b) Peripheral ramifications of the mature villous tree, consisting of a stem villus (1), which continues in a bulbous immature intermediate villus (3); the slender side branches (2) are the mature intermediate villi, the surface of which is densely covered with grape-like terminal villi (4). (c) Highly simplified light microscopic section of two terminal villi, branching off a mature intermediate villus (right). (d) Representation of an electron microscopic section of the placental barrier, demonstrating its typical layers. From Benirschke and Kaufmann, 1995a.
Most investigations of the dynamic processes occurring during human trophoblast differentiation have been performed using primary cultures of term trophoblast cell cultures and human choriocarcinoma cells as model systems. Numerous studies have shown that mononucleated CTB isolated by enzymatic dispersion of term placental tissue and cultured in medium containing fetal calf serum or bovine serum aggregate and fuse to form a multinucleated syncytium that synthesizes and secretes placental lactogen, chorionic gonadotropin, progesterone and many other proteins and steroids that are secreted by STB in vivo (Kliman et al., 1986; Daiter et al., 1996) (Figure 3). In most studies, human CTB cells prepared by enzymatic digestion are further purified to >95% homogeneity using a Percoll gradient separation or by negative selection using an antibody to the cell surface marker CD9. The in vitro system used in the author’s laboratory is similar to that utilized by other investigators but the culture medium containing 10% human pregnancy serum in place of fetal calf serum or other animal serums (Richards et al., 1994). CTB cultured in the presence of human maternal serum differentiate to a more mature phenotype than cells cultured in the presence of non-pregnant human serum or animal serums, and the cells synthesize much greater amounts of hPL and hCG (Richards et al., 1994). Furthermore, the patterns of hPL and hCG expression during the culture period more closely resemble the in vivo expression patterns of these hormones. Primary CTB from term human placentas preferentially differentiate into a STB phenotype, while CTB isolated from first trimester placentas preferentially differentiate to an invasive phenotype.
Figure 3.
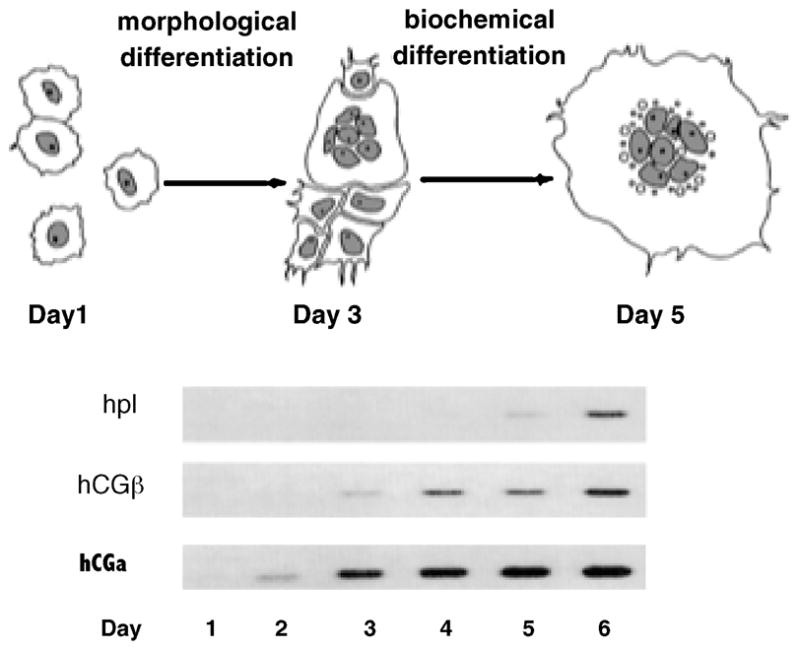
Expression of placental lactogen (hPL), hCG, and hCG mRNAs during in vitro differentiation of human cytotrophoblast cells. By day 1, the isolated cytotrophoblast cells are beginning to aggregate. By day 3, many of the cells have fused to form a syncytium; by day 5, syncytialization is nearly complete. hPL, hCG, and hCG mRNA levels were determined by Northern blot analysis. From Richards et al., 1994.
Cultured human choriocarcinoma cells have also been used as a model to study some aspects of trophoblast differentiation. However, there are some significant limitations that need to be considered before using these cells. For example, BeWo and other human choriocarcinoma cells do not normally form a syncytium, but can be induced to fuse and express syncytin by exogenous cAMP analogues or by factors that induce intracellular cAMP such as cholera toxin and forskolin. However, while studies of these cells have provided some information about the process of syncytialization, the investigations provide little information about terminal differentiation since most of the genes that are expressed in normal STB are not expressed in these or other choriocarcinoma cells following cAMP-induced cell fusion.
The in vitro differentiation of primary human CTB to a STB phenotype has been arbitrarily divided into two stages (Figure 3). During the initial stage, referred to as morphological differentiation, the villous CTB proliferate, aggregate and fuse to form a syncytium. During the second stage, referred to as biochemical differentiation, the cells begin to express genes involved in substrate transport, hormone synthesis and secretion, metabolism, and other functions of terminally differentiated STB. Several studies have demonstrated that the two phases of differentiation are linked and that morphological differentiation is required before the cells undergo biochemical differentiation. Frendo and co-workers (Frendo et al., 2003b) demonstrated that human CTB cultured in the presence of an antisense oligonucleotide to syncytin do not fuse to form a syncytium and express less than 20% of the hCG of control cells. Kudo and coworkers (Kudo et al., 2003a; Kudo et al., 2003c) showed that BeWo choriocarcinoma cells treated with an antisense oligonucleotide to CD45 did not syncytialize in response to forskolin and expressed greater than 80% less hCG than control cells not treated with the antisense oligonucleotide. In addition, we have recently shown that disruption of CTB fusion with an antiserum to syncytin prevents the induction of hPL, hCG and other genes normally expressed by terminally differentiated STB.
At present, the hormonal and other factors that regulate the differentiation of CTB to STB are poorly understood. Because human and murine placentas have many similarities in function and some homologies in structure, some investigators have proposed that the murine placenta is a good model system to understand human placental development (Cross et al., 1994; Rossant and Cross, 2001). Knockout experiments in the mouse have identified many transcription factors that are important in the differentiation of the various cell types constituting the murine placenta (Cross et al., 1994; Morrish et al., 1998; Cross, 2000; Scott et al., 2000), including HOXB6, HOXC5, HOXC6, HOX3E, HB24, GCM1, GAX, MSX2, DLX3, Pit-1, HAND1, TF-1, TEF5, c-Ets1 and several other transcription factors, many of which are helix-loop-helix (bHLH) proteins. ID-2, a member of a family of inhibitors of bHLH binding, acts in trophoblast cells as a dominant/negative bHLH transcription factor (Janatpour et al., 2000); and constitutive overexpression prevents differentiation of the cells. However, the roles for homologs of these transcription factors in human placental development are not known (Knofler et al., 2000). Furthermore, very little or nothing is known about the upstream factors in the murine and human placenta that modulate the expression of the regulatory factors and the downstream genes and pathways that are induced and/or repressed in response to the factors.
Consequently, while much has been learned about the transcriptional regulation of placental developmental in the mouse, the relevance of many of these studies to the human placenta is unclear. The subject of animal models for human placentation was recently reviewed extensively by Carter (2007) who pointed out the striking differences between the human and mouse in implantation, the yolk sac, trophoblast invasion of uterine arteries, transformation of uterine arteries, placental exchange, the interhemal barrier (one layer in the human, three in the mouse) and the striking differences in placenta hormones (such as chorionic gonadotropin and placental lactogen). Carter emphasized that most of the hormonal activity in the mouse placenta is achieved by the trophoblast layer that is adjacent to maternal blood, whereas the transport and barrier functions are achieved by the other two trophoblast layers, which are linked by gap junctions. Furthermore, our laboratory has observed that the DNA binding sites on the promoters of the most induced genes during human CTB differentiation are poorly conserved in the mouse, strongly suggesting that these genes are regulated differently in the two species. In addition, there are fundamental differences in the biological actions and regulation of many of the proteins that belong to homologous gene families. Unlike the human placenta, the mouse placenta synthesizes and secretes two distinct placental lactogens that differ significantly in structure, function and gene regulation from hPL. Consequently, extrapolation of studies in the mouse placenta to the human placenta may lead to erroneous conclusions.
Studies from many laboratories using primary human CTB cells and choriocarcinoma cells implicate EGF (Matsuo et al., 1993), hCG (Shi et al., 1993), LIF (Kojima et al., 1995), CSF-1 (Garcia-Lloret et al., 1994), IGF-I (Maruo et al., 1995), cAMP (Strauss et al., 1992), members of the TGFβ superfamily (including TGFβ and TGIF) (Peng, 2003), the Wnt/β-catenin pathway (Getsios et al., 2000; Getsios et al., 2001; Pollheimer et al., 2006), the transcription factors PPARγ (Schaiff et al., 2000), Ikaros (Nomura et al., 2005), GATA-2/3, and several other factors in the differentiation process. Oxygen has also been shown to be a critical factor in the differentiation process. Prior to 10 weeks of gestation, when CTB differentiate primarily to an invasive phenotype, the placental intervillous oxygen content is less than 20 mm Hg, which is equivalent to less than 3% oxygen. After ten weeks, when CTB primarily differentiate to a STB phenotype, oxygen tension rises sharply and reaches steady state levels of approximately 8–10% from mid-gestation to parturition (Many et al., 2000; Red-Horse et al., 2004). In collaboration with Dr. Jared Robins (formerly Department of Ob/Gyn, University of Cincinnati; currently Ob/Gyn, Brown University), we observed that oxygen tension directs the pathway of in vitro CTB differentiation (Robins et al., 2007) (Figure 4). CTB incubated in 21% oxygen spontaneously differentiated to form a syncytium and expressed abundant amounts of syncytin and hPL. In contrast, cells incubated in 1% oxygen expressed little syncytin or hPL (p<0.001) and did not fuse. Furthermore, the cells incubated at 1% expressed abundant amounts of HLA-G (a marker of invasive CTB), as demonstrated by immunohistochemistry and real-time PCR, while the cells incubated at 21% oxygen did not. These results suggest that low oxygen tension directs differentiation along the extravillous trophoblast cell pathway, while greater oxygen tension directs differentiation along the villous trophoblast cell pathway.
Figure 4.
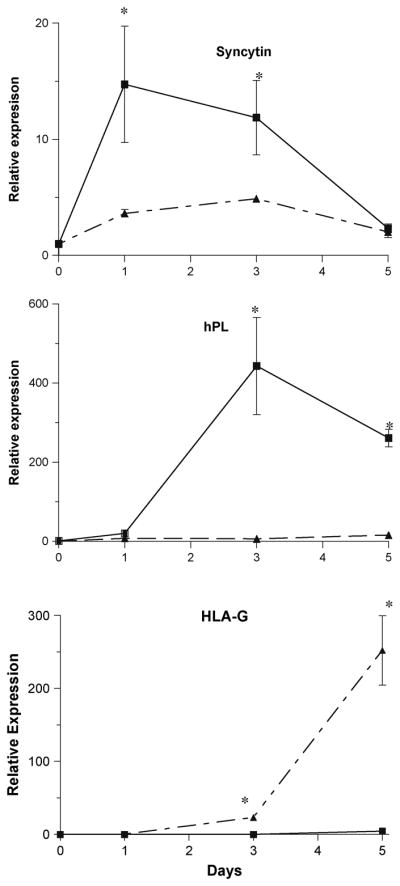
Effect of oxygen tension on syncytin, hPL and HLA-G expression. Cytotrophoblast cells were incubated in 20% (solid line) or 1% (broken line) oxygen tension for 5 days. Real-time PCR was performed for markers of villous trophoblast differentiation (syncytin and hPL) and extravillous trophoblast differentiation. Gene expression was normalized to β-actin and expressed relative to day 0. *, p < 0.05 compared to time 0. From Robins et al., in press.
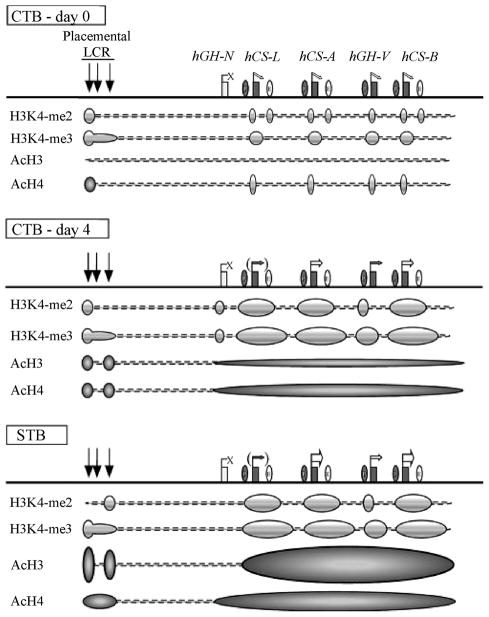
Chromatin modification has been shown to be important in trophoblast differentiation. Placental lactogen, one of the most abundant proteins expressed by syncytiotrophoblast cells, is a member of the hGH/hPL gene cluster that contains a single pituitary growth hormone (hGH-N) gene and four placenta-specific paralogs – the placenta growth hormone (hGH-V) and the three placental lactogen genes. In collaboration with the Drs. Nancy Cooke and Stephen Liebhaber (University of Pennsylvania), we tracked structural changes over time in the hGH chromatin locus during placental gene activation in CTB undergoing differentiation to a STB phenotype (Kimura et al., 2007)(Figure 5). The data reveal that gene activation is initiated by H3K4 methylation of HSIII-HSV of each individual placental gene repeat (PGR) unit. Subsequent transcriptional activation is accompanied by acetylation of histones H3 and H4 encompassing the entire placenta-expressed region of the cluster. The distribution and progression of chromatin modifications suggested that each PGR independently initiates transcription. Initial activating chromatin modifications are nucleated within the individual PGR units; and subsequent transcriptional induction relies on additional determinants and more extended chromatin modifications.
Fig 5.
Summary of epigenetic modifications during the transition of human placental CTBs to STBs. (Top) The histone modification patterns in freshly prepared primary CTBs are diagrammed below the map. Prior to terminal STB differentiation, all the placental LCR HSs were already formed and the placental genes were expressed at trace levels, whereas hGH-N was totally inactive. Moderate levels of histone H3K4 dimethylation were observed at discrete regions, including HSV, the sites of the promoters of the placental genes, and the sites of the putative enhancers. Histone H3K4 trimethylation was also established at this point within the PGR regions. In contrast, only minimum levels of H3 acetylation were observed across the locus, and H4 acetylation was limited to HSV and at the placental-gene promoters. (Middle) After 4 days of culture, most of the CTBs fused to form a syncytium and the expression of the placental genes, especially hCS-A (hPL-A) and hCS-B (hPL-B), was dramatically enhanced. Histone H3K4 di- and trimethylation patterns were well established, and the histone H3/H4 acetylation levels increased modestly at the LCR and at the cluster region in a block encompassing the four placental genes. (Bottom) In the full-term placental STBs, histone H3/H4 acetylation increased to maximum levels and the epigenetic patterns necessary for full activation of the placental genes were completed. The four placental genes are expressed at the highest levels, whereas hGH-N, lacking the epigenetic modifications, remains inactive in STBs. From Kimura et al., 2007
Maintenance of structural integrity has also been shown to be critical for the differentiation of CTB to STB. Getsios and co-workers (38) showed that E-cadherin and cadherin 11 are differentially expressed during the aggregation, fusion and terminal differentiation of isolated human CTB. E-cadherin expression was highest in CTB and decreased as the cells underwent fusion and terminal differentiation. In contrast, cadherin-11 expression increased during syncytium formation. Overexpression of cadherin-11 in JEG-3 human choriocarcinoma cells results in the formation of a multinucleated syncytium, a reduction of cell proliferation, a reduction in E-cadherin and α-, β-, and γ-catenin protein in the cytoplasm, an increase in β-catenin in the nucleus and an increase in hCG expression. Taken together, these observations strongly support the hypothesis that catenins play a critical role in controlling transcription and transducing cell and tissue structural status to the transcriptional machinery.
Gene expression profiling of genes that are regulated during differentiation of human CTB to STB phenotype
To study the genetic program that directs human placental differentiation, my laboratory performed DNA microarray analyses on a highly purified preparation of human CTB undergoing spontaneous differentiation (Aronow et al., 2001; Handwerger and Aronow, 2003). RNA samples were analyzed at frequent intervals over a 6 day period. Initial analyses were performed using the Incyte Human GEM V microarray, which contains 7197 probe sets, while later analyses were performed with the Affymetrix U133 chip, which contains 54,675 probe sets. There was an excellent agreement between the two microarray platforms in that 94% of the regulated genes detected by the Incyte microarray were also detected by the Affymetrix microarray. During the first 2–3 days, the highly purified CTB spontaneously aggregated and fused to form a multinucleated syncytium. During the subsequent culture days, the cells expressed abundant amounts of placental lactogen, human chorionic gonadotropin, and other proteins normally expressed by terminally differentiated STB. Of the 54675 genes present on the genome-wide Affymetrix microarray, 1062 were induced and 1592 were downregulated by more than 2-fold. The dynamically regulated genes fell into nine distinct kinetic patterns of induction or repression as detected by the K-means algorithm.
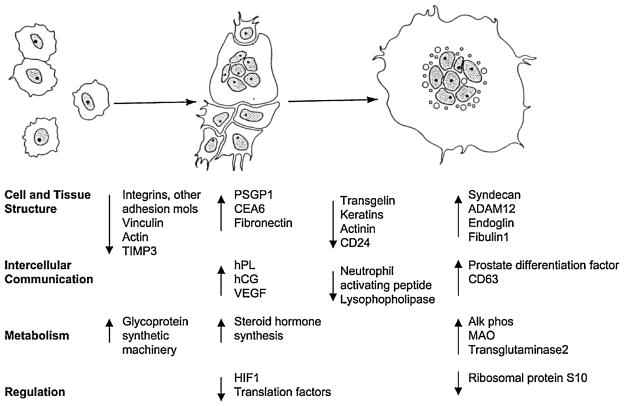
The regulated genes were divided into six overall categories based on functional characteristics: cell and tissue structural dynamics, cell cycle and apoptosis, intercellular communication, metabolism, regulation of gene expression, and expressed sequence tags with function unknown. Gene expression changes within key functional categories were tightly coupled to the morphological changes that occurred during trophoblast differentiation (Figure 6). Within several key gene categories (e.g., cell and tissue structure), many genes were strongly activated, while others with related function were strongly repressed. These findings suggest that trophoblast differentiation is augmented by “categorical reprogramming” in which the ability of induced genes to function is enhanced by diminished expression of other genes within the same category. Taken together, these findings point to a fundamental role for simultaneous induction and repression of mRNAs that encode functionally related proteins during the differentiation process. Of the 102 most induced genes detected during trophoblast differentiation, 92 have one or more TFAP2 binding sites, 75 have NR2F2 binding sites and 82 have FOXF1 binding sites in their promoter regions. Interestingly, none of the 100 most repressed genes contained an TFAP2 binding site. Syncytin, TFAP2A, NR2F2 and FOXF1 mRNA levels increased during the first day of differentiation when the cells were aggregating and beginning to syncytialize. These observations, along with the studies described below, support a role for TFAP2A, NR2F2 and FOXF1 in the regulation of trophoblast differentiation. Bioinformatic analysis of the NR2F2 and FOXF1 promoters also revealed the presence of common transcription factor binding sites, suggesting that the transcription factors that bind to these common binding sites may be important in coordinating the expression of the NR2F2 and FOXF1 genes during trophoblast differentiation. These studies provide the critical information necessary to delineate the genes and pathways involved in STB development.
Figure 6.
Categorical reprogramming-based scheme for CTB cell differentiation. Major genes and functional groups that exhibit dynamic expression changes are depicted in relationship to the morphological changes that accompany CTB cell formation during the 6-day culture period. Integrins refers to integrins-a2, -a6, and -b6. Adhesion proteins refers to cadherin 1, 3 and 5, annexins A3 and A8, CD24, and transgelin. TIMP3, tissue inhibitor of metalloproteinase 3; CEA6, carcinoembryonic antigen gene family member 6; and MAO, monoamine oxidase A. We hypothesize that there is categorical reprogramming of gene expression, particularly within the category of cell and tissue structure genes, which is necessary to accomplish the marked cell morphology changes that occur during trophoblast differentiation. Categorical reprogramming represents the simultaneous activation, repression or degradation of mRNAs from within a given functional group. From Aronow et al., 2001.
Role of syncytin in cell fusion and syncytium formation
Although relatively little is known about the factors that mediate the fusion of CTB to form a syncytium, several proteins have been shown to be critical for the fusion process, including caspase 8 and syncytin. Syncytin is the envelope protein of a recently identified human endogenous defective retrovirus (HERV-W) that is expressed in the human in STB and, to a lesser amount, in testes but not other cell types. Syncytin induces syncytium formation on interaction with the D type mammalian retrovirus receptor (RDR, also known to be the neutral amino acid transporter ATB0/ASCT2/SLC1A5) (Blond et al., 2000; Chen et al., 2006; Huppertz et al., 2006). Expression of recombinant syncytin in a wide variety of cell types induces formation of giant syncytia, and fusion of a human trophoblast cell line expressing endogenous syncytin can be inhibited by an anti-syncytin antiserum. Syncytin transcription in BeWo cells increases 5-fold or more in response to treatment with forskolin, correlating well with the increased fusion observed under these conditions. In contrast, JEG3 cells, which do not fuse in response to forskolin, show very low syncytin expression. Fusion of BeWo cells with COS cells can be achieved in the absence of forskolin if BeWo cells are first transfected with a syncytin expression plasmid, indicating that the expression of syncytin is sufficient to produce the fusion of the two cell types (Figure 7). In recent experiments, we have confirmed that syncytin mRNA is upregulated during forskolin-induced differentiation of BeWo cells, and our laboratory and others (Potgens et al., 2004; Chang et al., 2005) have shown that syncytin is also induced during in vitro differentiation of villous CTB.
Figure 7.
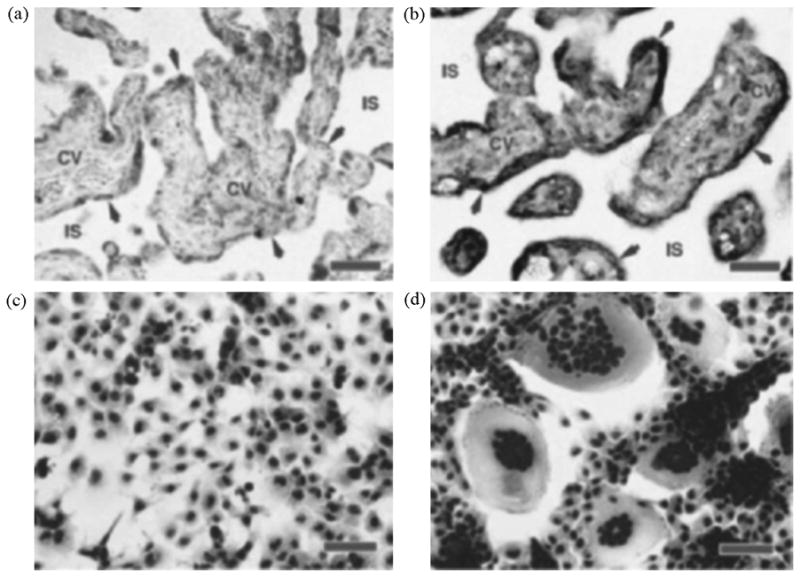
In situ hybridizations and syncytin-mediated COS cell fusion. a,b, In situ hybridizations of sections taken through the villous region of human term placenta. a, Negative control, section probed with digoxigenin-labelled sense-strand syncytin RNA. b, Section probed with digoxigenin-labelled antisense-strand syncytin RNA. Arrowheads, syncytiotrophoblast layer; CV, chorionic villi (fetal tissue); IS, intervillous space (maternal blood space). c,d, Syncytin-mediated cell fusion. c, COS cells transfected with a vector containing the syncytin gene in the reverse orientation for expression (negative control). d, COS cells transfected with a vector containing the syncytin gene in the sense orientation. Red scale bars, 100 mm. From Mi et al., 2000.
Another human endogenous retrovirus, HERV-FRD, has also been shown to encode an envelope protein in human placenta. Because of its similarities to syncytin 1, this protein has been designated as syncytin 2 (Blaise et al., 2003; Malassine et al., 2006). Syncytin 2 is detected only in the villous trophoblast of the chorionic villi, at the level of CTB. Immunostaining for syncytin 2, however, is not observed in all CTB, with staining frequently at the membrane level at the interface between the CTB and STB. In vitro detection of HERV-FRD transcripts is restricted to villous trophoblastic cells and decreases significantly with time in culture. While these results suggest that syncytin 2 might play a role in human trophoblastic cell fusion, direct evidence to support this hypothesis is lacking. Several other factors have been demonstrated to be involved directly in trophoblast cell fusion, including the phosphatidylserine flip (Morrish et al., 2001), connexin 43 (Frendo et al., 2003a; Knerr et al., 2005), cadherin 11 (Getsios and MacCalman, 2003), CD98 (Kudo et al., 2004), and caspase 8 (Black et al., 2004), but the possible interaction of these factors with syncytin 1 and syncytin 2 are unknown.
Since the syncytin promoter had not been identified, my laboratory isolated the 5′-flanking region of the syncytin gene from human genomic DNA by PCR and identifyed several cis-acting elements on the promoter that are important for transcription (Cheng et al., 2004). The major transcription initiation site was identified 56 base pairs (bp) downstream from a putative CCAAT box. Deletion analysis indicated that the proximal 148 bp of the 5′-flanking region are essential for minimal promoter activity and that regions of the promoter from nt −1519 to −984 and nt −294 to −148 are required for maximal expression in normal trophoblast cells. DNase I footprint analysis of the region between nt −252 and +110 revealed three protected regions, FP1-FP3. Mutation of the CCAAT motif and the octamer protein (Oct) binding site in FP2 decreased promoter activity while mutation of the ecdysone receptor (EcR) response element in FP2 increased basal promoter activity. Gel shift and supershift assays indicated that CCAAT-binding factor (CBF) binds to the CCAAT motif and that Oct-1 binds to the Oct binding site. Taken together, these findings indicate that the syncytin promoter is located in the 5′ long terminal repeat (LTR) of the HERV-W gene and that binding sites for CBF and Oct-1 in the proximal promoter are critical for transcriptional regulation of the gene in trophoblast cells.
We (Cheng and Handwerger, 2005) subsequently identified a more distal 146 bp region of the 5′-flanking region of the human syncytin gene from nt−294 to −148 that is essential for basal gene expression in human BeWo and JEG3 choriocarcinoma cell lines but not in hepatoblastoma and kidney cell lines (Cheng et al., 2004). DNase I footprint assays indicated that nuclear extracts from BeWo but not HepG2 cells protected four regions (FP1-FP4) of the 146-bp fragment. Site-directed mutagenesis of an Sp1-binding site in FP3 and a GATA-binding site in FP4 significantly repressed promoter activity in the placenta cells (>80% for both). Overexpression of Sp1 and GATA-2/3 induced syncytin promoter activity, but had little or no effects on syncytin promoter fragments containing mutations in the Sp1 and GATA binding sites. GATA-2 and GATA-3 mRNA levels increased markedly during in vitro differentiation prior to cell fusion. These findings strongly suggest that the 146-bp region of the 5′-flanking region (nt−294/−148) of the human syncytin gene acts as a placenta-specific enhancer and that binding of SP1 and GATA family members to this enhancer is critical for cell-specific expression of the syncytin gene.
Since the syncytin promoter contains several putative nuclear hormone binding sites and the nuclear hormone receptors RXRA and NR2F2 are induced early during human CTB differentiation, we subsequently examined whether these transcription factors are involved in the induction of syncytin gene expression (submitted). Overexpression of NR2F2 alone and RXRA alone induced luciferase activity, and overexpression of NR2F2 and RXRA together induced luciferase activity to a greater extent than either NR2F2 or RXRA alone. Although, NR2F2 has been shown to inhibit transactivation of many other promoters by retinoic acid and other nuclear hormone receptors, these findings strongly suggest that NR2F2 and RXRA act synergistically to induce syncytin gene expression. In addition, retinoic acid, which is known to induce syncytialization of human CTB, may act, at least in part, by activation of RXRα and NR2F2.
Since the syncytin promoter also contains a binding site for members of the ETS family of transcription factors, we examined whether ETS1 is important for the basal activity of the syncytin promoter and whether ETS1 can modulate the transactivation of the promoter by NR2F2 (unpublished observations). Forced overexpression of ETS1 alone had no effect on basal syncytin promoter activity but caused a dose-dependent inhibition of NR2F2-induced syncytin promoter activity. The presence of an ETS1 binding site on the syncytin promoter, however, was not necessary for the inhibitory action of ETS1 since ETS1 inhibited NR2F2-induced transactivation of a syncytin fragment (nt −148/+24) lacking an ETS binding site. The inhibitory effect of ETS1 on transactivation of the syncytin promoter by NR2F2 strongly suggests a role for ETS1 in modulation of cytotrophoblast cell fusion.
Since the syncytin promoter contains a putative FOXF1 binding site, we examined whether FOXF1 also transactivates the syncytin promoter. In transient transfection studies, overexpression of FOXF1 but not FOXF2, induced syncytin promoter activity. Since FOXF2 binds to the same DNA elements as FOXF1, this finding suggests that transcription activation by FOXF1 may require the binding of one or more co-activators that do not activate FOXF2. Since FOXF1 mRNA levels are induced in the early stage of differentiation prior to cell fusion, these findings strongly suggest a role for FOXF1 in the induction of human CTB differentiation
Role of TFAP2A in terminal trophoblast differentiation
Recent studies from our laboratory indicate that TFAP2A plays a critical role in the induction of human placenta-specific genes and human trophoblast differentiation. While syncytin appears to be critical for morphological differentiation of CTB, the transcription factor TFAP2A appears to be critical for biochemical differentiation. In our initial studies, we showed that TFAP2A transactivates the hPL (Richardson et al., 2000), hCGα and hCGβ (Johnson et al., 1997) promoters and that mutagenesis of the TFAP2 binding sites on the promoters of these genes prevents the induction of hPL and hCG gene expression during trophoblast differentiation. TFAP2A potentiates the transactivation of the corticotropin releasing hormone (CRH) promoter by CREB However, the induction of promoter activity was not due to the binding of TFAP2A to the promoter but was due to the binding of TFAP2A to CREB and the potentiation of CREB-mediated transactivation. These findings strongly suggest that TFAP2A can induce gene expression during trophoblast differentiation by both binding directly to cis-acting DNA elements and binding to CREB as a co-activator, thus potentiating the actions of CREB on gene expression (Cheng and Handwerger, 2002). Moreover, TFAP2A also stimulates expression of the genes for aromatase cytochrome P-450 (CYP11A1)(Yamada et al., 1995), germ cell alkaline phosphatase (Wada and Chou, 1993) and 17β-hydroxysteroid dehydrogenase type 1 (Piao et al., 1997) in trophoblast cell lines. A role for TFAP2A in ovine placental differentiation is suggested by the observation that TFAP2A induces placenta lactogen gene expression in the sheep (Liang et al., 1999); and a role in bovine placental differentiation is suggested by a global gene expression study showing a role for TFAP2A in the regulation of PL and many other genes expressed by terminally differentiated bovine placental cells (Ushizawa et al., 2007).
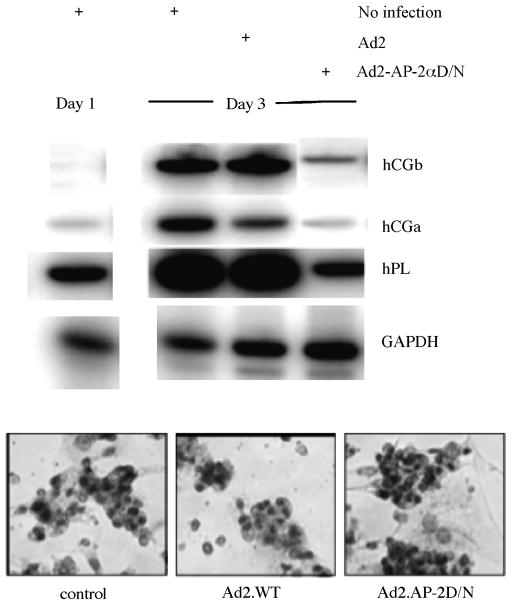
In an additional set of experiments to determine whether TFAP2A is a critical component of the genetic program that directs human trophoblast differentiation, we used DNA microarray analyses to characterize the effects of a dominant-negative form of the TFAP2 protein (Ad2.TFAP2D/N, which binds to DNA but lacks an activating domain) on in vitro differentiating CTB (Cheng et al., 2004) (Figure 8). DNA microarray analyses using Affymetrix human U95Av2 GeneChips were performed on RNA extracted from primary human CTB cells immediately prior to and after 3 days of cell culture. Cells infected with Ad2.TFAP2D/N or Ad2.WT (wild type) underwent morphological differentiation similar to that of uninfected cells, with greater than 90% of the cells in each group fusing to form multinucleated STB. However, Ad2.TFAP2D/N markedly inhibited the induction or repression of many genes that were regulated in the noninfected and Ad2.WT-infected cells during differentiation. Eighteen of the 25 most induced genes and 17 of the 20 most repressed genes during differentiation were TFAP2 dependent, with the majority of these related to extracellular organization, cellular communication, and signal transduction. Taken together, these findings strongly suggest that TFAP2 plays a critical role for both the induction and repression of genes that play a role in postsyncytialization gene expression programs of trophoblast differentiation and maturation. TFAP2, however, is not required for the fusion of CTB to form a syncytium or the expression of syncytin. In a recent preliminary experiment, we observed that when CTB were simultaneously infected with Ad2.WT and Ad2.TFAP2D/N, the overexpression of TFAP2A could rescue the inhibitory effect of TFAP2D/N on the mRNA levels of the STB-specific genes hPL, hCG, CRH and PSG1. This experiment indicates that the effect of the D/N protein was mediated by binding to TFAP2 binding sites.
Figure 8.
Effect of Ad2.AP-2D/N on placental lactogen (hPL) and chorionic gonadotropin (hCG and hCG) mRNA levels during trophoblast differentiation. RNA was isolated from the three groups of cells. HPL, hCG, and hCG mRNA levels were then determined by RT-PCR. Note that hPL, hCG,hCG, and GAPDH mRNA levels increased markedly in the control and Ad2.WT-infected cells. Ad2.AP-2D/N cells, however, had little or no increase in these mRNAs. From Cheng et al., 2004.
The TFAP2 transcription factor family consists of three isoforms (α, β and γ) that are produced by separate genes (Bar-Eli, 1999). Two of the isoforms, TFAP2A and TFAP2γ, are expressed in the human placenta. Numerous investigations have shown that TFAP2A is important in differentiation of many cell types, including the differentiation of keratinocytes and neural crest cells as well as the differentiation of 3T3-LI fibroblasts to adipocytes (Zeng et al., 1997). Most studies suggest that TFAP2A and TFAP2G bind to the same DNA element (GCCNNNGGC)(Williams and Tjian, 1991). However, there appear to be significant differences in the biological actions mediated by the two isoforms. For example, TFAP2A knockout mice die at birth, while TFAP2γ knockout mice die at about 7.5 days of gestation before complete formation of the placenta (Auman et al., 2002). This finding suggests that the two isoforms are activating different targets, perhaps due to the binding of the transcription factors of different partners, co-activators and/or co-repressors (Imhof et al., 1999).
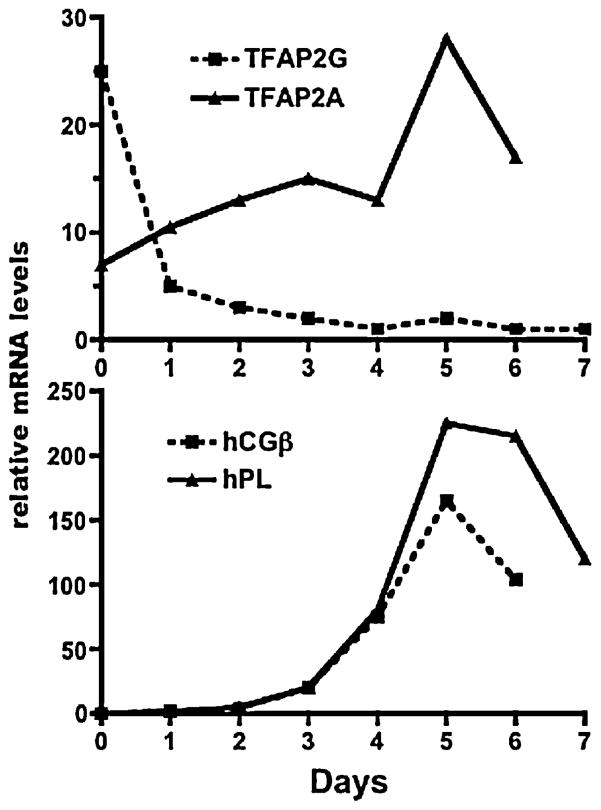
At present, the factors that regulate TFAP2A gene expression in the placenta are poorly understood. As discussed in detail below, TFAP2A gene expression in some, but not all cell types, is induced by cAMP, prostaglandins, retinoic acid, thyroid hormone, inflammatory cytokines and estrogen (French et al., 1994; Micali et al., 1996; Wanner et al., 1996; Bourbonniere et al., 1997; Oyama et al., 1999). In addition, the transcription factor Sp1, the retinoblastoma (Rb) protein, the glucocorticoid receptor (GR), β-catenin (Li and Dashwood, 2004) and other proteins form complexes with TFAP2A in some cells to synergize or repress the activity of TFAP2A (Ebert et al., 1998; Wu and Lee, 1998). However, the effects of these factors on the activity of TFAP2G in the placenta and other cell types are unknown. The recent observation that forskolin induces TFAP2A but not TFAP2γ expression in JEG-3 choriocarcinoma cells suggests that the two TFAP2 family members are differentially regulated in normal trophoblast cells (Wu and Lee, 1998). Our studies of the ontogeny of the TFAP2 isoforms in human CTB during differentiation reveal markedly different patterns for TFAP2A and TFAP2G (Figure 9). TFAP2A mRNA levels significantly increase during CTB differentiation, with the increase paralleling the increases in hPL, hCGα and hCGβ mRNA levels. TFAP2γ mRNA levels, on the other hand, markedly decrease during differentiation, suggesting that TFAP2γ is the predominant TFAP2 isoform in CTB and TFAP2A is the predominant isoform during CTB differentiation and in fully differentiated STB cells. At present, the cellular processes modulated by TFAP2A during STB formation are unknown. Several investigators have observed that TFAP2A induces apoptosis by downregulating Bcl-2 via a BAX/cytochrome c/Apaf1/caspase/9-dependent mitochondrial pathway (Muller et al., 2004; Wajapeyee et al., 2006). Hilger-Eversheim observed that TFAP2A inhibits cell growth by inducing both cell cycle arrest and apoptosis (Hilger-Eversheim et al., 2000). Overexpression of TFAP2A was found to inhibit cell division and colony formation, whereas down-regulation of TFAP2A with a dominant-negative TFAP2A mutant was found to increase invasiveness and tumorigenicity (McPherson et al., 2002). The inhibition of cell proliferation by TFAP2A was due to the induction of G(1) and G(2) cell cycle arrest in the presence of p53. TFAP2A has also been shown to regulate genes involved in the maintenance of cell structure such as E-cadherin (Batsche et al., 1998), metalloproteinases and connexin (Hammani et al., 1996; Seul et al., 1997). TFAP2A also functions as a tumor suppresser in breast cancer (Turner et al., 1998), colon cancer (Schwartz et al., 2007) and malignant melanoma (Bar-Eli, 1999). Since the abundance of TFAP2A in choriocarcinoma cells is very low, diminished TFAP2A action in choriocarcinoma cells may contribute to the malignant transformation of the cells and the failure to differentiate to a STB phenotype.
Figure 9.
Marked differences in TFAP2G and TFAP2A mRNA expression profiles during human trophoblast cell differentiation. Human trophoblast cells, prepared by enzymatic digestion of term placental tissue and purified by negative selection with an antiserum to CD9, were cultured for seven days. The top panel shows the amounts of TFAP2G and GAPDH mRNA levels, determined by RT-PCR, at the beginning of the culture period (day 0) and at days 1–7. The amounts of hPL and hCGβ mRNAs, determined by RT-PCR from the same mRNA samples as TFAP2A and TFAP2G (and normalized to GAPDH mRNA) are shown in the bottom panel. Similar patterns of TFAP2G mRNA levels were observed in two other experiments using trophoblast cells from different placentas. The patterns for TFAP2A, hPL and hCG mRNAs are nearly identical to that observed earlier From Richardson et al., 2001.
Since NR2F2 transactivates the syncytin promoter, we examined whether NR2F2 also transactivates the TFAP2A promoter. HepG2 cells, which do not express TFAP2A, were transfected with a plasmid containing the TFAP2A promoter coupled to a luciferase reporter gene in the presence or absence of an NR2F2 expression plasmid. Luciferase activity in the cells cotransfected with the NR2F2 expression plasmid was much greater than that of cells co-transfected with the plasmid lacking the NR2F2 promoter. A NR2F2 siRNA markedly blocked the expression of the mRNAs for NR2F2, TFAP2A, hPL, CRH and pregnancy specific glycoprotein 1 (PSG1). NR2F2 significantly potentiated retinoid receptor induced transactivation of the TFAP2A promoter. The effect of NR2F2 on TFAP2A promoter activity, however, was not potentiated by overexpression of vitamin D3R, PPARγ or T3Rβ. Taken together, these results suggest that NR2F2 may regulate trophoblast differentiation by activation of TFAP2A gene expression and by potentiation of the effects of RARα and RXRα. The induction of trophoblast differentiation by retinoids may also be mediated, at least in part, by activation of TFAP2A expression. These experiments and our transient transfection studies with NR2F2 provide compelling evidence that NR2F2 is a component of the transcriptional network that directs the differentiation of human cytotrophoblast cells to a syncytiotrophoblast phenotype.
A summary of the regulation of villous trophoblast differentiation by syncytin and TFAP2A is shown in Figure 10.
Figure 10.
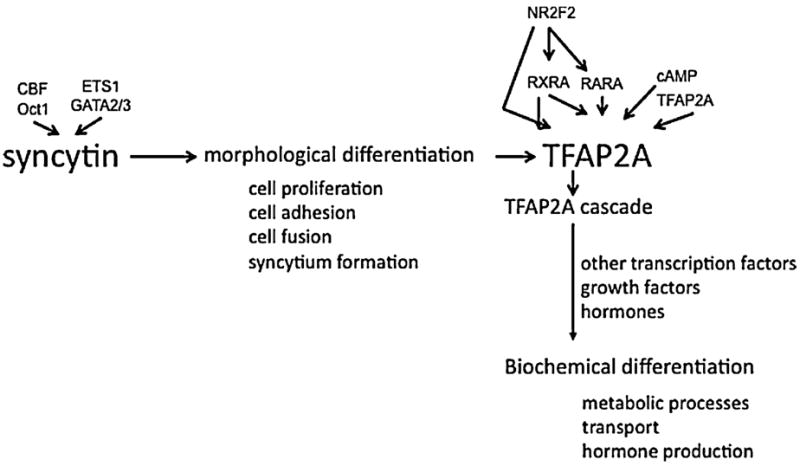
Schematic representation of the regulation of villous trophoblast differentiation by syncytin and TFAP2A. Some of the transcription factors and other signalling molecules that modulate syncytin and TFAP2A expression are indicated.
Gene expression in placentas from patients with pre-eclampsia, choriocarcinoma and other pathologic conditions of pregnancy
Several studies have demonstrated abnormalities in CTB differentiation in choriocarcinoma, pre-eclampsia and a few other pathologic conditions of pregnancy. Choriocarcinoma cells have a phenotype of CTB that have undergone incomplete differentiation. The cells do not fuse to form a syncytium but express abundant amounts of hCG. However, many other proteins normally expressed by terminally differentiated STB, such as hPL, hGH-v and pregnancy-specific glycoprotein 1 are not expressed or are expressed at very low levels. Treatment of choriocarcinoma cells with forskolin and other agents that increase intracellular cAMP levels induce syncytin, cell fusion and hCG expression. However, TFAP2A levels remain very low, and there is no induction of most of the genes expressed by terminally differentiated STB cells. In pre-eclampsia, there is incomplete differentiation of CTB to both invasive and villous CTB cells. Incomplete differentiation to invasive CTB results in shallow invasion of the uterus; and incomplete villous CTB differentiation results in abnormal syncytin expression. Lee and co-workers noted that syncytin 1 expression in the pre-eclamptic placenta is 96% less than that in normal placentas and the localization of syncytin in the STB of pre-eclamptic placentas is at the apical microvillus membrane while the localization in normal placentas is at the basal microvillus membrane (Lee et al., 2001). These findings have now been confirmed in numerous other studies (Knerr et al., 2002; Kudo et al., 2003a; Kudo et al., 2003b; Kudo et al., 2003c; Chen et al., 2006). This decrease in syncytin expression and localization is thought to be responsible for the characterized disturbances in STB noted in pre-eclampsia, such as increased numbers of syncytial knots (abnormal accumulations of syncytiotrophoblast nuclei) and the progressive proliferation of villous CTB (Lee et al., 2001). In addition, the lack of efficient cell fusion is likely responsible for the unstable villous structure and increased trophoblast deportation into the maternal peripheral blood that has been reported in pregnancies affected by pre-eclampsia (Chua et al., 1991). Newhouse and co-workers (3) recently reported a study in which primary term villous CTB from uncomplicated pregnancies and pregnancies complicated with IUGR alone, IUGR with preeclampsia and preeclampsia alone were cultured for five days and the extent of differentiation into STB measured in terms of syncytialization and secretion of hCG and hPL. Normal and IUGR-preeclampsia cells showed low syncytialization and decreased release of hCG and hPL as compared to term cells obtained from women with normal pregnancies. IUGR cells showed the highest level of syncytialization and secretion, and preeclampsia cells showed high syncytialization but low secretion. In a recent study, our laboratory collaborated with Professor Mark Schleiss (University of Minnesota, formerly at the University of Cincinnati) observed that human placentas infected with cytomegalovirus express low levels of TFAP2A and many genes normally expressed by mature STB cells (Schleiss et al., 2007). TFAP2A mRNA levels in the CMV-infected cells were approximately 80% less than that of non-infected cells, and the mRNA levels of most of the TFAP2A-dependent genes were 50–80% less. While there is no evidence at present to indicate that the low TFAP2A expression in these placentas is the cause of decreased expression of the other genes, this possibility should be considered.
Since abnormal differentiation of CTB is a prominent feature of choriocarcinoma and pre-eclampsia, a comprehensive understanding of the transcription factors and signaling molecules that direct normal trophoblast differentiation and the syncytin-TFAP2A pathway should provide new insights into the molecular pathophysiology of these conditions and the development of new therapeutic modalities.
Acknowledgments
I thank the many postdoctoral fellows, collaborators and technicians who have contributed to these studies. Special thanks go to You-Hong Cheng, Anastasis Stephanou, Brian Richardson, Michael Hubert, Susan Sherritt, Jared Robins, Bruce Aronow, Anil Jegga, Stephen Liebhaber and Nancy Cooke. Supported by NIH grant HD-07447.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronow BJ, Richardson BD, Handwerger S. Microarray analysis of trophoblast differentiation: gene expression reprogramming in key gene function categories. Physiological Genomics. 2001;6:105–116. doi: 10.1152/physiolgenomics.2001.6.2.105. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Bar-Eli M. Role of AP-2 in tumor growth and metastasis of human melanoma. Cancer Metastasis Rev. 1999;18:377–385. doi: 10.1023/a:1006377309524. [DOI] [PubMed] [Google Scholar]
- Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol. 1998;18:3647–3658. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. 1995a. [Google Scholar]
- Benirschke K, Kaufmann P. Early development of the human placenta. In: Benirschke K, Kaufmann P, editors. Pathology of the Human Placenta. Springer-Verlag; New York: 1995b. pp. 49–56. [Google Scholar]
- Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004;11:90–98. doi: 10.1038/sj.cdd.4401307. [DOI] [PubMed] [Google Scholar]
- Blaise S, de Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 2003;100:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonniere M, Shekarabi M, Nalbantoglu J. Enhanced expression of amyloid precursor protein in response to dibutyryl cyclic AMP is not mediated by the transcription factor AP-2. J Neurochem. 1997;68:909–916. doi: 10.1046/j.1471-4159.1997.68030909.x. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation--a review. Placenta. 2007;28(Suppl A):S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Castellucci M, Scheper M, Scheffen I, Celona A, Kaufmann P. The development of the human placental villous tree. Anatomy & Embryology. 1990;181:117–128. doi: 10.1007/BF00198951. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chuang HC, Yu C, Yao TP, Chen H. Stimulation of GCMa transcriptional activity by cyclic AMP/protein kinase A signaling is attributed to CBP-mediated acetylation of GCMa. Mol Cell Biol. 2005;25:8401–8414. doi: 10.1128/MCB.25.19.8401-8414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. Bjog. 2006;113:152–158. doi: 10.1111/j.1471-0528.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Aronow BJ, Hossain S, Trapnell B, Kong S, Handwerger S. Critical role for transcription factor AP-2alpha in human trophoblast differentiation. Physiol Genomics. 2004;18:99–107. doi: 10.1152/physiolgenomics.00181.2003. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Handwerger S. AP-2alpha modulates human corticotropin-releasing hormone gene expression in the placenta by direct protein-protein interaction. Mol Cell Endocrinol. 2002;191:127–136. doi: 10.1016/s0303-7207(02)00081-3. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Richardson BD, Hubert MA, Handwerger S. Isolation and characterization of the human syncytin gene promoter. Biol Reprod. 2004;70:694–701. doi: 10.1095/biolreprod.103.023473. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Handwerger S. A placenta-specific enhancer of the human syncytin gene. Biol Reprod. 2005;73:500–509. doi: 10.1095/biolreprod.105.039941. [DOI] [PubMed] [Google Scholar]
- Chua S, Wilkins T, Sargent I, Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991;98:973–979. doi: 10.1111/j.1471-0528.1991.tb15334.x. [DOI] [PubMed] [Google Scholar]
- Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–13. doi: 10.1006/scdb.2000.0156. [MEDLINE record in process] [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Daiter E, Omigbodun A, Wang S, Walinsky D, Strauss JF, 3rd, Hoyer JR, Coutifaris C. Cell differentiation and endogenous cyclic adenosine 3′,5′-monophosphate regulate osteopontin expression in human trophoblasts. Endocrinology. 1996;137:1785–1790. doi: 10.1210/endo.137.5.8612515. [DOI] [PubMed] [Google Scholar]
- Ebert SN, Ficklin MB, Her S, Siddall BJ, Bell RA, Ganguly K, Morita K, Wong DL. Glucocorticoid-dependent action of neural crest factor AP-2: stimulation of phenylethanolamine N-methyltransferase gene expression. J Neurochem. 1998;70:2286–2295. doi: 10.1046/j.1471-4159.1998.70062286.x. [DOI] [PubMed] [Google Scholar]
- French RP, Warshawsky D, Tybor L, Mylniczenko ND, Miller L. Upregulation of AP-2 in the skin of Xenopus laevis during thyroid hormone-induced metamorphosis. Dev Genet. 1994;15:356–365. doi: 10.1002/dvg.1020150407. [DOI] [PubMed] [Google Scholar]
- Frendo JL, Cronier L, Bertin G, Guibourdenche J, Vidaud M, Evain-Brion D, Malassine A. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J Cell Sci. 2003a;116:3413–3421. doi: 10.1242/jcs.00648. [DOI] [PubMed] [Google Scholar]
- Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003b;23:3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lloret MI, Morrish DW, Wegmann TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokine-placental interactions: CSF- 1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Experimental Cell Research. 1994;214:46–54. doi: 10.1006/excr.1994.1232. [DOI] [PubMed] [Google Scholar]
- Getsios S, Chen GT, MacCalman CD. Regulation of beta-catenin mRNA and protein levels in human villous cytotrophoblasts undergoing aggregation and fusion in vitro: correlation with E-cadherin expression. J Reprod Fertil. 2000;119:59–68. doi: 10.1530/jrf.0.1190059. [DOI] [PubMed] [Google Scholar]
- Getsios S, Chen GT, MacCalman CD. alpha-, beta-, gamma-catenin, and p120(CTN) expression during the terminal differentiation and fusion of human mononucleate cytotrophoblasts in vitro and in vivo. Mol Reprod Dev. 2001;59:168–177. doi: 10.1002/mrd.1019. [DOI] [PubMed] [Google Scholar]
- Getsios S, MacCalman CD. Cadherin-11 modulates the terminal differentiation and fusion of human trophoblastic cells in vitro. Dev Biol. 2003;257:41–54. doi: 10.1016/s0012-1606(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Hammani K, Blakis A, Morsette D, Bowcock AM, Schmutte C, Henriet P, DeClerck YA. Structure and characterization of the human tissue inhibitor of metalloproteinases-2 gene. J Biol Chem. 1996;271:25498–25505. doi: 10.1074/jbc.271.41.25498. [DOI] [PubMed] [Google Scholar]
- Handwerger S, Aronow B. Dynamic changes in gene expression during human trophoblast differentiation. Recent Prog Horm Res. 2003;58:263–281. doi: 10.1210/rp.58.1.263. [DOI] [PubMed] [Google Scholar]
- Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260:1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Bartz C, Kokozidou M. Trophoblast fusion: Fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006 doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Imhof A, Schuierer M, Werner O, Moser M, Roth C, Bauer R, Buettner R. Transcriptional regulation of the AP-2alpha promoter by BTEB-1 and AP-2rep, a novel wt-1/egr-related zinc finger repressor. Molecular & Cellular Biology. 1999;19:194–204. doi: 10.1128/mcb.19.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatpour MJ, McMaster MT, Genbacev O, Zhou Y, Dong J, Cross JC, Israel MA, Fisher SJ. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- Johnson W, Albanese C, Handwerger S, Williams T, Pestell RG, Jameson JL. Regulation of the human chorionic gonadotropin alpha- and beta-subunit promoters by AP-2. J Biol Chem. 1997;272:15405–15412. doi: 10.1074/jbc.272.24.15405. [DOI] [PubMed] [Google Scholar]
- Kimura AP, Sizova D, Handwerger S, Cooke NE, Liebhaber SA. Epigenetic activation of the human growth hormone gene cluster during placental cytotrophoblast differentiation. Mol Cell Biol. 2007;27:6555–6568. doi: 10.1128/MCB.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3d Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Knerr I, Beinder E, Rascher W. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am J Obstet Gynecol. 2002;186:210–213. doi: 10.1067/mob.2002.119636. [DOI] [PubMed] [Google Scholar]
- Knerr I, Schubert SW, Wich C, Amann K, Aigner T, Vogler T, Jung R, Dötsch J, Rascher W, Hashemolhosseini S. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 2005;579:3991–3998. doi: 10.1016/j.febslet.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Knofler M, Kalionis B, Huelseweh B, Bilban M, Morrish DW. Novel genes and transcription factors in placental development--a workshop report. Placenta. 2000;21(Suppl A):S71–3. doi: 10.1053/plac.1999.0531. [DOI] [PubMed] [Google Scholar]
- Kojima K, Kanzaki H, Iwai M, Hatayama H, Fujimoto M, Narukawa S, Higuchi T, Kaneko Y, Mori T, Fujita J. Expression of leukaemia inhibitory factor (LIF) receptor in human placenta: a possible role for LIF in the growth and differentiation of trophoblasts. Human Reproduction. 1995;10:1907–1911. doi: 10.1093/oxfordjournals.humrep.a136205. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Kimura H, Cook PR, Redman CW, Sargent IL. Quantifying the syncytialisation of human placental trophoblast BeWo cells grown in vitro. Biochim Biophys Acta. 2003a;1640:25–31. doi: 10.1016/s0167-4889(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Millo J, Sargent IL, Redman CW. Manipulation of CD98 expression affects both trophoblast cell fusion and amino acid transport activity during syncytialization of human placental BeWo cells. J Physiol. 2003b;550:3–9. doi: 10.1113/jphysiol.2003.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Sargent IL, Redman CW. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim Biophys Acta. 2003c;1638:63–71. doi: 10.1016/s0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Sargent IL, Redman CW, Lee JM, Freeman TC. An analysis using DNA microarray of the time course of gene expression during syncytialization of a human placental cell line (BeWo) Placenta. 2004;25:479–488. doi: 10.1016/j.placenta.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22:808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- Li Q, Dashwood RH. Activator protein 2alpha associates with adenomatous polyposis coli/beta-catenin and Inhibits beta-catenin/T-cell factor transcriptional activity in colorectal cancer cells. J Biol Chem. 2004;279:45669–45675. doi: 10.1074/jbc.M405025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Limesand SW, Anthony RV. Structure and transcriptional regulation of the ovine placental lactogen gene. Eur J Biochem. 1999;265:883–895. doi: 10.1046/j.1432-1327.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- Malassine A, Blaise S, Handschuh K, Lalucque H, Dupressoir A, Evain-Brion D, Heidmann T. Expression of the Fusogenic HERV-FRD Env Glycoprotein (Syncytin 2) in Human Placenta is Restricted to Villous Cytotrophoblastic Cells. Placenta. 2006 doi: 10.1016/j.placenta.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000;156:321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo T, Murata K, Matsuo H, Samoto T, Mochizuki M. Insulin-like growth factor-I as a local regulator of proliferation and differentiated function of the human trophoblast in early pregnancy. Early Pregnancy. 1995;1:54–61. [PubMed] [Google Scholar]
- Matsuo H, Maruo T, Murata K, Mochizuki M. [Epidermal growth factor regulates trophoblast proliferation and differentiation in an autocrine/paracrine manner] Nippon Sanka Fujinka Gakkai Zasshi. 1993;45:23–30. [PubMed] [Google Scholar]
- McPherson LA, Loktev AV, Weigel RJ. Tumor suppressor activity of AP2alpha mediated through a direct interaction with p53. J Biol Chem. 2002;277:45028–45033. doi: 10.1074/jbc.M208924200. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, Jr, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Micali A, Medici N, Sottile A, Venza M, Venza I, Nigro V, Puca GA, Teti D. Prostaglandin E2 induction of binding activity to CRE and AP-2 elements in human T lymphocytes. Cell Immunol. 1996;174:99–105. doi: 10.1006/cimm.1996.0298. [DOI] [PubMed] [Google Scholar]
- Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39:179–195. doi: 10.1016/s0165-0378(98)00021-7. [DOI] [PubMed] [Google Scholar]
- Morrish DW, Dakour J, Li H. Life and death in the placenta: new peptides and genes regulating human syncytiotrophoblast and extravillous cytotrophoblast lineage formation and renewal. Curr Protein Pept Sci. 2001;2:245–259. doi: 10.2174/1389203013381116. [DOI] [PubMed] [Google Scholar]
- Muller FU, Loser K, Kleideiter U, Neumann J, von Wallbrunn C, Dobner T, Scheld HH, Bantel H, Engels IH, Schulze-Osthoff K, Schmitz W. Transcription factor AP-2alpha triggers apoptosis in cardiac myocytes. Cell Death Differ. 2004;11:485–493. doi: 10.1038/sj.cdd.4401383. [DOI] [PubMed] [Google Scholar]
- Nomura S, Ito T, Yamamoto E, Sumigama S, Iwase A, Okada M, Shibata K, Ando H, Ino K, Kikkawa F, Mizutani S. Gene regulation and physiological function of placental leucine aminopeptidase/oxytocinase during pregnancy. Biochim Biophys Acta. 2005;1751:19–25. doi: 10.1016/j.bbapap.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Oyama N, Iwatsuki K, Homma Y, Kaneko F. Induction of transcription factor AP-2 by inflammatory cytokines in human keratinocytes. J Invest Dermatol. 1999;113:600–606. doi: 10.1046/j.1523-1747.1999.00734.x. [DOI] [PubMed] [Google Scholar]
- Peng C. The TGF-beta superfamily and its roles in the human ovary and placenta. J Obstet Gynaecol Can. 2003;25:834–844. doi: 10.1016/s1701-2163(16)30674-0. [DOI] [PubMed] [Google Scholar]
- Piao YS, Peltoketo H, Vihko P, Vihko R. The proximal promoter region of the gene encoding human 17beta- hydroxysteroid dehydrogenase type 1 contains GATA, AP-2, and Sp1 response elements: analysis of promoter function in choriocarcinoma cells. Endocrinology. 1997;138:3417–3425. doi: 10.1210/endo.138.8.5329. [DOI] [PubMed] [Google Scholar]
- Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knofler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgens AJ, Drewlo S, Kokozidou M, Kaufmann P. Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum Reprod Update. 2004;10:487–496. doi: 10.1093/humupd/dmh039. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, Hartman SM, Handwerger S. Human cytotrophoblast cells cultured in maternal serum progress to a differentiated syncytial phenotype expressing both human chorionic gonadotropin and human placental lactogen. Endocrinology. 1994;135:321–329. doi: 10.1210/endo.135.1.8013368. [DOI] [PubMed] [Google Scholar]
- Richardson B, Cheng Y-H, Langland RA, Handwerger S. Differential expression of AP-2 gamma and AP-2 alpha during human trophoblast differentiation. Life Sciences. 2001 doi: 10.1016/s0024-3205(01)01299-1. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Langland RA, Bachurski CJ, Richards RG, Kessler CA, Cheng Y, Handwerger S. Activator protein-2 regulates human placental lactogen gene expression. Mol Cell Endocrinol. 2000;160:183–192. doi: 10.1016/s0303-7207(99)00209-9. [Record as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- Robins J, Heizer A, Hardiman A, Hubert MA, Handwerger S. Oxygen tension dirests the differentiation pathway of cytotrophoblast cells. Placenta. doi: 10.1016/j.placenta.2007.05.006. in press. [DOI] [PubMed] [Google Scholar]
- Robins JC, Heizer A, Hardiman A, Hubert M, Handwerger S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta. 2007;28:1141–1146. doi: 10.1016/j.placenta.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab. 2000;85:3874–3881. doi: 10.1210/jcem.85.10.6885. [DOI] [PubMed] [Google Scholar]
- Schleiss MR, Aronow BJ, Handwerger S. Cytomegalovirus infection of human syncytiotrophoblast cells strongly interferes with expression of genes involved in placental differentiation and tissue integrity. Pediatr Res. 2007;61:565–571. doi: 10.1203/pdr.0b013e318045be6d. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Melnikova VO, Tellez C, Mourad-Zeidan A, Blehm K, Zhao YJ, McCarty M, Adam L, Bar-Eli M. Loss of AP-2alpha results in deregulation of E-cadherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene. 2007;26:4049–4058. doi: 10.1038/sj.onc.1210193. [DOI] [PubMed] [Google Scholar]
- Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seul KH, Tadros PN, Beyer EC. Mouse connexin40: gene structure and promoter analysis. Genomics. 1997;46:120–126. doi: 10.1006/geno.1997.5025. [DOI] [PubMed] [Google Scholar]
- Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387–1395. doi: 10.1210/endo.132.3.7679981. [DOI] [PubMed] [Google Scholar]
- Strauss JF, 3rd, Kido S, Sayegh R, Sakuragi N, Gafvels ME. The cAMP signalling system and human trophoblast function. Placenta. 1992;13:389–403. doi: 10.1016/0143-4004(92)90047-w. [DOI] [PubMed] [Google Scholar]
- Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, Glazer PM, Hurst HC, Haffty BG, Williams T. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res. 1998;58:5466–5472. [PubMed] [Google Scholar]
- Ushizawa K, Takahashi T, Hosoe M, Ishiwata H, Kaneyama K, Kizaki K, Hashizume K. Global gene expression analysis and regulation of the principal genes expressed in bovine placenta in relation to the transcription factor AP-2 family. Reprod Biol Endocrinol. 2007;5:17. doi: 10.1186/1477-7827-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Chou JY. Characterization of upstream activation elements essential for the expression of germ cell alkaline phosphatase in human choriocarcinoma cells. J Biol Chem. 1993;268:14003–14010. [PubMed] [Google Scholar]
- Wajapeyee N, Britto R, Ravishankar HM, Somasundaram K. Apoptosis induction by activator protein 2alpha involves transcriptional repression of Bcl-2. J Biol Chem. 2006;281:16207–16219. doi: 10.1074/jbc.M600539200. [DOI] [PubMed] [Google Scholar]
- Wanner R, Zhang J, Henz BM, Rosenbach T. AP-2 gene expression and modulation by retinoic acid during keratinocyte differentiation. Biochem Biophys Res Commun. 1996;223:666–669. doi: 10.1006/bbrc.1996.0952. [DOI] [PubMed] [Google Scholar]
- Williams T, Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991;5:670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Wu F, Lee AS. Identification of AP-2 as an interactive target of Rb and a regulator of the G1/S control element of the hamster histone H3.2 promoter. Nucleic Acids Res. 1998;26:4837–4845. doi: 10.1093/nar/26.21.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Harada N, Honda S, Takagi Y. Regulation of placenta-specific expression of the aromatase cytochrome P-450 gene. Involvement of the trophoblast-specific element binding protein. Journal of Biological Chemistry. 1995;270:25064–25069. doi: 10.1074/jbc.270.42.25064. [DOI] [PubMed] [Google Scholar]
- Zeng YX, Somasundaram K, el-Deiry WS. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nat Genet. 1997;15:78–82. doi: 10.1038/ng0197-78. [DOI] [PubMed] [Google Scholar]