Abstract
The androgen receptor gene (AR) plays an important role in molecular signaling and regulation and the subsequent cellular growth of prostate cancer. In addition, it is a highly variable region of the genome. We used direct nucleotide sequencing to genotype the entire exogenous coding region of the androgen receptor in ten commonly used prostate cancer cell lines. Our analysis confirmed the presence or absence of several known SNPs in the cell lines studied. We also assayed the number of CAG-repeat and GGC-repeat sequences for each for the ten cell lines.
Our analysis identified three new mutations, one each in the DU145, LnCAP, and RWPE-2 cell lines. In DU145, the DNA isolated in our lab was heterozygous at G527G (T>C transition), a polymorphism not previously reported. The LnCAP cells cultured in our lab were found to have a T>C transition (heterozygous), resulting in a S641P change that was not present in the ATCC cell line DNA. Lastly, a homozygous G>T transversion was found in RWPE-2 cells, resulting in the S187I change. This is potentially significant for use in cell culture and future cell model development.
Keywords: androgen receptor, prostate cancer, LnCAP, SNP, CAG repeat, genotype, DU145
INTRODUCTION
It has been well established that the androgen receptor gene (AR) plays an important role in molecular signaling and regulation and the subsequent cellular growth of prostate cancer.1 Testosterone is converted by 5-α-reductase to dihydrotestosterone, which binds to and activates the AR protein in the cytoplasm before the complex translocates into the nucleus.2 Once inside the nucleus, the activated AR protein recruits a host of transcriptional cofactors to aid in the expression of AR-target genes.2 These genes are known to affect cellular function in a variety of ways, such as increasing PSA levels and decreasing levels of p21.2, 3
The AR gene is notable for being extremely variable, with one study finding missense base changes in no fewer than 44% of tumors studied.4 Concomitantly, a number of these variations have been shown to lead to changes in the expression or function of the AR protein.5–8 In addition to the single base changes, there are two areas of tri-nucleotide repeats (CAG and GGC) in exon 1. Some studies have found an association between shorter sections of polyglutamate (CAG) repeats and an increased risk of prostate cancer while others have found no such relationship, as reviewed by Montgomery et al.9 Another study describes an association showing that those patients with a repeat count of approximately 40+ are more at risk for Kennedy’s disease, a neuronal disease characterized by progressive muscle weakness and atrophy.10 Additionally, the number of CAG repeats in a specific cell line is highly variable and is known, on occasion, to become larger with passage from parent to child.11, 12 This phenomenon is hypothesized to occur due to the large number of cell divisions involved in spermatogenesis.11 We thus hypothesize that repeat number can also change with multiple passages in a cell line. Herein we sequenced the entire exonic region of the androgen receptor in 10 different prostate cancer cell lines. These data are novel and have the potential to be of significant value to those investigators who use these model systems.
MATERIALS AND METHODS
Genomic DNA was obtained for each of the 10 cell lines studied. Genomic DNA was purchased for the following cell lines from the American Type Culture Collection (ATCC; Manassas, VA): DU-145, LnCAP, MDA-PCa 2b, PC-3, RWPE-1, RWPE-2, PZ-HPV-7 and 22Rv1. For DU-145, LnCAP, PC-3, PC-3M, and CWR-22, gDNA was extracted from established cell lines within our laboratory using the Qiagen Midi Blood Kit per manufacturer’s protocol. ATCC lot and passage numbers are listed in Table 1. For some of the cell lines, multiple passage numbers were tested to confirm the presence or absence of any genetic variations found (Table 1).
Table 1.
Cell Lines Assayed, Passage Number, and Source of Cells
| Cell Line | Passage # | Source | Site of Origin | Year | Reference |
|---|---|---|---|---|---|
| DU145 | 65 | isolated 9/21/05 from ATCC cells | brain metastasis | 1978 | [14] |
| DU145 | 62 | ATCC Lot #4603874 | brain metastasis | 1978 | [14] |
| LnCAP | 57 | isolated 9/08 from ATCC cells | lymph node metastasis | 1983 | [15] |
| LnCAP | 20 | ATCC Lot #57805057 | lymph node metastasis | 1983 | [15] |
| MDA- PCa 2b | 35 | ATCC Lot #3287807 | bone metastasis | 1997 | [16] |
| PC3 | 27 | isolated 9/08 from ATCC cells | bone metastasis | 1979 | [17] |
| PC3 | unknown by ATCC | ATCC Lot #4605552 | bone metastasis | 1979 | [17] |
| PC3M | 34 | isolated 1/28/00 from ATCC cells | variant line of PC3 | 1984 | [18] |
| RWPE1 | 50 | ATCC Lot #4419188 | transfected using HPV-18 | 1998 | [19] |
| RWPE2 | 45 | ATCC Lot #3287803 | RWPE-1 cells transfected with Ki-ras | 1998 | [19] |
| PZHPV7 | 29 | ATCC Lot #3287806 | transfected using HPV-18 | 1994 | [20] |
| 22RV1 | unknown by ATCC | ATCC Lot #3287808 | primary, xenograft CWR-22R- 2152 | 1999 | [21] |
| CWR22 | 19 | isolated 7/26/02 from ATCC cells | androgen dependent xenograft model derived from a primary human prostatic carcinoma maintained in athymic nude mice | 1994 | [22] |
Polymerase chain reaction (PCR) was performed on each cell line for each exon of the AR. Briefly, 1X PCR buffer, four deoxynucleotidetriphosphates (dNTPs [2mM/each]), 1.5mM MgCl2, 20uM primers (Table 2), 1 unit of Platinum Taq DNA Polymerase, water and DNA were mixed to a final volume of 50.0uL. Samples were heated to 94°C for 5 minutes, followed by 40 cycles of 94°C (30 seconds), the appropriate annealing temperature (30 seconds), and 72°C (30 seconds). There was a final elongation period of 7 minutes at 72°C, and samples were cooled to 4°C. An exception to the above method was the amplification of exon 1, part 4. Samples in this amplicon were heated to 98°C for 3 minutes, followed by 10 cycles of 98°C (40 seconds), the calculated annealing temperature less 5 degrees (30 seconds), and 72°C (1 minute). This was followed by 20 cycles of 98°C (40 seconds), the appropriate annealing temperature less 5 degrees (30 seconds), and 72°C (1 minute plus 10 seconds with each additional cycle). There was a final elongation period of 10 minutes at 72°C, and samples were then cooled to 4°C. Amplification was achieved using the Herculase II Fusion DNA Polymerase (Stratagene, La Jolla, CA) according to manufacturer’s protocol for GC-Rich Targets 1–10kb titrated to 4% DMSO and using PCR Boost (Biomatrica, San Diego, CA) in replacement of water. Direct nucleotide sequencing was conducted using Big Dye Terminator Cycle Sequencing Ready Reaction kit V3.1 (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 3130xl Genetic Analyzer. All sequences were analyzed in the forward and reverse directions except for RWPE-1 exon 1 part 4 and RWPE-2 exon 1 part 1, and exon 5 (as noted). Sequences were visually confirmed via electropherogram. All previously unreported sequence changes were reamplified and resequenced for confirmation, with the exception of the mutation in the DU145 cell line. This was successfully amplified only once but was resequenced to confirm the mutation. We were unable to sequence DU145 DNA obtained from ATCC for confirmation.
Table 2.
Amplification and Sequencing Primers
All sequences were confirmed through forward and reverse sequencing, with the exception of some exon 1 sequences and exon 5, which was confirmed through two separate sequences in the reverse direction. Annealing temperatures are calculated based on primer composition, with TA = 4°C*(number of C/G basepairs) + 2°C*(number of A/T basepairs). Temperatures were adjusted for optimal amplification.
| Exon | Primer Sequence | Annealing Temperature °C |
|---|---|---|
| 1–1 | F 5′-cgcctggttaggctgcacgcg-3′ R 5′-catccacgttgtccctgctgg-3′ |
68 |
| seq F 5′-cagctagctgcagcgactacc-3′ seq R 5′-cagccgcagtcggccctggag-3′ | ||
| 1–2 | F 5′-aatctgttccagagcgtgcgc-3′ R 5′-ccttgtgccccattggccgaat-3′ |
68 |
| seq F 5′-gagagaggttgcgtcccagag-3′ seq R 5′-gtggaggcgttggagcatctg-3′ | ||
| 1–3 | F 5′-ccagcaccatgcaactccttc-3′ R 5′-tcctggcacactctcttcacag-3′ |
66 |
| seq F 5′-ctccaaggacaattacttagggggc-3′ seq R 5′-cagtgccgctatggggacct-3′ | ||
| 1–4 | F 5′-accagagtcgcgactactacaactttcc-3′[23] R 5′-gggcagagtcactctgtgttctgg-3′[23] |
60 |
| seq F 5′-actggctctggccggaccg-3′ seq R 5′-gggcagagtcactctgtgttctgg-3′ | ||
| 1-AR | F 5′-acagcctgttgaactcttctgag-3′ R 5′-catccacgttgtccctgctgg-3′ |
70 |
| seq F 5′-tctaccctcggccgccgtccaag-3′ seq R 5′-cagccgcagtcggccctggag-3′ | ||
| 2 | F 5′-cacgaagctaaagcaaggaacat-3′ R 5′-ctactctgctggctagtaaagga-3′ |
66 |
| seq F 5′-gcctggacaccaccttcagtt-3′ seq R 5′-ttaggcagtgaaggtggtccca-3′ | ||
| 3 | F 5′-aaaggggtgacaagttccacaat-3′ R 5′-ggacttaatgatactggcctgatg-3′ |
66 |
| seq F 5′-tttggtgccatactctgtccac-3′ seq R 5′-gaccagccatgctctagaca-3′ | ||
| 4 | F 5′-caggtatgaatactgaaggctgc-3′ R 5′-tacagggatcgaaactcagaaag-3′ |
66 |
| seq F 5′-aaccagtgttgaatgagcacttg-3′ seq R 5′-ggtgcttttctgcccattaactc-3′ | ||
| 5 | F 5′-agagttcactcatataagcagtc-3′ R 5′-caactggcaggagcccagga-3′ |
64 |
| seq R 5′-gacagtgaagcttagctcatttga-3′ * | ||
| 6 | F 5′-tcctggccagagaagatgagta-3′ R 5′-tgtatggcagccaaggaacttt-3′ |
64 |
| seq F 5′-agcaccagcaggagaaacagc-3′ seq R 5′-cactctactctctctcagcat-3′ | ||
| 7 | F 5′-ataggatttgtatggcagccaagga-3′ R 5′-gactccatggagaccatttctt-3′ |
68 |
| seq F 5′-cagatcggatccagctatcctt-3′ seq R 5′-ctggctttgagtgtggtcca-3′ | ||
| 8 | F 5′-agagatggagtgcggaggctt-3′ R 5′-gagagctaagattatctggggaa-3′ |
66 |
| seq F 5′-cagaggttggggaagaggcta-3′ seq R 5′-cctctattgatgtacagtctgt-3′ |
RESULTS
As shown in Table 3, our analysis confirmed the presence of the homozygous L57Q SNP in the 22RV1 cell line and the homozygous H874Y SNP in the 22RV1 and CWR22 cell lines, as previously stated by Chlenski et al.5 In addition, we confirmed the homozygous L701H SNP in MDA-PCa 2b cells and the homozygous T877A SNP in the LnCAP and MDA-PCa 2b cell lines.6, 7 The homozygous E211E SNP was found in the DU145 cell line, as previously reported by Lu et al.8 The CAG-repeat and GGC-repeat sequences are highly variable11, 12 among cell lines, and we have included our results for the repeat count of each for the ten cell lines in Table 3.
Table 3.
SNPs Found in Androgen Receptor of Several Common Prostate Cancer Cell Lines
Variations in the androgen receptor of several prostate cancer cell lines are shown as a nucleotide change, followed by the corresponding amino acid change. Novel SNPs are denoted by italics.
| (1-1) A1-F | (1-1) AR-F | (1-1) AR-R | (1-1) A2-R | (1–2) | (1–3) | (1–4) A4-F | (1–4) A4-R | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22RV1 | - | CTG>CAG L57Q |
26 CAGs (CAG)n | - | - | - | - | 12 GGCs (GGN)n | - | - | - | - | - | - | C>T/T H874Y |
| CWR22 | - | CTG>CAG L57Q | 26 CAGs (CAG)n | - | - | - | - | 12 GGCs (GGN) | - | - | - | - | - | - | C>T/T H874Y |
| DU145 | - | - | 18 CAGs (CAG)n | - | G>A/A in both NIH lab and ATCC E211E | - | - | T>T/C in NIH lab G527G | - | - | - | - | - | - | - |
| 16 GGCs (GGN)n | |||||||||||||||
| LnCAP | - | - | 26 CAGs (CAG)n | - | - | - | - | 15 GGCs in NIH lab 16 GGCs in ATCC (GGN)n |
- | - | T>C/T in NIH lab T/T in ATCC S641P | - | - | - | A>G/G in both home and ATCC T877A |
| MDA- PCa 2b | - | - | 24 CAGs (CAG)n | - | - | - | - | 16 GGCs (GGN)n | - | - | T>A/A L701H | - | - | - | A>G/G T877A |
| PC3 | - | - | 26 CAGs (CAG)n | - | - | - | - | 16 GGCs (GGN)n | - | - | - | - | - | - | - |
| PC3M | - | - | 26 CAGs (CAG)n | - | - | - | - | 16 GGCs (GGN)n | - | - | - | - | - | - | - |
| PZHPV7 | - | - | 24 CAGs (CAG)n | - | - | - | - | 16 GGCs (GGN)n | - | - | - | - | - | - | - |
| RWPE1 | - | - | 25 CAGs (CAG)n | - | - | - | - | 12 GGCs (GGN)n | - | - | - | - | - | - | - |
| RWPE2 | - | - | 25 CAGs (CAG)n | - | G>T/T S187I | - | - | 12 GGCs (GGN)n | - | - | - | - | - | - | - |
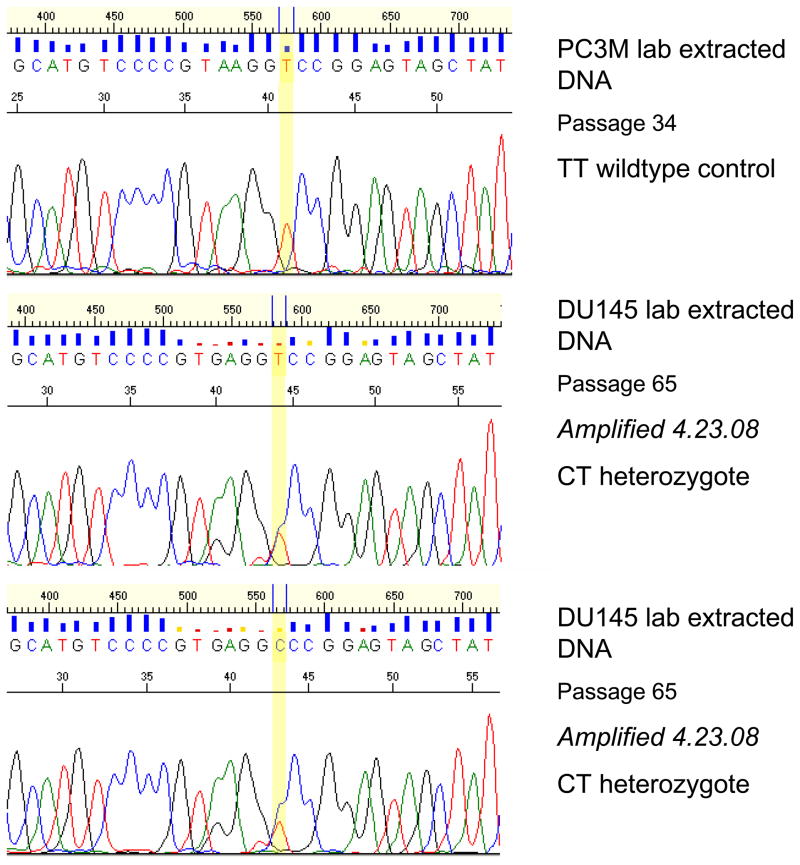
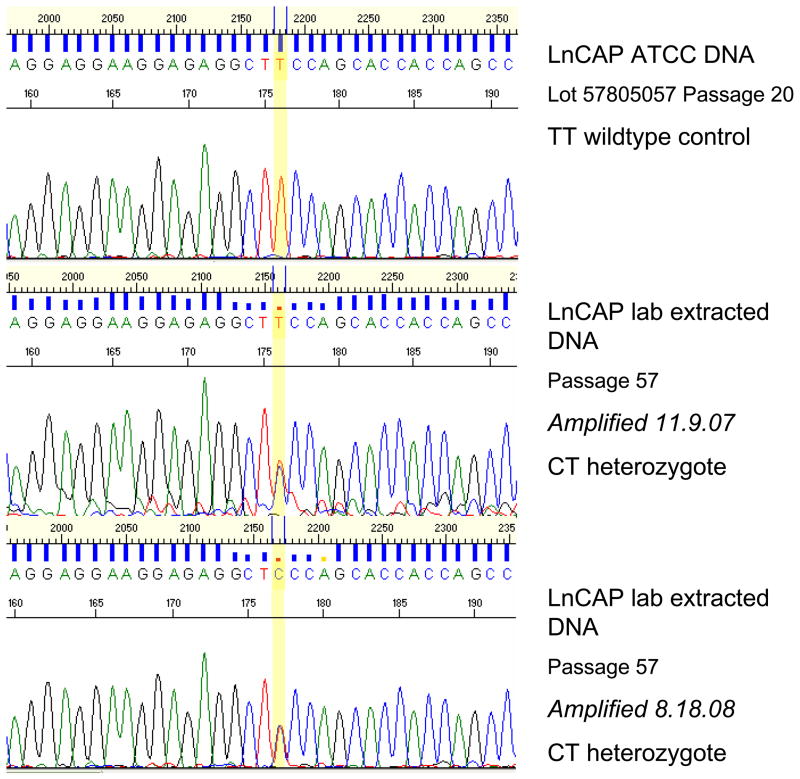
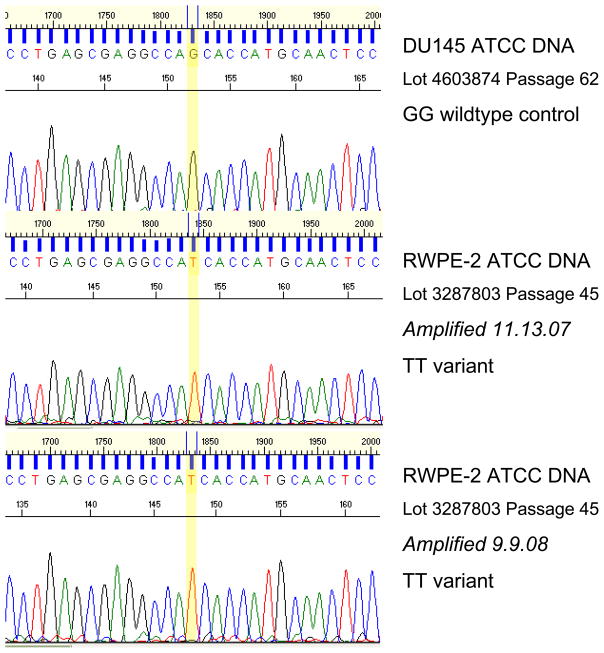
Interestingly, our analysis identified three new mutations, one each in the DU145, LnCAP, and RWPE-2 cell lines (Table 3). In DU145, the DNA isolated in our lab (3 passages more than the ATCC DNA) was heterozygous at G527G, a mutation that, to our knowledge, has not been reported previously (Figure 1). We were unable to confirm this with DNA purchased from ATCC. The LnCAP cells cultured in our lab, similarly, were found to have a T>C transition (heterozygous), resulting in S641P that was not present in the ATCC cell line DNA (Figure 2). Lastly, a homozygous G>T transversion resulting in the S187I change was found in ATCC RWPE-2 cells, the only source of RWPE-2 cells studied (Figure 3).
Figure 1.
DU145 lab extracted DNA shows CT heterozygote mutation (G527G) not present in other cell lines (PC3M sequence shown for comparison).
Figure 2.
LnCAP lab extracted DNA shows CT heterozygote mutation (S641P) not present in the ATCC DNA.
Figure 3.
RWPE-2 ATCC DNA shows a TT variant mutation (S187I). Also shown is a DU145 ATCC DNA sample with a wildtype (GG) genotype.
CONCLUSIONS
This is the first systematic characterization of AR exons in each of the common cell lines used world-wide in prostate cancer research. Though the alterations are not functionally studied herein, we now know that these variations exist. Some of these have been previously annotated and some have not. Given genomic instability in cell lines derived from the ATCC as compared to those cultured in our facility, we have documented that changes can occur with passage. Thus we urge caution in extrapolating results from ATCC-derived cell lines if the cell lines have been subjected to repetitive passage.
Several SNPs previously described by others were verified in our analysis of the common prostate cancer cell lines. The homozygous C>T H874Y SNP in the 22rv1 cell line, previously described by Bokhoven et al, was found in our analysis.13 Our analysis of CWR22 was also consistent with the literature, in that the homozygous C>T H874Y SNP was reported by Chlenski et al. 5 Our analysis of the LnCAP cell line DNA (both ATCC extracted and extracted by our lab) revealed a homozygous A>G SNP (T877A) previously reported by Veldscholte et al. 6 Finally, analysis of the MDA-PCa 2b cell line DNA confirmed the homozygous T>A (L701H) and homozygous A>G (T877A) SNPs previously published by Zhao et al. 7
There are two highly polymorphic regions of trinucleotide repeats in the first exon of the androgen receptor. The CAG trinucleotide repeat is highly varied, and we provide counts for each cell line studied. However, only the repeat numbers for the PC3 and LnCAP cell lines have been verified by the literature. 12 Our analysis of the number of CAG repeats in DU145 differs from that which was previously published, but by only one repeat unit. 12 The CAG repeat counts for 22RV1, CWR22, MDA-PCa 2b, PC3M, PZHPV7, RWPE1, and RWPE2 were not found in a literature search, and we believe that our data is novel in this respect. Similarly, no prior report could be found on the number of GGC repeats in the first exon for each of the cell lines studied. Thus our data represent the first report in the literature.
Several SNPs that were previously reported in the HapMap database were found in the 22RV1, CWR22, and DU145 cell lines. Specifically, the homozygous T>A (L57Q) SNP was located in the 22RV1 and CWR22 cell lines, and the homozygous G>A (E211E) SNP was located in the DU145 cell line. It is not clear whether these represent germline alterations present in the patient from whom the cell line was derived, or whether these represent somatic alterations.
The G527G, S641P, and S187I variations noted herein are not documented in the HapMap database, suggesting but not proving that they are somatic mutations which developed either in the patient’s tumor or in the cell line. To our knowledge, we are the first to identify the entire exonic structure of the androgen receptor with regards to SNPs and mutations for the prostate cancer cell lines commonly used in current laboratory research.
Acknowledgments
Financial Support
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Bethesda, Md.
The authors thank Southwest Oncology Group (SWOG) for the support during this study. This work is supported by National Cancer Institute, NIH, Department of Health and Human Services grant P01CA106451.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Bentel J, Tilley W. Androgen receptors in prostate cancer. J Endocrinol. 1996;151:1–11. doi: 10.1677/joe.0.1510001. [DOI] [PubMed] [Google Scholar]
- 2.Shen HC, Coetzee GA. The Androgen Receptor: Unlocking the Secrets of Its Unique Transactivation Domain. Vitamins and Hormones. 2005:301–19. doi: 10.1016/S0083-6729(05)71010-4. [DOI] [PubMed] [Google Scholar]
- 3.Wang LG, Ossowski L, Ferrari AC. Overexpressed Androgen Receptor Linked to p21WAF1 Silencing May Be Responsible for Androgen Independence and Resistance to Apoptosis of a Prostate Cancer Cell Line. Cancer Research. 2001;61:7544–51. [PubMed] [Google Scholar]
- 4.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the Androgen Receptor Gene Are Associated with Progression of Human Prostate Cancer to Androgen Independence. Clinical Cancer Research. 1996;2:277–85. [PubMed] [Google Scholar]
- 5.Chlenski A, Nakashiro K-i, Ketels KV, Korovaitseva GI, Oyasu R. Androgen Receptor Expression in Androgen-Independent Prostate Cancer Cell Lines. The Prostate. 2001;47:66–75. doi: 10.1002/pros.1048. [DOI] [PubMed] [Google Scholar]
- 6.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GGJM, Jenster G, Trapman J, et al. The Androgen Receptor in LnCAP Cells Contains A Mutation in the Ligand Binding Domain Which Affects Steroid Binding Characteristics and Response to Antiandrogens. J Steroid Biochem Molec Biol. 1992;41:665–9. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X-Y, Boyle B, Krishnan AV, Navone NM, Peehl DM, Feldman D. Two Mutations Identified in the Androgen Receptor of the New Human Prostate Cancer Cell Line MDA PCA 2A. The Journal of Urology. 1999;162:2192–9. doi: 10.1016/S0022-5347(05)68158-X. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Danielsen M. Short Report on DNA Marker at Candidate Locus. Clinical Genetics. 1996;49:323–4. [Google Scholar]
- 9.Montgomery JS, Price DK, Figg WD. The androgen receptor gene and its influence on the development and progression of prostate cancer. Journal of Pathology. 2001;195:138–46. doi: 10.1002/1096-9896(200109)195:2<138::AID-PATH961>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Spada ARL, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:1–92. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 11.Brooks BP, Fischbeck KH. Spinal and bulbar muscular atrophy: a trinucleotide-repeat expansion neurodegenerative disease. Trends in Neurosciences. 1995;18:459–61. doi: 10.1016/0166-2236(95)94497-s. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell S, Abel P, Ware M, Stamp G, Lalani E-N. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU International. 2000;85:932–44. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 13.Bokhoven Av, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, et al. Molecular Characterization of Human Prostate Carcinoma Cell Lines. The Prostate. 2003;57:205–25. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]