Abstract
Recent studies have demonstrated the abilities of solid-state NMR techniques to solve atomic-level resolution structures and dynamics of membrane-associated proteins and peptides. However, high-throughput applications of solid-state NMR spectroscopy are hampered by long acquisition times due to the low sensitivity of the technique. In this study, we demonstrate the use of a paramagnetic copper-chelated-lipid to enhance the spin-lattice relaxation and therefore to speed-up solid-state NMR measurements. Fluid lamellar phase bicelles composed of a lipid, detergent, copper-chelated-lipid, and containing a uniformly-15N-labeled antimicrobial peptide, subtilosin A, are used at the room temperature. The use of a chelating lipid reduces the concentration of free copper and limits RF-induced heating, a major problem for fluid samples. Our results demonstrate 6.2 times faster and 2.7 folds improvement of S/N for solid-state NMR experiments under MAS and static conditions respectively. Further, solid-state NMR measurements are shown to be feasible even on nanomole concentration of a membrane-associated peptide.
Atomic-level structure and dynamics are essential to understand the function of membrane proteins, their roles in biological processes, and for the development of compounds to treat related diseases. While these are challenging tasks to most techniques, recent studies have demonstrated that solid-state NMR spectroscopy is promising as it neither requires a single crystal nor imposes a restriction on the molecular-size to be investigated. Membrane proteins reconstituted in lipid bilayers are commonly used for solid-state NMR studies.1 The key difficulties that limit the high-throughput applications of solid-state NMR spectroscopy are: the requirement of a large amount of sample and/or a long data collection to enhance the signal-to-noise (S/N) ratio, radio frequency (RF) induced sample heating mainly due to a long experimental measurement that may denature expensive membrane proteins labeled with isotopes, need for stable NMR probes and electronics, and the demand on the continuous availability of a spectrometer. Therefore, the development of new approaches to speed up solid-state NMR measurements is essential. Previous studies successfully utilized copper salts and Cu-EDTA in crystalline samples at lower temperature2 and Gd-DTPA in glycosphingolipid bilayer samples.3 However, the presence of excess water and molecular mobilities in fluid lamellar-phase membranes complicates the direct utilization of paramagnetic ions. In this study, we overcome this limitation by the use of a copper-chelated lipid to significantly enhance the sensitivity of an NMR experiment on lipid bilayers under either aligned static or unaligned magic angle spinning (MAS) conditions.
Magnetically-aligned bicelles6 composed of a lipid DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), a detergent DHPC (1,2-dihexanoyl-sn-glycero-3-phosphocholine), and subtilosin A4 (Fig.S1) were used to demonstrate the paramagnetic effect of Cu2+ in reducing the spin-lattice relaxation (T1) and the recycle delay of NMR experiments in membranes. Cu2+ ions were introduced in bicelles either by adding Cu-EDTA or a copper-chelated-lipid (Figs. 1A, S2-S4). 31P NMR experiments were performed to optimize the alignment of bicelles (Fig. S5). 15N spectra of uniformly-15N-subtilosin-A embedded in bicelles are shown in Fig. 1B. T1 values of 1H and 13C nuclei from DMPC and 15N of subtilosin A were also measured (Fig. 2). These results suggested that a 2 s recycle delay provided the maximum S/N for the sample in the absence of Cu2+. On the other hand, the presence of a 2.56 mM copper-chelated-lipid in bicelles reduced T1 of protons by a factor of 10 and therefore ~0.2 s recycle delay would have been sufficient. However, RF heating and dehydration or denaturing effects (including degradation of magnetic alignment and protein stability) due to a very short recycle delay forced us to use a longer recycle delay. Nevertheless, it was found that a 1 s delay was sufficient to avoid RF heating and thus allowing 2 times faster data collection. The experimental results given in Fig. 1B suggest a ~2.7-fold increase in the S/N due to the use of copper-chelated-lipid in bicelles.
Fig. 1.
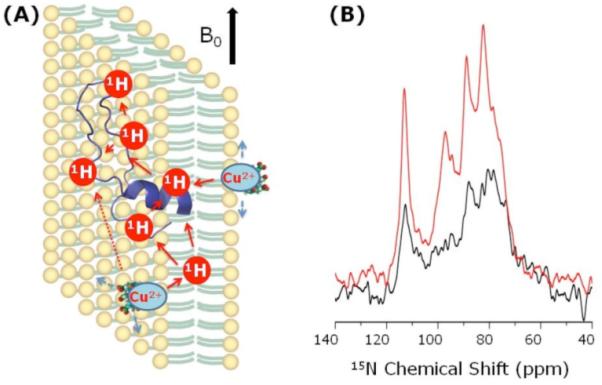
(A) A representation of lipid bilayers containing a paramagnetic copper-chelated lipid and subtilosin A. Subtilosin A is a 35-residue cyclic antimicrobial peptide (Fig. S1) that has been shown to interact with lipid bilayers with a membrane orientation as given in (A)4. (B) 15N spectra of aligned 7:3 DMPC:DHPC bicelles containing a 12-14% of uniformly-15N-labeled (only 70-82 nmoles) subtilosin A and with (red) and without (black) the 2.56 mM copper-chelated-lipid. The spectra were obtained from a 400 MHz Varian NMR spectrometer using a ramp-CP sequence5 with a 0.8 ms contact time under static conditions at 37 °C. The S/N dependence on the contact time and the recycle delay were optimized (Figs. S6 & S7). While the total data collection time was 8 hours for both spectra, the recycle delay was different for samples without (2s) and with (1s) a copper-chelated-lipid. The transfer of paramagnetic effect in T1 reduction for nuclei in the membrane via the proton spin diffusion is also indicated in (A).
Fig. 2.

NMR relaxation measurements on bicelles and 15N-labeled subtilosin A for the evaluation of paramagnetic doping effect: (A) with 2.56 mM copper-chelated lipid; (B) with 30 mM copper-EDTA. (C) Difference in spin-lattice relaxation rates with (R1) and without (R1’) copper-chelated lipid; labels I and II correspond to 15N signal from the rigid transmembrane (78.3 ppm) and flexible membrane-surface (112.6 ppm) regions respectively. (D) Difference in signal intensities obtained from bicelles with and without copper-chelated lipid. (E) Data showing the RF-induced heating on bicelles measured as explained in Fig. S8. (F-H) A comparison of 13C CP signal intensities measured from bicelles with no copper (E), 30 mM Cu-EDTA (blue) and 2.5 mM Cu DMPE-DTPA (red).
Our results suggest that while the use of Cu-EDTA also decreased the T1 values effectively (as shown in Fig. 2B), it was ineffective in enhancing the S/N compared to the immobilized Cu-chelated-DMPE lipid (Figs. 2F-H) and also resulted in more RF heating (Fig. 2E) due to mobile Cu2+ ions; the heating effects were also observed in 31P spectra (Figs. S8 & S9). The use of ~15-fold less [Cu2+] in the chelated form is of considerable advantage for NMR studies.
Since the magnetic-alignment of bicelles is not needed and the sample temperature can be lowered in MAS experiments, the use of Cu2+−DMPE-containing bicelles can be extended for MAS experiments. 2D MAS experiments were performed on such bicelles (Fig. 3). An RFDR (radio frequency driven dipolar recoupling) technique was used in the mixing period to recouple 1H-1H dipolar couplings.7 Cross peaks connecting dipolar coupled protons can be seen in the spectra. While the spectra of bicelles without (Fig. 3A) and with (Fig. 3B) the copper-chelated-lipid are similar in overall S/N, the intensities of some of the peaks are different. The proximity of Cu2+ ion broadens peaks associated with the lipid head group whereas the intensities of peaks from the hydrophobic acyl chains are enhanced. Since no decoupling was used in these experiments, the much reduced RF-induced heating on bicelles enabled a further reduction in the recycle delay period to 0.2 s, unlike in the static experiment of Fig. 1. Therefore, a 6.2-fold overall reduction in the 2D experimental time was achieved.
Fig. 3.

2D 1H/1H chemical shift correlation spectra of bicelles without (A) and with (B) a 2.56 mM copper-chelated lipid obtained under 5 kHz MAS with a total data collection times of 1.77 and 11 hours respectively. A 6.2-fold reduction in data collection time was made possible due to the use of the copper-chelated lipid with a similar S/N ratio as can be seen from 1D spectral slices taken at 1.34 ppm (top) and 3.25 ppm (bottom) with (red) and without (black) a copper-chelating lipid. An RFDR7 sequence with a 100 ms mixing time and a 100 ms low-power pulse for water-saturation at 35°C was used. 512 t1 experiments with 32 scans were used with a recycle delay of 0.2 s (with a copper-chelating lipid) and 2 s (without).
In this study, we have shown that the inclusion of Cu2+ ions in bicelles results in a 10-fold T1 reduction. It is also demonstrated that the challenges posed by the molecular mobilities and presence of bulk water can be overcome by using a copper-chelated-lipid. About 2.5 and 6.2 folds increase in S/N of spectra obtained from static and MAS experiments (6.2 times faster experiments for MAS) respectively. While this enhancement is not as dramatic as that reported for crystalline solids,2 given the tremendous challenges in the investigation of fluid membranes, this S/N enhancement is significant and will be of considerable use in the structural studies of membrane proteins. For example, a satisfactory completion of 2D experiments typically used on aligned lipid bilayers containing a 15N-labeled membrane protein takes about 3 days time. The use of Cu-DMPE, on the other hand, could enable the completion of such experiments in <1.5 days. There are a number of membrane disrupting/permeating systems (such as antimicrobial peptides, toxins, amyloid peptides, and fusion peptides) whose concentration cannot be increased as they result in non-lamellar conditions; such systems tremendously benefit from the use of the approach proposed in this study to reduce the amount of peptide/protein used in the experiment.
Interestingly, further reduction in the measurement time would be possible in the following cases: (a) lowering the sample temperature to avoid heating like in MAS experiments on frozen samples, (b) use of efficient electric-field-free probes,8 (c) use of bicelles that align at low temperatures (as shown in Fig. S10), and (d) use of cryoprotectants. Therefore, we believe that the approach presented in this study will have broad impacts on the structural studies of a variety of membrane-associated peptides and proteins that are scarcely available and/or their production could be very expensive and are quite unstable.
Supplementary Material
Acknowledgement
This research was supported by NIH (GM084018 and RR023597 to A.R.) and CRIF-NSF, and Natural Sciences and Engineering Research Council (to J.C.V.). We thank Dr. Brender for critical reading of this manuscript.
Footnotes
Supporting Information Available: 31P and 15N NMR spectra of bicelles and spectra related to relaxation measurements. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M. Nature. 2006;440:959–962. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]; (b) Dürr UH, Waskell L, Ramamoorthy A. Biochim. Biophys. Acta. 2007;1768:3235–3259. doi: 10.1016/j.bbamem.2007.08.007. [DOI] [PubMed] [Google Scholar]; (c) Lorieau JK, Day LA, McDermott AE. Proc. Natl. Acad. Sci. 2008;105:10366. doi: 10.1073/pnas.0800405105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu JD, Dürr UH, Im SC, Gan ZH, Waskell L, Ramamoorthy A. Angew. Chem. Intl. Ed. 2008;47:7864–7867. doi: 10.1002/anie.200801338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Ganapathy S, Naito A, McDowell CA. J. Am. Chem. Soc. 1981;103:6011–6015. [Google Scholar]; Wickramasinghe NP, Kotecha M, Samoson A, Past J, Ishii Y. J. Magn. Reson. 2007;184:350–356. doi: 10.1016/j.jmr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LW, Past J, Samoson A, Ishii Y. Nat. Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wickramasinghe NP, Shaibat M, Ishii Y. J. Am. Chem. Soc. 2005;127:5796–5797. doi: 10.1021/ja042188i. [DOI] [PubMed] [Google Scholar]; (e) Weliky DP, Bennett AE, Zvi A, Anglister J, Steinbach PJ, Tycko R. Nat. Struct. Biol. 1999;6:141–145. doi: 10.1038/5827. [DOI] [PubMed] [Google Scholar]; (f) Linser R, Chevelkov V, Diehl A, Reif BJ. J. Magn. Reson. 2007;189:209–216. doi: 10.1016/j.jmr.2007.09.007. [DOI] [PubMed] [Google Scholar]; (g) Cai S, Seu C, Kovacs Z, Sherry AD, Chen Y. J. Am. Chem. Soc. 2006;128:13474–8. doi: 10.1021/ja0634526. [DOI] [PubMed] [Google Scholar]
- (3).DeMarco ML, Woods RJ, Prestegard JH, Tian F. J. Am. Chem. Soc. 2010;132:1334–1338. doi: 10.1021/ja907518x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Kawulka K, Sprules T, McKay RT, Mercier P, Diaper CM, Zuber P, Vederas JC. J. Am. Chem. Soc. 2003;125:4726–4727. doi: 10.1021/ja029654t. [DOI] [PubMed] [Google Scholar]; (b) Thennarasu S, Lee DK, Poon A, Kawulka KE, Vederas JC, Ramamoorthy A. Chem. Phys. Lipids. 2005;137:38–51. doi: 10.1016/j.chemphyslip.2005.06.003. [DOI] [PubMed] [Google Scholar]
- (5).Metz G, Wu X, Smith SO. J. Magn. Reson. A. 1994;110:219–227. [Google Scholar]
- (6).(a) Sanders CR, Hare BJ, Howard KP, Prestegard JH. Prog. Nucl. Magn. Reson. Spec. 1994;26:421–444. [Google Scholar]; (b) Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Biochemistry. 2006;45:8453–8465. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]; (c) Inbaraj JJ, Cardon TB, Laryukhin M, Grosser SM, Lorigan GA. J. Am. Chem. Soc. 2006;128:9549–9554. doi: 10.1021/ja0622204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yamamoto K, Soong R, Ramamoorthy A. Langmuir. 2009;25:7010–7018. doi: 10.1021/la900200s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Prosser SR, Volkov VB, Shiyanovskaya IV. Biophys. J. 1998;75:2163–2169. doi: 10.1016/S0006-3495(98)77659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Dvinskikh SV, Yamamoto K, Dürr UHN, Ramamoorthy A. J. Magn. Reson. 2007;184:228–235. doi: 10.1016/j.jmr.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bennett AE, Ok JH, Griffin RG, Vega S. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- (8).(a) McNeill SA, Gor’kov PL, Shetty K, Brey WW, Long JR. J. Magn. Reson. 2009;197:135–144. doi: 10.1016/j.jmr.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gor’kov PL, Chekmenev EY, Li C, Cotten M, Buffy JJ, Traaseth NJ, Veglia G, Brey WW. J. Magn. Reson. 2007;185:77–93. doi: 10.1016/j.jmr.2006.11.008. [DOI] [PubMed] [Google Scholar]; (c) Stringer JA, Bronnimann CE, Mullen CG, Zhou DH, Stellfox SA, Li Y, Williams EH, Rienstra CM. J. Magn. Reson. 2005;173:40–48. doi: 10.1016/j.jmr.2004.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


