Abstract
Cancer is the second leading cause for mortality in US only after heart disease and lacks a good or effective therapeutic paradigm. Despite the emergence of new, targeted agents and the use of various therapeutic combinations, none of the treatment options available is curative in patients with advanced cancer. A growing body of evidence is supporting the idea that human cancers can be considered as a stem cell disease. Malignancies are believed to originate from a fraction of cancer cells that show self renewal and pluripotency and are capable of initiating and sustaining tumor growth. The cancer-initiating cells or cancer stem cells were originally identified in hematological malignancies but is now being recognized in several solid tumors. The hypothesis of stem cell-driven tumorigenesis raises questions as to whether the current treatments, most of which require rapidly dividing cells are able to efficiently target these slow cycling tumorigenic cells. Recent characterization of cancer stem cells should lead to the identification of key signaling pathways that may make cancer stem cells vulnerable to therapeutic interventions that target drug-effluxing capabilities, anti-apoptotic mechanisms, and induction of differentiation. Dietary phytochemicals possess anti-cancer properties and represent a promising approach for the prevention and treatment of many cancers.
Keywords: Colon, pancreas, CD133, DCAMKL-1, Notch, Hedgehog, Curcumin, Resveratrol
INTRODUCTION
Cancer is a hyperproliferative disorder in which invasion and angiogenesis leads to tumor metastasis. The World Health Organization (WHO) has estimated cancer cases for the year 2009, men 766,130 and women 713,220 in the United States. The estimated death rate in 2009 was 292,540 men and 269,800 women in the United States [1]. Although progress has been made in the early detection of cancer and in improvements of cancer therapies, the ability to provide long-term survival has been limited. Increasing evidence suggests that biologically unique population of cancer stem cells (CSCs) exists in most neoplasms and may be responsible for tumor initiation, progression, metastasis, and relapse. Tumors comprise from heterogeneous populations of cells that have varying degrees of tumorigenic potential. Only a subset of tumor cells are thought to initiate and promote tumorigenesis [2] and recent evidence has implicated a pluripotent subset of cells that have the capacity to seed the cellular heterogeneity seen in tumors [3]. Further characterization of cancer stem cells may lead to the identification of key cellular activities. Once pathways are established in these cells, therapeutic interventions could potentially be designed that target drug-effluxing capabilities, stem cell pathways, anti-apoptotic mechanisms, and induction of differentiation. These cancer stem cells are the earliest undifferentiated progenitors with an unlimited capacity to propagate [4]. Given their potential role in tumorigenesis, cancer stem cells may represent important targets for prevention and therapy.
COLON CANCER
Colorectal cancer is a second leading cause of death in the United States and is a major health problem globally [5]. Colorectal cancer affects over 146,970 individuals yearly, and accounts for around 49,920 deaths [1,6]. Screening for colon cancer can be done by colonoscopy to find polyps, and removing these polyps at an early stage can prevent cancer progression. When the polyps are allowed to persist in the colon for a long time, they may develop into cancer. Hence regular colonoscopy is recommended in the United States for those over 50 years of age [7]. Recurrence of colon cancer is common with an estimated 40% of cases returning in 3 to 5 years for treatment. Chemotherapeutic compounds currently being used for the treatment of colorectal cancer include 5-flurouracil, Oxaliplatin and Gemcitabine [8]. Because conventional therapies, including surgical resection, chemotherapy, and radiation are often inadequate in treating this disease, new treatment options are critically needed. Despite the emergence of novel targeted agents and the use of various therapeutic combinations, no treatment options are available that are curative in patients with advanced cancer. More recently, the cancer stem cell concept is gaining importance because it suggests new approaches to anti-cancer therapies [9,10]. This concept is very similar to that of normal tissue stem cells, namely that the majority of cells in a tumor are heterogeneous, but a rare number of cells within the tumor exist termed cancer stem cells or tumor initiating cells that are capable of tumor maintenance and/or regrowth following therapeutic interventions [9]. Potential markers of colorectal cancer stem cells have been proposed, including CD133, CD166, integrin β1, signal transducer CD24, CD44, ALDH1 and LGR5 [10, 11].
PANCREATIC CANCER
Pancreatic cancer is an aggressive malignancy with one of the worst outcomes among all cancers. It is the fourth leading cause of cancer death in the United States with less than 5% five-year survival rate. The lifetime risk of developing pancreatic cancer in both men and women is about 1 in 79 (1.27%) [12,13]. The American Cancer Society (ACS) estimated that new cases of 42,470 Americans (21,050 men and 21,420 women) would be diagnosed with pancreatic cancer during 2009. The ACS also estimated that 35,240 Americans (18,030 men and 17,210 women) would die of pancreatic cancer in 2009 [1]. Despite advances in molecular pathogenesis, problems such as drug resistance and susceptibility for metastasis make pancreatic cancer a major unsolved health problem in the United States [14]. Unfortunately, pancreatic cancer is a rapidly invasive, metastatic tumor that is resistant to standard therapies [15,16]. At present, single agent based chemotherapy (e.g. Gemcitabine) is the mainstay treatment for metastatic pancreatic adenocarcinoma. Recent data indicate that in addition to Gemcitabine, 5-FU plus a platinum agent such as Oxaliplatin could be used as a therapeutic paradigm for early stage cancer patients [17]. However, none of the available current chemotherapeutic agents have objective response rates of over 10% [18,19]. The magnitude of this problem mandates the need for novel therapeutic agents. Recently, cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT)-type cells, which share molecular characteristics with CSCs, have been postulated to play critical roles in drug resistance and cancer metastasis in pancreatic cancer [4,20]. Recent studies suggest that CD44+CD24+ESA+ (epithelial specific antigen) and ALDH1 could potentially be pancreatic cancer stem cell markers [21,22]. In addition, we have determined that the recently identified intestinal stem cell marker DCAMKL-1 is also expressed in a small proportion of cells in the pancreas and in pancreatic cancer stem cell marker [23], unpublished observation]. However, the role of DCAMKL-1 as a bonafide stem maker remains to be elucidated.
BREAST CANCER
Breast cancer is the most common form of cancer diagnosed in women world wide, affecting an estimated 10% of the subjects [24,25]. Although the rate of mortality as a result of breast cancer has decreased in Western countries in part due to early detection, ACS estimates that 192,370 new breast cancer cases will be identified in the United States in 2009 with an estimated death rate of 40,170 (15%) [1]. Breast cancer is a very heterogeneous disease at both the histological and molecular levels. At least six distinct subtypes have been described on the basis of gene expression profiling, with the most important determinants of these subtypes being the presence or absence of expression of the estrogen receptor or the progesterone receptor, or the amplification/overexpression of the HER2/ERBB2 locus [26]. Despite the ability of these subtypes to predict outcome, patient response to chemotherapy or targeted therapy remains variable. The current standard of therapy for breast cancer include surgical resection, radiation, and chemotherapeutic agents such as cisplatin, pacliataxel, carboplatin, bevacizumab, doxorubicin, cyclophosphamide, docetaxel, and epirubicin [27].
Our current understanding of CSCs comes primarily from studies on breast cancer stem cells (BrCSC). These have been isolated from human breast tumors or breast cancerderived pleural effusions using flow cytometry for a specific pattern of cell surface marker expression (CD44+, CD24−/low, ESA+ [28,29]. Many groups have attempted to confirm that the minimum surface phenotype for a tumorigenic BrCSC is CD44+/CD24− [30]. In addition, CD133 (also a marker of CSC population in other tumors) and in some cases, selected members of the integrin family of receptors (beta1, alpha6 or beta3 integrins), alone or in conjunction with the CD44+/CD24− phenotype have also been used to isolate the BrCSCs [31]. Aldehyde dehydrogenase (ALDH) expression has also been used as a marker for BrCSCs [32]. While these have been exciting, it remains to be seen whether a single cell isolated by this method can develop new tumors in animal models.
STEM CELLS
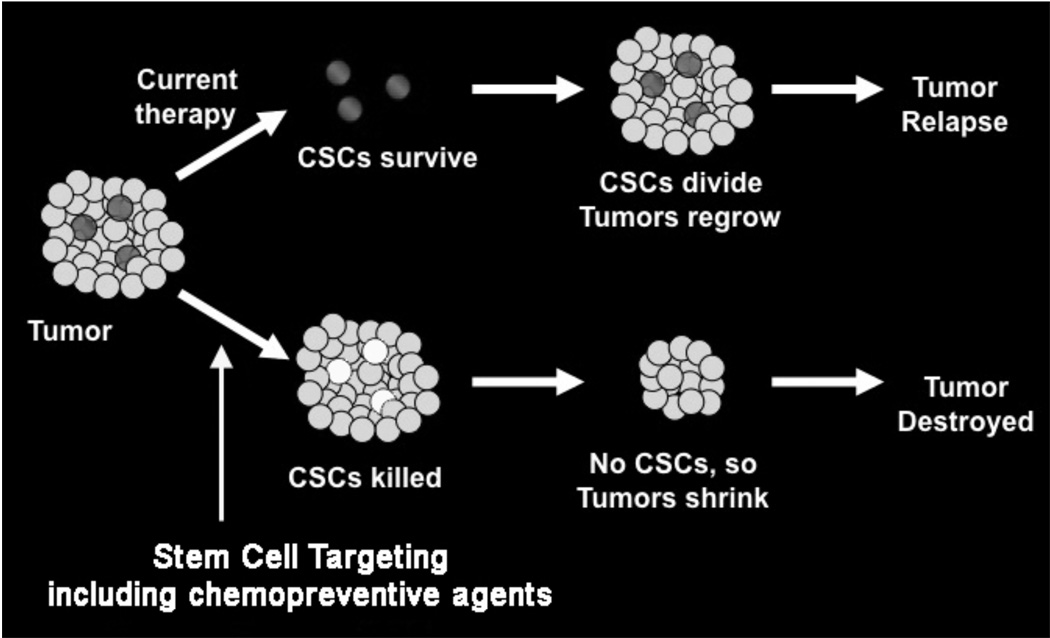
During tumor development, tissues accumulate a series of mutations over the years and these populations of cells would have to self-renew, clonally expand, and acquire additional mutations. It is now widely believed that long lived, uncommon cells are tissue stem cells (SCs) or cell derived from them that acquire the ability to self–renew. Self-renewal, one of the defining characteristics of stem cells, is a cell division in which one or both of the resulting daughter cells remain undifferentiated, retaining the ability to give rise to another stem cell with the same capacity to proliferate as the parental cell [33,34]. In addition to self-renewal, stem cells have the capacity to differentiate, generating cells in each organ. When mutated, they can become cancer stem cells (CSCs). Cancer stem cells are defined by similar characteristics, mainly their abilities to self-renew, a characteristic that drives tumorigenesis, and to (aberrantly) differentiate, a property that generates the bulk of cells within a tumor. These self-renewing ‘cancer stem cells’ might constitute only a small fraction of the cells within a tumor, with the bulk of the tumor composed of more differentiated cells that lack self-renewal capacity (Fig. 1) [35]. CSCs may account for only a small fraction of cells (approximately 1%) in any given tumor [36,37]. Progress in stem cell research and the identification of potential esophageal, gastric, intestinal, colonic, hepatic and pancreatic stem cells provides hope for the use of stem cells in regenerative medicine and treatments for disease [38,39].
Fig. (1).
Cancer Stem Cell Hypothesis. Conventional therapies are directed towards rapidly dividing cells and fail to eliminate the rare cancer stem cells (CSCs) resulting in regrowth of the tumor. However, recent studies suggest that when CSCs are targeted and killed, it will lead to the elimination of the tumor.
a. Signaling Pathways
Although pathways that regulate self-renewal are tightly controlled in normal stem cells, in tumor-initiating cells they may be constitutively activated or improperly regulated through genetic and/or epigenetic changes, leading to uncontrolled growth [40]. Several studies show that BMI1 (polycomb ring finger oncogene) and the Wnt signaling molecule β-catenin regulate the self-renewal and the proliferation of leukemia-initiating cells [41,42]. Actually, many leukemia-initiating cells have a higher self-renewal capacity than normal hematopoietic stem cells. In addition, it was recently shown that maintenance of cutaneous tumor-initiating cells is dependent on Wnt and β-catenin signaling.
The molecular mechanisms that control self-renewal of stem cells are an essential element for tumor survival and propagation. Specific pathways identified to play a key role for self-renewal of embryonic stem cell include the Wnt/β-catenin, Hedgehog (Hh), and Notch signaling pathways [40]. Although genes involved in these pathways are expressed in normal stem cells, they are frequently mutated or aberrantly activated in cancers, thus making them potential therapeutic targets. Table 1 lists the major signaling pathways for normal and cancer stem cells. A few natural compounds have been identified that affect these pathways, but their efficacy in targeting stem cells is currently unknown. Further work is therefore required before these preventive and/or therapeutic agents target stem cells to efficiently inhibit tumorigenesis. In addition, further work is also need to determine whether such agents have differential effects against cancer versus normal stem cells.
Table 1.
Major Stem Cell Signaling Pathways and Compounds
WNT SIGNALING PATHWAY
In a normal mucosa, the Wnt pathway controls proliferation and migration within a colonic crypt via the expression of transcription factors and adhesion molecules [43]. This pathway however, is dysregulated in colorectal cancers. Patients with familial adenomatous polyposis coli (APC) have a malfunctioning APC protein that allows for the accumulation of β-catenin in the cytoplasm. β-catenin is normally bound to membranous E-cadherin. When β-catenin enters the nucleus not only does it trigger the cell cycle, it precipitates the loss of membranous E-cadherin and suppresses its expression [44]. Thus, cell–cell contact is reduced, thereby permitting migration of cells from the epithelium to the mesenchyme. This process has been coined 'epithelial to mesenchymal transition' (EMT) [44,45]. EMT is an essential process in certain physiological circumstances such as embryogenesis and wound healing. In epithelial cells, loss of E-cadherin leads to diminished cell–cell contact, allowing for motility and migration of cells.
In colorectal cancers, the highest concentration of nuclear β-catenin is found at the advancing margin in free tumor cells that have lost E-cadherin expression [44,45]. Presence of these cells has been associated with metastasis and poor survival. Lower levels of β-catenin are found in adenomas and within the central tumor. To further enhance the oncogenic potential, nuclear β-catenin increases the expression of survivin, a protein that promotes cellular proliferation and resistance to apoptosis [46]. Another second study also proposed that colorectal cancer stem cells are located at the tumor margin and become mobile through their high nuclear β-catenin and subsequent suppression of adhesion molecules [47]. These cells form a 'front' that functions as a 'germinal layer' and can break away to metastasize to form new colonies of cancer cells. Mutations in the Wnt/β-catenin pathway are associated with a number of cancers [48] and implicated in controlling cancer SC self-renewal capabilities [49].
Several phytochemicals, such as curcumin, (−)-epigallocatechin-3-gallate (EGCG), and resveratrol have been recently shown to inhibit Wnt signaling in cancers and could potentially be excellent candidates for targeting cancer SCs [50,51]. EGCG has been shown to alter Wnt/β-catenin signaling in breast cancer cells [52]. Moreover, EGCG inhibited Wnt-induced gene expression responses such as reduced activity of TCF/LEF binding and decreased c-Myc expression. This attenuation of Wnt/β-catenin activity was mediated through the stabilization of HBP-1, a transcriptional repressor of Wnt/β-catenin signaling and a suppressor of oncogenesis [48]. EGCG also inhibited tumor formation in APCmin/+ mice [53]. EGCG treatment resulted in a significant reduction in nuclear β-catenin levels, further implicating the Wnt/β-catenin signaling pathway.
NOTCH SIGNALING PATHWAY
Notch and its signaling components are implicated in a wide variety of developmental processes, such as central nervous system development, vasculature system development, organogenesis, and adult-type hematopoietic stem cell generation [54]. Notch ligand binding to Notch family receptor induces transcriptional activation of target genes. Notch signaling is activated by direct cell–cell contact. Activation of Notch leads to proteolytic cleavage of the intracellular domain of Notch (NICD). The NICD cleavage product then translocates to the nucleus and coordinates transcription factor complexes and co-activators of the Mastermind-like family of proteins [54,55]. Among Notch ligand genes, JAG1 gene is predicted to be an evolutionarily conserved target of the canonical WNT signaling pathway based on the conservation of double TCF/LEF–binding sites within the 5′ promoter region of mammalian JAG1 orthologues [56,57]. Notch4 is activated due to Mouse mammary tumor virus integration during mammary carcinogenesis [58] where WNT signaling pathway is also activated [59]. Notch and WNT signaling pathways are both necessary for the self-renewal of hematopoietic stem cells [60]. Notch and WNT signaling pathways synergize to inhibit terminal differentiation of intestinal epithelial cells partially through downregulation of ATOH1/HATH1 bHLH transcription factor [56]. Together, these facts indicate that Notch and WNT signaling pathways keep the homeostasis of stem and progenitor (transit-amplifying) cells through the inhibition of terminal differentiation. Notch signaling also promotes the expansion of neuronal and breast SCs [61,62]. Aberrant Notch signaling is implicated in controlling tumor self-renewal in medulloblastomas [63]. Notch expression is enriched in brain tumor SCs, and inhibition of Notch signaling resulted in apoptosis in the cancer SC population and blocked xenograft tumor formation [63].
The phytochemical curcumin, a common flavoring agent and an active ingredient of the spice turmeric inhibits Notch signaling pathway in both colon and pancreatic cancer (Subramaniam and Anant, unpublished observations) [64,65]. Similarly, the phytochemical resveratrol, which is found in grapes, berries and peanuts exhibits anti-cancer properties by affecting the Notch pathway [66]. Resveratrol treatment of Acute lymphoblastic leukemia cells resulted in decreased Notch protein expression [67]. Resveratrol appeared to affect Notch at the post-translational level because Notch mRNA levels were not affected. Moreover, mRNA levels of downstream effectors of Notch were decreased in the presence of resveratrol [67].
HEDGEHOG SIGNALING PATHWAY
The hedgehog (Hh) signaling pathway plays a crucial role in vertebrate embryogenesis by controlling cell fate, proliferation, survival and differentiation. In the adult organism, Hh signaling remains active and is involved in the regulation of tissue homeostasis, regeneration and stem cell maintenance [68]. Three Hh homologs have been identified in vertebrates, contrasting with the single Hh gene found in Drosophila. These gene homologs are called Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh), which are expressed at different stages of ontogeny in different tissues and may have distinct biological functions [69]. Hh signal transduction is initiated by the binding of the processed and lipid modified Hh ligand to its receptor Patched1 (Ptch1), a 12-pass transmembrane protein. In the absence of the Hh protein, Ptch1 represses signal transduction by inhibiting the seven transmembrane protein, Smoothened (Smo) [70]. The ultimate step in the pathway is mediated by the zinc finger transcription factors Gli1, Gli2 and Gli3, where Gli1 and Gli2 represent the main activators of Hh target genes and Gli3 acts mostly as a repressor [71]. This signaling is aberrantly activated in glioma, medulloblastoma, basal cell carcinoma, lung cancer, esophageal cancer, gastric cancer, pancreatic cancer, breast cancer, and other tumors [72].
Shh signaling proceeds through two transmembrane proteins, Patched, and Smoothened. At rest, Ptch1 inhibits Smo activity, but upon its binding to Shh, releases this inhibition, thus allowing Smo-mediated activation followed by Shh related transcriptional responses [73]. Mutations in this pathway are associated with an increase in cellular proliferation, transformation, and ultimately cancer. An oncogenic form of Shh has been identified in basal cell carcinoma and Shh is misregulated in pancreatic adenocarcinoma, prostate adenocarcinoma, esophageal and stomach cancer and nonsmall cell lung carcinoma [74]. Misregulated Shh signaling contributes to mechanisms whereby these cancers use both autocrine and paracrine signaling to affect proliferation and differentiation of their surrounding environment. Shh is a mediator of angiogenesis and has been shown to induce vessel formation in endothelial cells [75]. Shh was also shown to induce the expression of angiopoietins I and II and the family of VEGF signaling proteins from mesenchymal cells, highlighting the significance of tumor-associated fibroblasts in combination with Shh signaling to mediate blood vessel formation [76]. Inhibition of Shh signaling has been shown to reduce tumor burden and metastasis in both prostate and pancreatic adenocarinomas [77,78]. Recently, pancreatic cancer stem cells were shown to express high levels of Shh [79,80], which is interesting given the implications for Shh in adult stem cell renewal, in pancreatic ductal progenitor cells and also in adult hair follicle stem cells [59]. Perturbations in the Shh pathway are linked to cancer stem cells in multiple myeloma, pancreas, and breast [62,81]. Hedgehog signals induce stem cell markers BMI1, LGR5, CD44 and CD133 based on cross-talk with WNT and/or other signals.
The first phytochemical that was observed to inhibit the Hh pathway was cyclopamine [82, 83], a naturally occurring compound found in the plant Veratrum californicum, commonly called the corn lily. Cyclopamine targets the Shh pathway, specifically by inhibiting the activation of Smo [82]. Cyclopamine treatment of murine medulloblastoma blocked proliferation and induced neuronal differentiation, effectively depleting the cancer stem cell population [83]. This study further demonstrated that cyclopamine reduced tumor burden in a mouse tumor allograft and was cytotoxic to cultured human medulloblastoma cells. In addition, cyclopamine is effective in targeting cancer SCs of pancreatic cancer, breast cancer, and multiple myeloma [4,62]. Sonic Hh (Shh) expression is elevated in pancreatic cancer SCs [79], the putative mediators of pancreatic tumor invasion and metastasis [84]. Combination therapy of cyclopamine and gemcitabine inhibited metastatic spread and reduced primary tumor burden in pancreatic orthotopic xenografts [85]. In breast cancer and multiple myeloma SCs, cyclopamine reduced mammosphere formation and SC proliferation, respectively [81]. Recent study has shown that curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Moreover, curcumin inhibited the Shh-Gli1 signaling pathway by downregulating the Shh protein and its most important downstream targets Gli1 and Ptch1 [86]. Taken together, plant polyphenols target the self-renewal properties of cancer SCs, highlighting a potential and novel paradigm for cancer prevention.
OTHER SIGNALING PATHWAYS
Apart from the above three major signaling pathways like Notch, Wnt, and Hedgehog, there were other signaling pathways implicated in maintaining cancer stem cells including JAK-STAT, MAPK/ERK and PI3-K/Akt.
JAK-STAT PATHWAY
The Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway plays an important role in the signaling of various cytokines and growth factors affecting various cellular functions, including proliferation, growth, and immune response. JAK-STAT pathways have been implicated in various cancers. JAK-STAT pathway plays a role in self-renewal and continual maintenance of germ line stem cell population [87]. Moreover, autocrine JAK-STAT signaling has been shown to regulate the kidney stem cell self-renewal [88]. Curcumin has been shown to suppress JAK–STAT signaling in brain microglial cells [89]. The obvious requirement for STAT activation in stem cells and the link between tumorigenesis and cancer stem cells suggests the need to further understand this signaling pathway and identify the molecules that affect this pathway.
MAPK-ERK PATHWAY
The Mitogen-Activated Protein Kinase (MAPK) pathways transduce a wide variety of signals, leading to a variety of cellular responses including inflammation, growth, differentiation and cell death. These Ser/Thr protein kinases are phosphorylated and activated by MAPK-kinases (MAPKK), which are phosphorylated by MAPKK-kinases, which are in turn activated by interaction with the small GTPase family proteins connecting the MAPK component to cell surface receptors. Activated MAPK signaling appears to confer resistance to TGF-β–induced apoptosis in CD133+ cells compared with CD133− cells [90]. Moreover, increased MAPK signaling specifically in CD133+ CSCs resulted in activated ERK1/2. Furthermore, aberrant MAPK/ERK pathway in liver cancer stem cells may play a pivotal role in the initiation and development of HCC [90]. Alterations in the MAPK pathway with elevated ERK levels have also been described in Mat1a deletion mice, which develop HCC spontaneously by 18 months [91]. In contrast, inhibition of this pathway using specific inhibitors or antisense oligonucleotide was shown to inhibit HCC proliferation in a dose-dependent manner [92]. Interestingly, a recent report indicates that MAPK-2, another member of the MAPK/ERK pathway, was upregulated in prostate progenitor cells expressing CD133 [93]. Curcumin, Resveratrol, Silibinin and indole-3 carbinol has been shown to suppress MAPK activation in both inflammation and cancer [94–97]. However, the effect of these compounds on cancer stem cells needs to be discerned.
PI3-K-AKT SIGNALING
Phosphoinositide 3-kinase (PI3-K) plays a crucial role in effecting alterations in a broad range of cellular functions in response to extracellular signals. A key downstream effector of PI3-K is the serine-threonine kinase Akt, which in response to PI3-K activation phosphorylates and regulates the activity of a number of targets including kinases, transcription factors and other regulatory molecules. PI3-K is constitutively activated in human cancers by several mechanisms including through activation of tyrosine kinase growth factor receptors, loss of the inhibitor PTEN, by oncogenic RAS or by activation mutations in the PI3-K protein itself [98]. Several downstream targets of Akt have been identified that confirm the important role this protein in the regulation of growth and cell survival signaling pathways. It has been suggested that CD133 and nestin positive stem cells in gliomas and medulloblastomas survive radiation by activating the Akt pathway [99]. PI3-K pathway has also been shown to regulate post-irradiation survival of cancer stem cells residing in the perivascular niche in medulloblastoma [99]. Recent data suggests that mTOR plays an important role in PI3-K-AKT-mediated signaling for cancer stem cell self-renewal and resistance to chemotherapy or radiotherapy. This is believed to be the root cause of treatment failure and cancer recurrence and in the activation of metastatic activity. Curcumin and other compounds such as Resveratrol, Silibinin and Indole 3-carbinol regulate PI3-K-Akt pathway in colorectal cancer cells [100–103]. It would be interesting to determine if these compounds have differential effects on cancer stem cells, and if so understanding the mechanism of action would lead to development of novel therapeutic parameters for cancer.
b. Identification of cancer stem cells
Several proteins have emerged as potential markers for the identification of cancer stem cells in a variety of systems: CD44, prominin-1 (CD133), CD 166, integrins and new markers LGR5, DCAMKL-1 and BMI1 (Table 2).
Table 2.
Markers that have Been Proposed to Characterize Normal Intestinal SCs and Used to Target Cancer Stem Cells
| Marker | Function | Reference | |
|---|---|---|---|
| Normal Stem cells | DCAMKL-1 | Unknown | [23] |
| Musashi-1 | RNA binding protein | [170] | |
| Bmi-1 | Polycomb-repressor protein | [118] | |
| Lgr 5 | Unknown, WNT target gene | [114] | |
| Aldh-1 | Enzyme | [29] | |
| Cancer Stem cells | CD133 | Unknown | [38] |
| CD44 | Hyaluronic acid receptor | [104, 105] | |
| CD166 | Adhesion molecule | [106] | |
| Aldh-1 | Enzyme | [29] | |
CD44
CD44 is a class I transmembrane glycoprotein that can act as a receptor for extracellular matrices such as hyaluronic acid, and is a known downstream target of the Wnt/β-catenin pathway [104]. It was the first marker identified for a solid tumor stem cell found in a study of tumorigenic breast cancer [30]. Currently, it is not known whether all CD44 positive cells are stem cells, because a large population of cells within a tumor expresses CD44. Given that CD44 has many splice variants, the possibility exists that a specific splice variant is expressed in the stem cells. Colorectal cancer stem cells were similarly shown to express CD44 and the epithelial cell adhesion molecule EpCAM, and in several colorectal tumors CD166 could be used for further enrichment of colon cancer stem cells within the EpCAM/CD44-positive population [105,106]. In a recent study, they have identified CD44 as a gastric cancer stem cell marker. CD44-positive murine cells formed spheroid colonies, xenograft tumors, and also gave rise to CD44-negative cells and exhibited differentiation. The CD44 (+) gastric cancer cells demonstrated properties of chemo- and radio-resistance, which likely accounts for the resistance of this tumor type to standard treatment protocols. Moreover, CD44 expression correlated with the presence of dysplasia in murine and human gastric cancer [104]. This stem cell marker emphasizes the necessity of novel therapeutic approaches to target a better clinical outcome for patients with cancer.
CD133
Recent lineage-tracing studies of adult Prominin-1 (also called CD133; a pentaspan transmembrane glycoprotein that localizes to membrane protrusions) showed that some Prominin-1-positive cells are located at the base of crypts in the small intestine, co-express LGR5 and can generate the entire intestinal epithelium [39]. Prominin-1 is thought to function in maintaining stem cell properties by suppressing differentiation. Although it was first reported as a specific cancer stem cell marker for glioblastoma [107,108], it was subsequently shown to be a useful cancer stem cell marker for many gastrointestinal tumors including colorectal cancer. Several groups identified human colon cancer-initiating cells using Prominin-1 as a marker [38]. This finding was later challenged by the finding that both Prominin-1-positive and prominin-1-negative colon cancer cells could initiate tumorigenesis. A recent study has shown that a single prominin-1+/CD24+ colon cancer stem cell can self-renew and reconstitute a complete and differentiated carcinoma [109]. Interestingly, spheroid cultures of these colon cancer stem cells contain expression of prominin-1, CD166, CD44, integrin β1, signal transducer CD24, LGR5, and nuclear β-catenin, which have all been suggested to be marker for the cancer stem cell population [110]. Prominin-1 has also been reported to be a marker for cancer stem cells in primary pancreatic cancers and pancreatic cancer cell lines [84].
ALDH1
Aldehyde dehydrogenase 1 (ALDH1) has been reported as a cancer stem cell marker in pancreatic cancer, breast cancer, prostate cancer, lung cancer, multiple myeloma, and leukemia [22]. However, it is not clear whether ALDH1 is expressed only in the stem cells or also in other progenitor cells within a tumor. Nevertheless, ALDH1 can be used in fluorescence activated cell sorting as a method to enrich stem cells. There are several members in the ALDH family. They catalyze the oxidation of a wide variety of aldehydes to carboxylic acids, and are known to play an important role in endobiotic and xenobiotic metabolism. Consequently, ALDHs have been known to provide resistance to hematopoietic stem cells against alkylating agents of the oxazaphosphorines family, such as cyclophosphamide and its derivatives [22].
DCAMKL-1
Recently, Doublecortin and Ca2+/calmodulin-dependent kinase-like-1 (DCAMKL-1) was identified to be a bonafide stem cell marker [23]. In the normal intestine, DCAMKL-1 positive cells were identified to be in the +3 to +6 position and in occasional colon-based columnar (CBC) epithelial cells [23]. Many previous studies have demonstrated that the stem cell is located in the +3 to +6 position [111]. The adenomatous polyposis coli (APC)/multiple intestinal neoplasia (APCMin/+) mouse has an autosomal dominant heterozygous nonsense mutation of the mouse APC gene and exhibit spontaneous gastrointestinal tumors similarly found in humans with germ line and somatic APC mutations [112]. When expression of DCAMKL-1 was examined in the adenomas in the APCMin/+ mice, DCAMKL-1-expressing cells were negative for proliferating cell nuclear antigen and nuclear β-catenin in normal-appearing intestine. However, β-catenin was nuclear in DCAMKL-1-positive cells in adenomas. Thus, nuclear translocation of β-catenin distinguishes normal and adenoma stem cells. Targeting DCAMKL-1 may therefore represent a strategy for developing novel chemotherapeutic agents [113].
LGR5
The LGR 5 (a leucine-rich orphan G-protein-coupled receptor, also called Gpr49) gene encodes for a receptor in the Wnt signaling pathway. Using lineage tracing studies in mice, this gene has been shown to be a potential marker for normal adult intestinal stem cells and this protein specifically labels stem cells in the mouse small intestine as well as other adult tissues [110,114]. Also, mice generated from a LGR5-EGFP-IRES-Cre-ERT2 strain crossed with the Rosa-LacZ strain demonstrated that LGR5+ crypt based columnar cells are multipotent for all mature intestinal epithelial cell types, undergo self-renewal, persist for at least 60 days based on LacZ expression, and are resistant to irradiation. Furthermore, LGR5 marked ISCs that were rapidly cycling (dividing every 24 hours) under homeostatic conditions [115]. LGR5 has greater resistance to radiation and therefore may also be a marker for colorectal cancer stem and/or progenitor cells. Subsequent studies, however have demonstrated that the protein is expressed in multiple cells in the intestinal epithelium raising the question of whether LGR5 is a bonafide marker for stem cells or that of progenitors [23].
BMI1
BMI1 is a transcriptional repressor belonging to the polycomb gene family and its suppressor functions are involved in maintaining neuronal, hematopoietic and mammary gland stem cells [116,117]. A study published in 2009 suggested that BMI1 labels a subpopulation of differentiated acinar cells capable of self-renewing for more than 1 year [118] . BMI1 seems to be expressed within each acinus in one or more differentiated acinar cells that retain proliferative potential and replace the surrounding dying cells. This study, along with the characterization of BMI1 in the small intestine, suggests a more general role for BMI1 in self-renewal of stem cells as well as in the maintenance of the proliferative ability of differentiated cells [39].
TARGETING DRUG RESISTANCE
Although chemotherapy can reduce tumor mass, an aggressive population of cancer stem cells within the tumor may be capable of resisting chemotherapeutic drugs [119], leading to relapse and multi-drug resistance [120]. For example, cancer stem cells from brain tumors express the neural stem cell surface marker CD133+ were resistant to standard chemotherapeutic drugs [107]. Cancer stem cells isolated from human glioblastomas were more resistant to chemotherapeutic agents (e.g., temozolomide, carboplatin, paclitaxel, and etoposide) than were their non-cancer SC counterparts (CD133−). Additionally, CD133 expression was higher in recurrent glioblastomas as compared to that in a newly diagnosed patient, suggesting the CD133+ cancer SCs were better able to survive therapy. Finally, there is a close association between CD133 and MDR expression in glioblastoma tissue [121]. Greater MDR expression has also been associated with cancer stem cells from melanomas, pancreas, and breast [41,122,123].
CHEMOPREVENTIVE AGENTS AND CANCER
To prevent, the onset of cancer, the National Institutes of Health (NIH) in the United States recommended a high fiber, low fat diet, consisting more fruits and vegetables. Epidemiological studies suggest that diet plays a major role in the prevention of many cancers [124].
CURCUMIN
Curcumin is a common flavoring agent and an active ingredient of Indian spice turmeric, which has been used in Indian folk medicine to treat a number of ailments. It may be a dietary component responsible for lower rates of colorectal cancer in certain part of India [125]. Extensive research over the last half century has revealed several important functions of curcumin. By modulating the activation of various transcription factors, curcumin regulates the expression of inflammatory enzymes, cytokines, adhesion molecules and cell survival proteins. Curcumin has no discernable toxicity, inhibits the growth of transformed cells [126] and has also been shown to suppress initiation, promotion, and progression of colon carcinogenesis in carcinogen-induced rodent models [127]. Curcumin also down regulates cyclin D1, cyclin E and MDM2 and up regulates p21, p27 and p53 [128,129]. The recent preclinical and clinical studies have demonstrated the antitumor, anti-angiogenic properties of curcumin [130,131]. The anti-tumor properties including inhibition of tumorigenesis and induction of apoptosis have been demonstrated in both in vitro and in vivo studies [132,133]. Moreover, curcumin has been shown to have potent anti-angiogenic activity through its ability to inhibit proliferation of vascular endothelial cells, and capillary tube formation and growth in vivo [134,135]. Pilot Phase I clinical trials have shown that curcumin is safe even when consumed at a daily dose of 12 g for 3 months [128,129]. The anti-tumor properties of curcumin have been attributed, at least in part, to its ability to inhibit the expression and activity of COX-2 [136, 137]. In a phase 1 clinical trial, curcumin was found to be effective in inhibiting the growth of a variety of tumors [138].
Colon CSCs have been shown to express surface markers CD44, CD166, CD133, and epithelial-specific antigen (also known as EpCAM) [105]. CSCs also show resistance to a number of conventional therapies [139], which may explain why it is difficult to completely eradicate cancer and why recurrence is an ever-present threat. Thus, therapeutic strategies that specifically target colon CSCs are likely to be effective in eradicating tumors and in reducing the risk of relapse and metastasis. 5-Fluorouracil or FOLFOX, which remains the backbone of colorectal cancer chemotherapeutics, shows limited success. Recent studies have demonstrated that curcumin enhances the effects of 5-FU and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGFR [140]. Also, curcumin by itself or in combination with the conventional colon cancer chemotherapeutic regimen could be an effective therapeutic strategy to prevent the emergence of chemoresistant colon cancer cells by reducing/eliminating the CSCs [141]. Recent studies in breast cancer cells demonstrated that curcumin and piperine inhibited Wnt signaling. Curcumin and piperine separately, and in combination, inhibit breast cancer stem cell self-renewal but do not cause toxicity to differentiated cells. Both curcumin and piperine inhibited mammosphere formation, serial passaging, and percent of ALDH+ cells by 50% in normal and malignant breast cells. There was no effect on cellular differentiation. These compounds could be potential cancer preventive agents. Mammosphere formation assays may be a quantifiable method to assess cancer preventive agent efficacy and Wnt signaling assessment can be a mechanistic biomarker for use in human clinical trials [142].
RESVERATROL
Resveratrol (3,4',5-tri-hydroxy-trans-stilbene), which is mostly found in red wine, is a natural polyphenol [143]. It possesses several pharmacologic effects that are closely related to health therapies, including cardiac protection as well as antiviral, anti-inflammatory, and antiaging activities [144]. Recent studies reported that resveratrol has an anticancer effect and inhibits tumorigenesis by inducing apoptosis via Fas, p53, and p21WAF/CIP1-mediated pathways [145, 146]. It exhibits a broad spectrum of antiproliferative effects against human cancer cells [147]. Resveratrol can also increase radiosensitivity of cancer cell lines [148, 149]. In addition, resveratrol treatment decreased the expression of VEGF and fibroblast growth factor-2 in bladder cancer xenografts, which might also contribute to the inhibition of tumor growth [150]. More recently, resveratrol was shown to synergize with curcumin to inhibit colon cancer growth in mouse models, suggesting that the combination of chemopreventive agents might have greater efficacy [151]. In addition, resveratrol has also been shown to inhibit Notch signaling and PI3-K/Akt pathways, and to activate the proapoptotic/tumor suppressor p53 signaling pathway [67].
A recent study reported that CD133+ tumor initiating cells isolated from tissue samples of atypical teratoid/rhabdoid tumors (AT/RT) patients shared characteristics with stem-like cells and were refractory to radiation therapy compared with CD133− cells. Resveratrol could both inhibit the proliferation and tumorigenicity of these CD133+ cells in vitro and in vivo and further enhance radiosensitivity and IR-induced apoptosis [144]. Resveratrol treatment induces both anticancer and radiosensitizing effects on medulloblastoma CSCs, [152]. This improves the response to radiation therapy for patients with pediatric brain tumors [152]. Resveratrol prevents cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells [153].
DIINDOLYMETHANE OR INDOLE-3-CARBINOL
The active and beneficial substances in cruciferous vegetables have been shown to contain absorbable 3,3’-diindolylmethane (DIM) and its precursor, indole-3-carbinol (I3C) and it has been shown long history in cancer prevention research [154,155]. DIM was shown to inhibit the growth of breast tumor xenografts, in part through inhibiting tumor angiogenesis [156]. The compound has also been shown to reduce the risk for developing breast cancer [157]. Moreover, disappearance of cervical dysplasia has been observed when treated with this compound [158]. More recently, B-DIM, a formulated DIM with greater bioavailability was observed to be much more effective as an antitumor agent when combined with erlotinib in an orthotopic pancreatic tumor model, when compared with either agent alone [159]. Further studies are now necessary to identify stem cells in the cervical and pancreatic cancers and to determine whether B-DIM and/or I3C alone or in combination with Erlotinib can be potent inhibitor of these cells.
SILIBININ
Silymarin is an active extract from the seeds of the plant milk thistle (Silybum marianum (L.) (Asterceae), and contains approximately 65–80% silymarin flavonolignans (silymarin complex) with small amounts of flavonoids and approximately 20–35% fatty acids and other polyphenolic compounds. The major component of the silymarin complex is silibinin [160]. Silibinin (or its crude form silymarin) is also a flavonoid compound having anticancer efficacy against various cancers including colon cancer [161,162]. Recent studies suggest that silibinin suppresses the growth while inducing apoptotic death of human colorectal carcinoma cells in culture and in tumor xenografts [163]. In many animal studies, it has been observed that silibinin/silymarin feeding to mice up to 2 gm/kg dose does not show any apparent signs of toxicity, and that the agent is physiologically available in different organs of the mice [164]. The non-toxic nature of the compound coupled with its anticancer potential makes silibinin a suitable candidate for cancer chemoprevention studies.
CRUCIFEROUS VEGETABLE COMPOUNDS
Consumption of cruciferous vegetables such as broccoli, cabbage, kale, Brussels sprouts, radish is more strongly associated with cancer protection [165]. Benzyl isothiocyanate (BITC) and Sulforaphane are cruciferous vegetable compounds, which have been shown to inhibit various animal models of cancers including chemically induced cancers and genetic models of cancer [166–169]. Recent studies suggest that sulforaphane protects against tumor development during the post-initiation phase, the mechanism of suppression being cell cycle arrest and apoptosis [169]. As before, studies are required to determine whether compounds isolated from cruciferous vegetables have any effects on CSCs.
CONCLUSION
The cancer stem cell hypothesis is gaining acceptance after the accumulation of extensive research evidence suggesting that the small subset of the tumor mass is responsible for the sustained growth of the tumor. Furthermore, it is becoming apparent that the cancer stem cells are responsible for disease relapse and resistance to the existing therapies. Identifying new drugs that can specifically target cancer stem cells could lead to a new generation of anti-cancer medicines and a new strategy of treatment. Dietary phytochemicals are natural products found in our diet and can be used to target cancer stem cells. As the phytochemicals sensitizes the chemotherapy resistant cells, and targets aberrantly expressed molecules and various signaling pathways in the cancer stem cells, developing novel therapeutic molecules from these lead compounds will target the highly resistant cancer stem cells, thereby preventing the recurrence. Thus identification of such cancer stem cell targeting therapy and their use in combination with standard chemotherapy agents will curtail this dreadful disease. The next level of challenge will be the protection of normal stem cells and targeting the cancer stem cells specifically.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J. Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ichim CV, Wells RA. First among equals: the cancer cell hierarchy. Leuk. Lymphoma. 2006;47(10):2017–2027. doi: 10.1080/10428190600733325. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki BT, Hurt EM, Mistree T, Farrar WL. Targeting cancer stem cells with phytochemicals. Mol. Interv. 2008;8(4):174–184. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson N, Scott-Conner CE. Surgical therapy for colorectal adenocarcinoma. Gastroenterol. Clin. North Am. 2008;37(1):253–267. doi: 10.1016/j.gtc.2007.12.012. ix. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S. Colorectal polyps: the scope and management of the problem. Am. J. Med. Sci. 2008;336(5):407–417. doi: 10.1097/MAJ.0b013e31817d2402. [DOI] [PubMed] [Google Scholar]
- 7.Young WF, McGloin J, Zittleman L, West DR, Westfall JM. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J. Rural Health. 2007;23(3):238–245. doi: 10.1111/j.1748-0361.2007.00096.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutr. Cancer. 2009;61(6):842–846. doi: 10.1080/01635580903285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365(1537):155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thenappan A, Li Y, Shetty K, Johnson L, Reddy EP, Mishra L. New Therapeutics Targeting Colon Cancer Stem Cells. Curr. Colorectal Cancer Rep. 2009;5(4):209. doi: 10.1007/s11888-009-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69(20):8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Daily total physical activity level and total cancer risk in men and women: results from a large-scale population-based cohort study in Japan. Am. J. Epidemiol. 2008;168(4):391–403. doi: 10.1093/aje/kwn146. [DOI] [PubMed] [Google Scholar]
- 13.Mysliwiec P, Kedra B. [Causes of delayed diagnosis of pancreatic cancer. Own study and proposed algorithm] Przegl. Lek. 2008;65(7–8):345–348. [PubMed] [Google Scholar]
- 14.Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13(5):562–5676. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 15.Duffy JP, Reber HA. Pancreatic neoplasms. Curr. Opin. Gastroenterol. 2003;19(5):458–466. doi: 10.1097/00001574-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Real FX. A "catastrophic hypothesis" for pancreas cancer progression. Gastroenterology. 2003;124(7):1958–1964. doi: 10.1016/s0016-5085(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 17.Petrelli F, Borgonovo K, Ghilardi M, Cabiddu M, Barni S. What Else in Gemcitabine-Pretreated Advanced Pancreatic Cancer? An Update of Second Line Therapies. Rev. Recent Clin. Trials. 2010 doi: 10.2174/157488710790820553. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur. J. Cancer. 1997;33 Suppl 1:S18–S22. doi: 10.1016/s0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 19.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40(7):643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J. Clin. Oncol. 2008;26(17):2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 22.Saini V, Shoemaker RH. Potential for therapeutic targeting of tumor stem cells. Cancer Sci. 2009;101(1):16–21. doi: 10.1111/j.1349-7006.2009.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeatcontaining G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27(10):2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imyanitov EN, Hanson KP. Molecular pathogenesis of bilateral breast cancer. Cancer Lett. 2003;191(1):1–7. doi: 10.1016/s0304-3835(02)00523-2. [DOI] [PubMed] [Google Scholar]
- 25.Imyanitov EN, Togo AV, Hanson KP. Searching for cancerassociated gene polymorphisms: promises and obstacles. Cancer Lett. 2004;204(1):3–14. doi: 10.1016/j.canlet.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. U S A. 2003;100(18):10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isakoff SJ. Triple-Negative Breast Cancer: Role of Specific Chemotherapy Agents. Cancer J. 2010;16(1):53–61. doi: 10.1097/PPO.0b013e3181d24ff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang WW, Lee CH, Lee P, Lin J, Hsu CW, Hung JT, Lin JJ, Yu JC, Shao LE, Yu J, Wong CH, Yu AL. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc. Natl. Acad. Sci. U S A. 2008;105(33):11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, Lee EY, Chang KJ, Lee WH. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One. 2009;4(12):e8377. doi: 10.1371/journal.pone.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res. Treat. 2009;118(2):241–254. doi: 10.1007/s10549-009-0524-9. [DOI] [PubMed] [Google Scholar]
- 32.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010;16(1):45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 2004;14(1):43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 35.Huang EH, Wicha MS. Colon cancer stem cells: implications for prevention and therapy. Trends Mol. Med. 2008;14(11):503–509. doi: 10.1016/j.molmed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J. Clin. Oncol. 2008;26(17):2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 37.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J. Clin. Oncol. 2008;26(17):2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 38.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J. Mol. Med. 2009;87(11):1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 39.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 2009;6(12):724–737. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 41.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 2009;8(10):806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 42.Rizo A, Olthof S, Han L, Vellenga E, de Haan G, Schuringa JJ. Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. Blood. 2009;114(8):1498–1505. doi: 10.1182/blood-2009-03-209734. [DOI] [PubMed] [Google Scholar]
- 43.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307(5717):1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 44.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179(1–2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 45.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J. Pathol. 2009;217(2):307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 46.Salama P, Platell C. Colorectal cancer stem cells. ANZ J. Surg. 2009;79(10):697–702. doi: 10.1111/j.1445-2197.2009.05054.x. [DOI] [PubMed] [Google Scholar]
- 47.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009;27(2):186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 48.Reguart N, He B, Taron M, You L, Jablons DM, Rosell R. The role of Wnt signaling in cancer and stem cells. Future Oncol. 2005;1(6):787–797. doi: 10.2217/14796694.1.6.787. [DOI] [PubMed] [Google Scholar]
- 49.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 50.Rao CV, Cooma I, Rodriguez JG, Simi B, El-Bayoumy K, Reddy BS. Chemoprevention of familial adenomatous polyposis development in the APC(min) mouse model by 1,4-phenylene bis(methylene)selenocyanate. Carcinogenesis. 2000;21(4):617–621. doi: 10.1093/carcin/21.4.617. [DOI] [PubMed] [Google Scholar]
- 51.Mishra L, Shetty K, Tang Y, Stuart A, Byers SW. The role of TGF-beta and Wnt signaling in gastrointestinal stem cells and cancer. Oncogene. 2005;24(37):5775–5789. doi: 10.1038/sj.onc.1208924. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006;281(16):10865–11075. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 53.Bose M, Hao X, Ju J, Husain A, Park S, Lambert JD, Yang CS. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (−)-epigallocatechin-3-gallate and fish oil. J. Agric. Food Chem. 2007;55(19):7695–7700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 54.Chiba S. [Regulation of hematopoietic stem cells and development of leukemia] Nippon Rinsho. 2007;65 Suppl 1:53–59. [PubMed] [Google Scholar]
- 55.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 56.Katoh M, Katoh M. Notch signaling in gastrointestinal tract (review) Int. J. Oncol. 2007;30(1):247–251. [PubMed] [Google Scholar]
- 57.Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 2006;17(4):681–685. [PubMed] [Google Scholar]
- 58.Robbins J, Blondel BJ, Gallahan D, Callahan R. Mouse mammary tumor gene int-3: a member of the notch gene family transforms mammary epithelial cells. J. Virol. 1992;66(4):2594–2599. doi: 10.1128/jvi.66.4.2594-2599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review) Int. J. Mol. Med. 2006;18(6):1019–1023. [PubMed] [Google Scholar]
- 60.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005;6(3):314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 61.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7(3):86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 64.Wang SF, Aoki M, Nakashima Y, Shinozuka Y, Tanaka H, Taniwaki M, Hattori M, Minato N. Development of Notchdependent T-cell leukemia by deregulated Rap1 signaling. Blood. 2008;111(5):2878–2886. doi: 10.1182/blood-2007-07-103119. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279(1):8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783–2840. [PubMed] [Google Scholar]
- 67.Cecchinato V, Chiaramonte R, Nizzardo M, Cristofaro B, Basile A, Sherbet GV, Comi P. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem. Pharmacol. 2007;74(11):1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat. Rev. Mol. Cell. Biol. 2005;6(4):306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 69.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 70.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur. J. Cancer. 2006;42(4):437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 71.Kasper M, Jaks V, Fiaschi M, Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30(6):903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 2009;9(7):873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 73.Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr. Biol. 2000;10(20):1315–1318. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- 74.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 75.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 2001;7(6):706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 76.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J. Biol. Chem. 2003;278(10):8244–8249. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 77.Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M, Maitra A, Feldmann G. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr. Relat. Cancer. 2007;14(3):865–874. doi: 10.1677/ERC-07-0108. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with Sonic Hedgehog-GLI1 signaling. Proc. Natl. Acad. Sci. U S A. 2004;101(34) doi: 10.1073/pnas.0404956101. 12561-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 80.Shao J, Zhang L, Gao J, Li Z, Chen Z. Aberrant expression of PTCH (patched gene) and Smo (smoothened gene) in human pancreatic cancerous tissues and its association with hyperglycemia. Pancreas. 2006;33(1):38–44. doi: 10.1097/01.mpa.0000222319.59360.21. [DOI] [PubMed] [Google Scholar]
- 81.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. U S A. 2007;104(10):4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297(5586):1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 84.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67(5):2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A, Aboussekhra A. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol. Carcinog. 2009 doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 87.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 88.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1(2):191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 2003;171(11):6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 90.Ding W, Mouzaki M, You H, Laird JC, Mato J, Lu SC, Rountree CB. CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis. Hepatology. 2009;49(4):1277–1286. doi: 10.1002/hep.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16(10):1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 92.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J. Am. Coll. Surg. 2004;198(3):410–421. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Shepherd CJ, Rizzo S, Ledaki I, Davies M, Brewer D, Attard G, de Bono J, Hudson DL. Expression profiling of CD133+ and CD133- epithelial cells from human prostate. Prostate. 2008;68(9):1007–1024. doi: 10.1002/pros.20765. [DOI] [PubMed] [Google Scholar]
- 94.Epstein J, Docena G, Macdonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br. J. Nutr. 2009:1–9. doi: 10.1017/S0007114509992510. [DOI] [PubMed] [Google Scholar]
- 95.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35(7):597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp. Toxicol. Pathol. 2010 doi: 10.1016/j.etp.2009.11.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 97.Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem Biophys. Res. Commun. 2007;354(1):165–171. doi: 10.1016/j.bbrc.2006.12.181. [DOI] [PubMed] [Google Scholar]
- 98.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev. 2008;4(3):203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 100.Binion DG, Heidemann J, Li MS, Nelson VM, Otterson MF, Rafiee P. Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297(2):G259–G268. doi: 10.1152/ajpgi.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L, Chopp M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol. 2009;8(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- 102.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K–Akt and MAPK signaling pathways. Chem. Biol. Interact. 2005;156(2–3):141–150. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin. Cancer Res. 2002;8(4):1228–1236. [PubMed] [Google Scholar]
- 104.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1(3):241–242. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 106.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu. Rev. Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 107.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 108.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 109.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 110.Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, Clevers H. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008;73:351–356. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- 111.Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc. Natl. Acad. Sci. U S A. 2008;105(11):4358–4363. doi: 10.1073/pnas.0800668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 113.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26(3):630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 114.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 115.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133(6):1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 116.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc. Natl. Acad. Sci. U S A. 2009;106(17):7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 120.Gottesman MM. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 121.Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26(5):535–542. doi: 10.1080/07357900801904140. [DOI] [PubMed] [Google Scholar]
- 122.Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res. 2008;314(5):1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N, Rechavi G. MDR1 expression identifies human melanoma stem cells. Biochem. Biophys. Res. Commun. 2008;368(4):930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 124.Mohandas KM. Dietary fiber and colorectal cancer. N. Engl. J. Med. 1999;340(24):1925. author reply 1926. [PubMed] [Google Scholar]
- 125.Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J. Gastroenterol. 1999;18(3):118–121. [PubMed] [Google Scholar]
- 126.Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J. Lab. Clin. Med. 1997;130(6):576–584. doi: 10.1016/s0022-2143(97)90107-4. [DOI] [PubMed] [Google Scholar]
- 127.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14(11):2219–2225. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 128.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem. Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol. Nutr. Food Res. 2008;52(9):1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 130.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 131.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. Aaps J. 2006;8(3):E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 133.Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1998;19(6):1039–1043. doi: 10.1093/carcin/19.6.1039. [DOI] [PubMed] [Google Scholar]
- 134.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- 135.Thaloor D, Singh AK, Sidhu GS, Prasad PV, Kleinman HK, Maheshwari RK. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9(4):305–312. [PubMed] [Google Scholar]
- 136.Du B, Jiang L, Xia Q, Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52(1):23–28. doi: 10.1159/000090238. [DOI] [PubMed] [Google Scholar]
- 137.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007;595:127–148. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 138.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 139.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N. Engl. J. Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 140.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 141.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl. Oncol. 2009;2(4):321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2009 doi: 10.1007/s10549-009-0612-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2007;2(2):133–138. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 144.Kao CL, Huang PI, Tsai PH, Tsai ML, Lo JF, Lee YY, Chen YJ, Chen YW, Chiou SH. Resveratrol-induced apoptosis and increased radiosensitivity in CD133-positive cells derived from atypical teratoid/rhabdoid tumor. Int. J. Radiat. Oncol. Biol. Phys. 2009;74(1):219–228. doi: 10.1016/j.ijrobp.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 145.Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest. New Drugs. 2005;23(2):111–119. doi: 10.1007/s10637-005-5855-8. [DOI] [PubMed] [Google Scholar]
- 146.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, Rodriguez AI, Koubi D, Hunter TJ, Corcoran GB, Seidman MD, Levine RA. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol. Cancer Ther. 2005;4(4):554–561. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 147.Wallenborg K, Vlachos P, Eriksson S, Huijbregts L, Arner ES, Joseph B, Hermanson O. Red wine triggers cell death and thioredoxin reductase inhibition: effects beyond resveratrol and SIRT1. Exp. Cell Res. 2009;315(8):1360–1371. doi: 10.1016/j.yexcr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 148.Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom's macroglobulinemia. Clin. Cancer Res. 2008;14(6):1849–1858. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 149.Johnson GE, Ivanov VN, Hei TK. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis. 2008;13(6):790–802. doi: 10.1007/s10495-008-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, Xie LP. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101(2):488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer. 2009;61(4):544–553. doi: 10.1080/01635580902752262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lu KH, Chen YW, Tsai PH, Tsai ML, Lee YY, Chiang CY, Kao CL, Chiou SH, Ku HH, Lin CH, Chen YJ. Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv. Syst. 2009;25(5):543–550. doi: 10.1007/s00381-009-0826-6. [DOI] [PubMed] [Google Scholar]
- 153.Gunther S, Ruhe C, Derikito MG, Bose G, Sauer H, Wartenberg M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett. 2007;250(1):25–35. doi: 10.1016/j.canlet.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 154.Bradlow HL, Sepkovic DW, Telang NT, Osborne MP. Multifunctional aspects of the action of indole-3-carbinol as an antitumor agent. Ann. N Y Acad. Sci. 1999;889:204–213. doi: 10.1111/j.1749-6632.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 155.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. Phytochemicals as modulators of cancer risk. Adv. Exp. Med. Biol. 1999;472:207–221. doi: 10.1007/978-1-4757-3230-6_18. [DOI] [PubMed] [Google Scholar]
- 156.Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26(4):771–778. doi: 10.1093/carcin/bgi018. [DOI] [PubMed] [Google Scholar]
- 157.Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J. Cell. Biochem. Suppl. 1997;28–29:111–116. doi: 10.1002/(sici)1097-4644(1997)28/29+<111::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 158.Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, Mayeaux EJ, Tucker A, Turbat-Herrera EA, Mathis JM. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol. Oncol. 2000;78(2):123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 159.Ali S, Banerjee S, Ahmad A, El-Rayes BF, Philip PA, Sarkar FH. Apoptosis-inducing effect of erlotinib is potentiated by 3,3'-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol. Cancer Ther. 2008;7(6):1708–1719. doi: 10.1158/1535-7163.MCT-08-0354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr. Cancer Ther. 2007;6(2):110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 161.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol. Cancer Ther. 2006;5(5):1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 162.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J. Surg. Res. 2007;143(1):58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 163.Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, Agarwal R. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol. Cancer Ther. 2009;8(8):2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22(51):8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 165.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733–748. [PubMed] [Google Scholar]
- 166.Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74(13):1570–1579. doi: 10.1055/s-2008-1081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol. Cancer Ther. 2004;3(10):1239–1248. [PubMed] [Google Scholar]
- 168.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21(12):2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 169.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269(2):291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, Chiba T. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535(1–3):131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]