Abstract
γδ T cells are generated in the thymus and traffic to secondary lymphoid organs and epithelial surfaces where they regulate immune responses. αβ T cells require the lipid receptor, S1P1 and CD62L, for thymic emigration and circulation through secondary lymphoid organs. Both of these genes are regulated by the transcription factor KLF2 in conventional αβ T cells. It is unclear if γδ T cells use similar mechanisms. In this study, we show that thymic γδ T cells express S1P1, and that it is regulated by KLF2. Furthermore, KLF2 and S1P1-deficient γδ T cells accumulate in the thymus and fail to populate the secondary lymphoid organs or gut, in contrast to the expectation from published work. Interestingly, KLF2 but not S1P1 deficiency led to the expansion of a usually rare population of CD4+ PLZF+ “γδ NKT cells”. Thus KLF2 is critically important for the homeostasis and trafficking of γδ T cells.
INTRODUCTION
T cell progenitors with an MHC restricted TCR undergo positive selection in the thymus at the CD4 CD8 double positive (DP) stage and become a CD4+ or CD8+ single positive (SP) αβ T cell as they enter the thymic medulla. After maturation, αβ T cells emigrate from the thymus to seed peripheral lymphoid organs. The sphingolipid receptor, sphingosine 1-phosphate receptor 1 (S1P1), is required for thymic emigration and is only expressed at high levels by fully mature thymocytes (1). Likewise, only mature thymocytes express CD62L (L-selectin), which is required to gain access to peripheral lymph nodes from the blood (2, 3). We recently showed that the transcription factor Krüppel-like factor 2 (KLF2, previously named LKLF) is required for expression of S1P1 and CD62L in thymocytes (4). KLF2 transactivates both S1P1 and CD62L promoters (4–6). Studies in KLF2-deficient mice showed an accumulation of CD4+ and CD8+ αβ thymocytes in the thymus and a lack of αβ T cells in secondary lymphoid organs (4). These findings suggest that a critical role of KLF2 in T cells is to induce expression of molecules required for naïve T cell trafficking.
γδ T cells from E17 fetal thymocytes have been reported to express S1P1 as determined by real-time PCR (7). However, it is unclear if S1P1 expression is dependent on KLF2 as it is in αβ T cells, or if it plays a functional role. Indeed, evidence with the S1P1 analogue FTY720 suggest that splenic γδ T cells rely on S1P1 but that gut homingγδ T cells do not (8). In this study, we report that KLF2 (and S1P1) are expressed in γδ thymocytes. Interestingly, we find that KLF2-deficiency in hematopoietic stem cells leads to a reduced frequency of conventional γδ T cells in the peripheral lymphoid pool, but an increased incidence of promyelocytic leukemia zinc finger (PLZF)+ γδ natural killer T cells (γδ NKT) (9, 10). Furthermore, we show that both KLF2 and S1P1 are required for localization of γδ T cells (and CD8αα+ αβ T cells) in the gut. Overall, our findings suggest that KLF2 regulates lymphoid homeostasis —affecting the composition and distribution of γδ T cell populations in steady state.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) and CD45.1+ congenic B6.SJL-Ptprca (B6.SJL) mice were purchased from Jackson Labs. Klf2GFP knock-in mice were previously described (11). Klf2fl/fl mice were obtained from Jerry Lingrel (University of Cincinnati, Ohio). S1pr1fl/fl mice were obtained from Richard Proia (National Institutes of Health NIDDK, Maryland). VavCre mice (12) were obtained from Dimitris Kioussis (National Institute for Medical Research, London), through Bruce Walcheck (University of Minnesota, Minnesota). All animal experimentation was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Mixed Bone Marrow Chimera
Mixed bone marrow chimeras were generated by preparing a 1:1 mixture of bone marrow from CD45.2+ Klf2fl/flVavCre, S1p1rfl/flVavCre, or VavCre together with marrow from C57BL/6.SJL (CD45.1+) animals and injecting it into lethally irradiated C57BL/6.SJL hosts. After eight weeks, thymus, lymph node, spleen and gut IELs were stained with FACS antibodies and analyzed by flow cytometry.
Purification of IELs
IELs were purified as described in (13). The small and large intestine were dissected from the mesentery and washed in RPMI 1640 supplemented with 10% Fetal Calf serum. Peyer’s patches were extracted and then the intestine was cut longitudinally, rinsed out, and cut into ~0.5cm pieces. Gut intraepithelial lymphocytes (IELs) were prepared via incubation of the small and large intestine in Ca++ Mg++ free HBSS with 1mM dithiothreitol shaking for 20min at 37°C three times. After each round, the supernatant was filtered with a 70μm nylon-filter. The resulting cell pellet was applied to a 70x/40x isotonic percoll gradient. After centrifugation at 2200 rpm for 25 min. at 25°C, cells at the interface were collected and washed.
Flow cytometry
Single-cell suspensions from spleen, lymph nodes, or thymus were prepared. Antibodies were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA) and included: CD4, CD8α, CD8β, CD25, CD45.1, CD45.2, CD62L, CD69, NK1.1, TCRβ, TCRγδ (GL3). Biotinylated CD1d-PBS-57 monomers (provided by the NIH Tetramer Facility) were incubated with Streptavidin-PE or Streptavidin-APC for 2 hours at room temperature to create fluorescent multimers. The anti-mouse/rat PLZF antibody (D-9) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and staining was performed according to Savage et al (14) using the Foxp3 staining buffer set (eBioscience, San Diego, CA). After washing, cells were then stained with anti-mouse IgG1-APC in 1× permeabilization buffer and re-washed. All cells were analyzed on Becton Dickinson LSR II instruments and the data was processed using FlowJo (Tree Star, Ashland, OR) software.
Cell Sorting and Real-time PCR
Fluorescence-activated cell sorting (FACS) was used to purify CD4+CD8+ DP, “dump” negative CD4+SP, and double negative GL3+ NK1.1/CD1d- CD25-γδ T cells. Each group was sorted in at least two independent experiments. For cell sorting, CD8 T cells were first depleted with anti-CD8 FITC using MACS magnetic beads (Miltenyl Biotec, Auburn, CA). Sorting was performed on a FACSVantage (Becton Dickinson) and was reliably >90% of target population. RNA was isolated from sorted populations using the RNeasy kit, Qiagen (Valencia, CA) and cDNA was produced using the SuperScriptIII Platinum Two-Step qRT-PCR kit, Invitrogen (Carlsbad, CA). cDNA was prepared at least twice from each sort. PCR products were amplified using QuantiTect SYBR Green PCR kit from Qiagen, and detected using ABI Prism 7000 Sequence Detection System (Applied Biosystems, USA). HPRT was used to normalize samples. Primers were as follows; HPRT (hypoxanthine-guanine phosphoribosyl transferase): CTTCCTCCTCAGACCGCTTT & ACCTGGTTCATCATCGCTAA, S1P1: GTGTAGACCCAGAGTCCTGCG & AGCTTTTCCTTGGCTGGAGAG, KLF2: AGCCTATCTTGCCGTCCTTT & CGCCTCGGGTTCATTTC, CD62L: GTGGAGCATCTGGAAACTGG & CGGCTACAGGAATGAAGAGG and β7 Integrin: GGACGACTTGGAACGTGTG and CGTTTTGTCCACGAAGGAG. Fold changes were calculated using the ΔΔCt method with DP values as baseline.
Statistical Analysis
Statistical analysis using unpaired student’s t-tests were performed with Prism 4.0a for Macintosh (Graphpad software, La Jolla, CA). A value of p≤0.05 was considered to be statistically significant.
RESULTS
S1P1 expression is dependent on KLF2 in γδ T cells
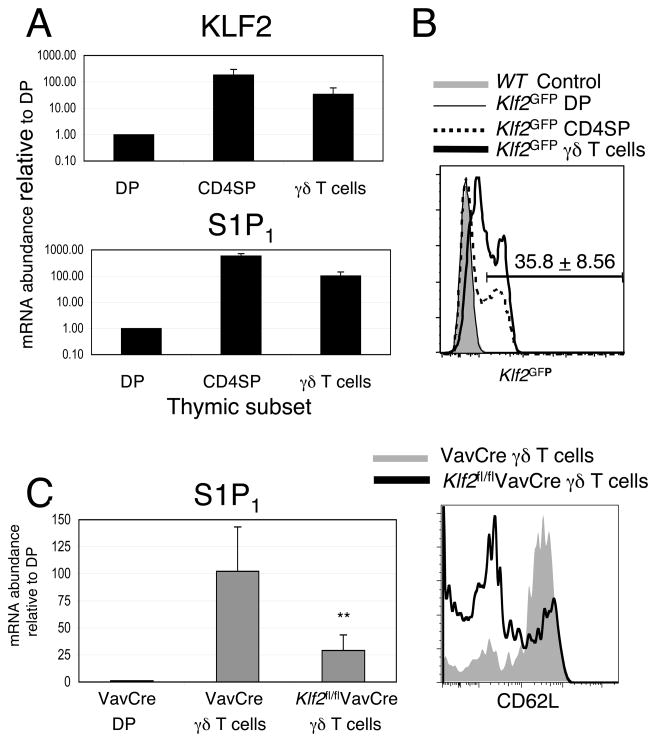
Using multicolor cell sorting and quantitative real-time PCR, we examined them expression of KLF2 and S1P1 mRNA in γδ T cells from adult thymi. As shown in Figure 1A, γδ T cells in the thymus express both KLF2 and S1P1 mRNA. To further determine the expression of KLF2 in individual cells, we utilized a knock-in mouse, which expresses a GFP-KLF2 fusion protein and allows for flow cytometric detection of KLF2 (11). Expression of KLF2 in γδ T cells is heterogeneous with approximately 35% of thymic γδ T cells expressing KLF2 (Figure 1B). These are likely the most mature γδ T cells, by analogy to αβ T cells, where KLF2 is expressed only in mature (Qa2hi, CD69low) CD4SP (11).
Figure 1. S1P1 expression is dependent on KLF2 in γδ T cells.
(A) Quantitative real-time PCR analysis for KLF2 and S1P1 was performed using RNA isolated from the sorted thymocytes. Double negative γδ T cells were sorted using a “dump strategy” to exclude CD4, CD8, TCRβ, NK1.1, and CD1d-aGal-cer tetramer-binding cells. Graphs show the mean difference ± standard deviation, log scale; n = 4. (B) Flow cytometric analysis of thymi from homozygous Klf2GFP mice showing the expression of a KLF2-GFP fusion protein in the indicated subsets. C57BL/6 mice or negative littermate mice were used as controls (shaded gray). Note that the Klf2GFP DP population is identical to negative littermates controls. Data in B are representative of analysis from at least three animals of each genotype, 5 independent experiments. Average percent (+/− standard deviation) of γδ T cells expressing Klf2 is indicated above the histogram. (C) Left Diagram: Quantitative real-time PCR analysis for S1P1 was performed using RNA isolated from sorted double positive thymocytes or double negative γδ + thymocytes from Klf2fl/flVavCre or control VavCre mice. Graph show the mean difference ± standard deviation, linear scale; n = 4. **p<0.02. Right Diagram: The expression of CD62L in γδ + thymocytes from VavCre (shaded gray) and Klf2fl/flVavCre (black line) mice. Data are representative of at least three animals per genotype, 4 independent experiments.
We have previously shown that S1P1 expression is dependent on KLF2 in αβ T cells. Similarly, we have shown that the basis for altered trafficking in KLF2-deficient T cells is due to reduced expression of essential trafficking molecules such as S1P1 and CD62L. As such, we sought to determine the expression of S1P1 and CD62L in KLF2-deficient γδ T cells. KLF2-deficient mice are embryonic lethal at day 13, due to the loss of hemodynamic regulation in response to fluid shear stress in endothelial cells (15). In the past, we and others have used fetal liver chimeras or RAG2 blastocyst chimeras to create a hematopoetic cell specific defect in KLF2 (4, 16). In this report, we utilized a VavCre system that resulted in the conditional deletion of the floxed KLF2 allele in all hematopoetic cells. Efficient deletion of KLF2 mRNA was confirmed on both sorted CD4+ SP and γδ T cells via real-time PCR (data not shown). Subsequent real-time PCR analysis of sorted γδ T cells from Klf2fl/flVavCre thymi demonstrated a 4-fold reduction of S1P1 mRNA (Figure 1C). Furthermore, Klf2fl/flVavCreγδ T cells showed reduced expression of CD62L mRNA (data not shown) and protein (Figure 1C). Thus, S1P1 and CD62L expression are dependent on KLF2 in γδ T cells, as in αβ T cells.
S1P1 deficiency causes peripheral γδ T cell lymphopenia
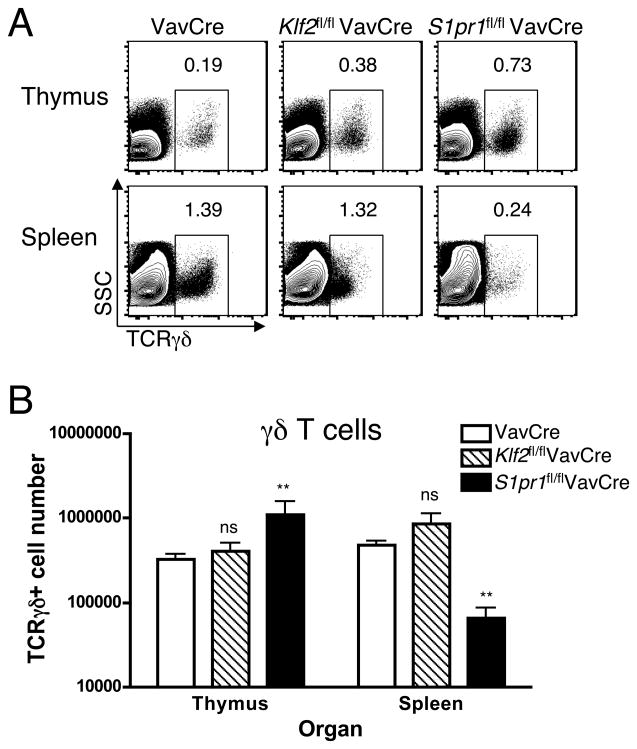
To determine the role of S1P1 in γδ T cells, we used a strategy similar to that used with KLF2 (VavCre transgenic mice crossed to mice with a floxed S1P1 allele). We observed a higher percentage and absolute number of γδ T cells in the thymus of S1pr1fl/flVavCre mice compared to control VavCre mice (Figure 2A and 2B). This accumulation is consistent with a block in thymic egress in the absence of S1P1. Furthermore, γδ T cells were severely reduced in percentage (Figure 2A) and absolute numbers (Figure 2B) in peripheral tissues of S1pr1fl/flVavCre mice. Hence, these data suggest that S1P1 is required for thymic emigration of γδ T cells.
Figure 2. S1P1, but not KLF2, deficiency causes peripheral γδ T cell lymphopenia.
Single cell suspensions from the thymus and spleen of VavCre (control), Klf2fl/flVavCre, or S1pr1fl/flVavCre mice were stained with antibodies to CD4, CD8α, TCRγδ and TCRβ and examined by flow cytometry. (A) γδ + T cells amongst total live cells in thymus and spleen from the indicated mice. (B) Bar graph represent the absolute number (log scale, mean +/−standard deviation, **p<0.02) of TCRγδ+ thymocytes (white bars) or splenocytes (black bars); 3–9 mice were analyzed per group, 5 independent experiments.
To determine the role of KLF2 in γδ T cells, we examined Klf2fl/flVavCre animals. Similar to S1pr1fl/flVavCre mice, we observed a significant increase in the percentage of γδ T cells in the thymus of Klf2fl/flVavCre mice (Figure 2A), though the absolute number was not significantly increased (Figure 2B). Surprisingly, γδ T cell numbers in the spleen were not reduced in Klf2fl/flVavCre mice (Figure 2B). Interestingly, the level of surface TCR γδ was consistently lower on peripheral γδ T cells from Klf2fl/flVavCre animals (Figure 2A).
Peripheral γδ T cell homeostasis is perturbed by KLF2 deficiency, but not S1P1 deficiency
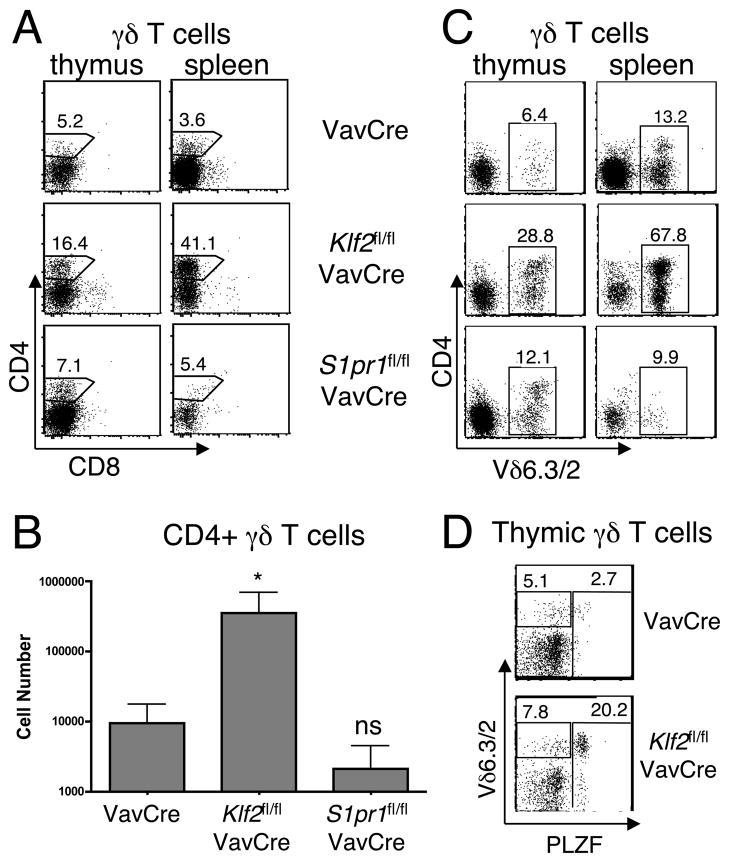
The lack of an effect on peripheral γδ T cell numbers in KLF2-deficient mice was surprising since S1P1 deficiency resulted in reduced numbers, and KLF2-deficient cells have reduced S1P1. It is possible that KLF2 deficient γδ T cells have sufficient residual surface S1P1 to mediate normal emigration. However, given that others have reported defective trafficking of T cells in S1P1+/− animals (where S1P1 mRNA expression is only ~50% reduced) (17), it seemed likely that the substantial (~75%) reduction of S1P1 observed in the Klf2fl/flVavCre γδ T cell pool would be functionally relevant. Furthermore, we noticed that a variable, and sometimes substantial, proportion of the γδ T cells present in the spleen of Klf2fl/flVavCre mice expressed CD4 (Figure 3A) and a reduced surface TCR level (Figure 2B). On average, we observed a 37-fold increase in the number of CD4+ γδ T cells in the spleen of KLF2-deficient mice compared to wild-type control (Figure 3B). This increase was not observed in S1P1-deficient mice (Figures 3A and 3B). Interestingly, a large fraction of these CD4+ γδ T cells expressed Vδ6.3/2 (Figure 3C), characteristic of a recently described subset of “γδ NKT” (18),(9).(10). Indeed, these cells express the PLZF transcription factor, similar to γδ and canonical natural killer T (NKT) cells (Figure 3D). Thus, KLF2 deficiency, but not S1P1 deficiency results in perturbed peripheral γδ homeostasis, with a marked expansion of γδ NKT.
Figure 3. CD4+ γδ NKT cells are expanded in KLF2-, but not S1P1-, deficient mice.
Single cell suspensions from the thymus and spleen of VavCre, Klf2fl/flVavCre, or S1pr1fl/flVavCre mice were stained with antibodies to CD4, CD8α, TCRγδ, TCRβ, Vδ6.3/2 and/or PLZF and examined by flow cytometry. (A), (C), and (D) Two-parameter dot plots are shown after gating on γδ T cells. Numbers indicate the percentage of cells within each gate. (B) Bar graph represents the absolute number (log scale, mean +/−standard deviation, *p<0.05) of CD4+ TCRδ+ splenocytes; 3–9 mice were analyzed per group, 5 independent experiments.
KLF2 is required for thymic emigration of conventional γδ T cells
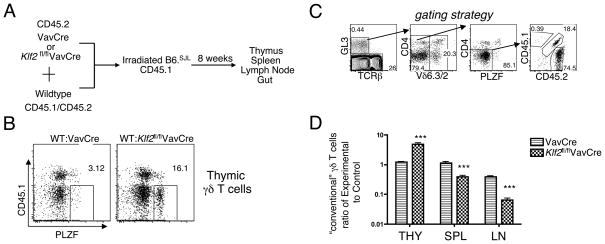
The expansion of a γδ NKT population in the periphery of KLF2-deficient mice is unlikely to be driven by or secondary to αβ T cell lymphopenia, as it was not observed in S1P1-deficient mice (Figure 3), which are equally severely lymphopenic (1). Nonetheless, to eliminate this variable, we employed a mixed bone marrow chimera approach. This approach also allows comparison of thymic emigration of wild-type and knockout cells directly within the same animal, which controls for variability in the microenvironment. We created mixed bone marrow chimeras in which KLF2-deficient (Klf2fl/flVavCre) and wild-type progenitors were mixed and used to reconstitute lethally irradiated recipients, using a congenic marker to distinguish wild-type progenitors from KLF2-deficient progenitors and host cells (Figure 4A). Indeed in mixed bone-marrow chimeras without lymphopenia, we still observed γδ NKT expansion in the KLF2 knockout-derived γδ T cell population and not in wild-type γδ T cells within the same mouse (Figure 4B), suggesting an autonomous effect. Importantly, we enumerated the “conventional” γδ T cells in both lymphoid and non-lymphoid organs using a gating strategy to exclude CD4+, Vδ6.3/2+, PLZF+ cells (Figure 4C). In this setting, KLF2 and S1P1-deficient γδ T cells accumulated in the thymus (3 fold) and were underrepresented in the periphery (3–6 fold) (Figure 4D). Hence, these data suggest that KLF2 deficiency impairs thymic emigration of conventional γδ T cells, an observation that was masked by the expansion of non-conventional γδ NKT cells.
Figure 4. KLF2 is required for thymic emigration of conventional γδ T cells.
Mixed bone-marrow chimeras were created using the strategy shown in (A). (B) Two-parameter dot plots showing the distribution of PLZF+ (γδ NKT cells) are shown after gating on γδ T cells. CD45.1-negative cells mark the experimental group. Numbers indicate the percentage of cells within each gate. (C) Gating strategy to define “conventional γδ T cells” in mixed bone marrow chimeras. (D) Analysis of the ratio of CD45.2+ “Experimental” to CD45.1+ “Control” cells amongst conventional γδ T cells from the thymus (THY), spleen (SPL) and lymph nodes (LN) of mixed bone marrow chimeras. To allow comparison between different experiments, we normalized all groups to the chimerism ratio of DN (double negative) progenitors in the thymus. Bar graph shows the average (log scale, mean +/− standard deviation) of three independent sets of chimeras with at least one mouse per genotype in each. Klf2fl/flVavCre ratios were significantly different from controls in all three tissues (***p < 0.005) using an unpaired student’s t-test.
KLF2 is not expressed by resident γδ or CD8αα+ αβ T cells in the gut yet is required for their gut localization
Recently, Kunisawa et al. proposed that γδ T cells and CD8αα+ αβ T cells migrated in an S1P-independent manner to the gut whereas conventional CD4+ αβ T cells and CD8αβ + αβ T cells required S1P1 for entry into intestinal epithelium (8). Hence, we wished to evaluate the role of KLF2 in regulation of gut trafficking of these populations.
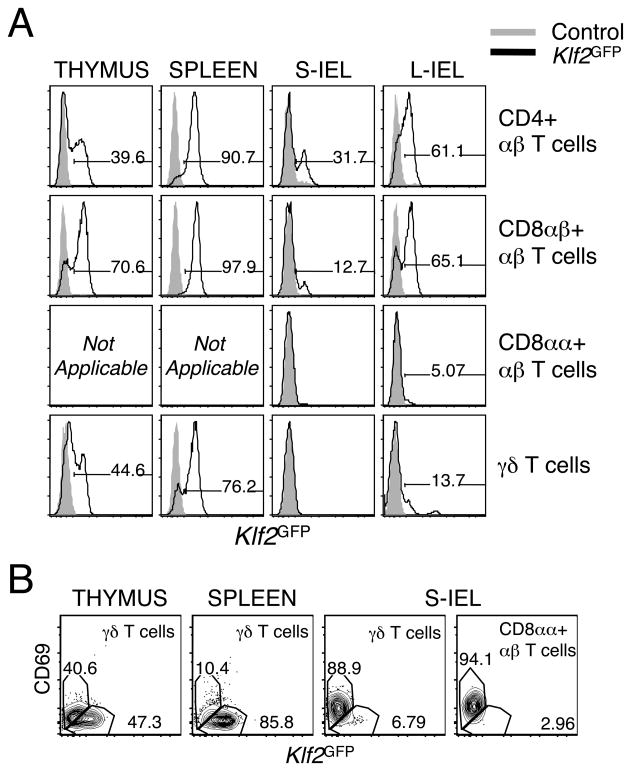
First, we determined the expression of KLF2 in multiple intraepithelial lymphocytes (IEL) subsets at the protein level via flow cytometry using the KLF2GFP reporter mice. We focused on four major subsets in the gut: CD4+ αβ T cells, CD8αβ +αβ T cells, CD8αα+ αβ T cells, and γδ T cells. Interestingly, KLF2 was not expressed on the majority of in CD8αα+ or γδ + IEL in the intestine (Fig. 5A). This correlates with a previous study that reported undetectable S1P1 mRNA in CD8αα+ IEL (8). In contrast, the majority of CD4+ and CD8αβ + αβ T cells in the large intestine do express KLF2 (Figure 5A). Interestingly, we observed an inverse correlation between KLF2 and CD69. In fact, the only IEL that expressed KLF2 were CD69 negative (Figure 5B).
Figure 5. KLF2 is not expressed in γδ or CD8αα+ αβ T cells in the gut.
Single cell suspensions from the thymus, spleen, small intestine (S-IEL), and large intestine (L-IEL) of Klf2GFP mice were stained with antibodies to CD4, CD8α, CD8β, CD69, TCRδ and TCRβ and examined by flow cytometry. (A) KLF2 expression in TCRβ CD4+, TCRβ CD8α+, TCRβ CD8β+, and TCRγδ+ T cells. Representative histograms from one of five independent experiments (except L-IEL for which n=2) (B) Representative dot plots of CD69 and KLF2 expression in TCRγδ+ T cells in the thymus, spleen and S-IELs, and on TCRβ CD8αα+ small intestine IELs. Data are representative of five independent experiments.
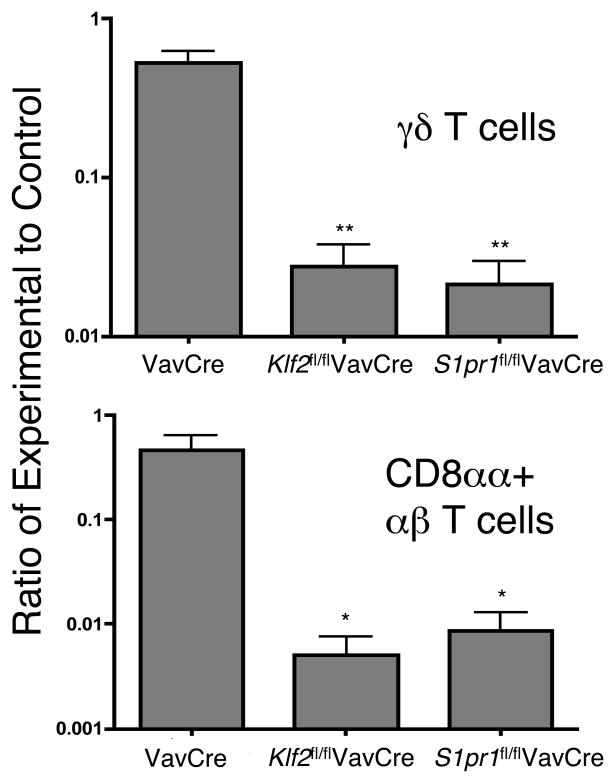
To determine if KLF2 or S1P1 are required for homing to the gut, we examined IEL in competitive mixed bone marrow chimeras (similar to Figure 4). Klf2fl/flVavCre cells and S1pr1fl/flVavCre cells were underrepresented amongst CD4+ and CD8αβ + αβ T cells (data not shown), as expected given that these cells require KLF2 (4) and S1P1 (8) for thymic emigration. Surprisingly, Klf2fl/flVavCre cells and S1pr1fl/flVavCre cells were also profoundly underrepresented (10–100 fold) amongst CD8αα+ αβ and γδ IELs in the gut (Figure 6 and data not shown for large intestine). These data show that KLF2 and S1P1 are required for γδ T cell and CD8αα+ αβ T cell localization to the gut in adult animals.
Figure 6. KLF2 and S1P1 are required for homing of γδ and CD8αα+ αβ IEL to the gut.
Mixed bone-marrow chimeras were created with equal ratios of wild-type competitor and VavCre (control), Klf2fl/flVavCre, or S1pr1fl/flVavCre marrow. Cells were isolated from thymus and small intestine and stained for CD4, CD8α, CD8β, CD45.1, CD45.2, TCRδ and TCRβ and analyzed by flow cytometry. To allow comparison between different experiments, we normalized the ratios relative to the ratio in DP thymocytes. The effect of KLF2 and S1P1 deficiency in γδ T cells (top panel) and CD8αα+ αβ T cells (bottom panel) are indicated. Error bars indicate the standard deviation. n = 3 chimeras per genotype and three independent experiments. **p < 0.001 (for γδ T cells),*p < 0.02 (for CD8αα+ αβ T cells)
DISCUSSION
KLF2 was originally reported as a regulator of T cell quiescence; this conclusion arising from the apparent collapse of the peripheral T cell pool in KLF2-deficient animals, and the ability of over-expressed KLF2 to restrain T cell proliferation and activation (16, 19, 20). Our subsequent studies argued that KLF2 was not required for maintenance of the naïve T cell pool, but rather acted to induce expression of key molecules, including S1P1 and CD62L, which are upregulated late in thymocyte maturation and thus to permit thymic emigration and peripheral trafficking to secondary lymphoid organs (4). The regulation of CD62L and S1P1 by KLF2 have been subsequently confirmed in other studies (5, 6, 21, 22).
In contrast to αβ T cells, γδ T cells are generated from thymic precursors in embryonic waves associated with trafficking to distinct tissue compartments (skin, reproductive tract, and gut). It was previously shown that embryonic thymic γδ T cells express S1P1, suggesting that they might use a similar emigration pathway as αβ T cells (7). However, subsequent data suggested that S1P1 was necessary for γδ T cell egress to the spleen and lymph node but that migration of γδ T cells or CD8αα+ αβ T cells into non-lymphoid sites (the gut specifically) was S1P-independent (8).
In this study, we observed KLF2 (and S1P1) mRNA in normal adult thymic γδ T cells. We showed that S1P1 mRNA levels were substantially reduced by KLF2 deficiency. In accordance with the role of KLF2 in αβ T cells, CD62L was also reduced in KLF2-deficient γδ T cells. CD62L expression was not completely absent, consistent with its control via multiple other transcriptional regulators (6). Since reduction of S1P1 expression by as little as 50% impairs T cell trafficking in vivo (17), it seemed likely that KLF2 deficiency would result in reduced thymic emigration of γδ T cells. However, despite having a phenotype suggestive of retention of γδ T cells in the thymus, a reduction of total γδ T cell numbers in the spleen was not observed. At least three factors confounded the interpretation of this result. First, KLF2 deficient mice are highly αβ lymphopenic, and it was possible that this lymphopenia caused the small number of γδ T cells in the periphery to expand. Second, KLF2-deficient mice display a cell-nonautonomous (bystander) effect, where the over-production of IL-4 causes multiple effects on wild-type bystander CD8 T cells, including the upregulation of CXCR3, and elevated levels of IgE (11). This overproduction of cytokine could also contribute to γδ T cell expansion. Finally, we observed an expanded population of γδ NKT cells (discussed below) in KLF2-deficient mice. For these reasons, we sought to test the trafficking of KLF2-deficient T cells in mixed bone marrow chimeras where wild-type competitor cells fill the peripheral niche and dilute bystander effects to an undetectable level (11). Using this approach, and applying a gating strategy that excluded γδ NKT cells, we report that KLF2-deficient conventional γδ T cells exhibit a thymic emigration defect, accumulating 4-fold in the thymus, and being reduced 3–6 fold in secondary lymphoid organs. A similar approach was employed with S1P1-deficient mixed bone marrow chimeras to show that S1P1, like KLF2, is required for thymic emigration of γδ T cells, which is in agreement with the requirement of KLF2 for optimal expression of S1P1 mRNA in γδ T cells. It is unclear at this point, if S1P1 is the exclusive target of KLF2 as relates to thymic emigration. We also showed that KLF2 controls CD62L expression in γδ T cells, so presumably entry into lymph nodes is coordinately regulated with thymic emigration in γδ T cells.
In the absence of KLF2, we observed a striking expansion of CD4+ γδ T cells. Typically CD4 is only expressed on a small percentage of γδ T cells (23). This expression appears to be enriched amongst a population of γδ T cells with a skewed TCR repertoire, showing an over-representation of TCRγ1.1 and TCRδ6.3 receptors (24). Such cells rapidly produce IFNγ and IL-4 cytokines, express NK1.1 (25), and express the transcription factor PLZF (26). Indeed, such cells require PLZF for their expansion (10). Because of these features, CD4+ γδ T cells have been called “γδ NKT” (18), although we note that like CD1d-tetramer binding NKT cells, not all “γδ NKT” express CD4 and NK1.1 (data not shown). Rather PLZF itself seems to be the most reliable marker for γδ NKT cells. γδ NKT cell expansion has been observed now in several mutant mouse models: TCRα-deficient mice (27), inducible T-cell kinase (ITK)-deficient mice (26, 28) and mice lacking the Id3 transcription factor (29, 30).
One consistent feature so far, is that mouse strains reported to have an expanded γδ NKT population (discussed above), including KLF2-deficient mice, all display peripheral lymphopenia to various extents. There is considerable evidence that T-cell lymphopenia can alter the composition and differentiation state of T cells (31, 32). However, because CD4+γδ T cells were not expanded in S1P1 deficient mice, it is unlikely that γδ NKT expansion was due solely to lymphopenia in KLF2-deficient mice. A second observation was that in mixed bone marrow chimeras with control and KLF2-deficient cells, where the emigration of control cells prevented overt lymphopenia, we still observed an expansion of KLF2-deficient γδ NKT. Thus, although many strains that display an expanded γδ NKT population also display lymphopenia, such lymphopenia was not a primary driver of γδ NKT expansion. It remains to be identified what factors all of these strains have in common that allow γδ NKT expansion.
The fact that γδ NKT cells are expanded in the periphery of KLF2-deficient mice may imply that they have different requirements for thymic emigration than conventional γδ T cells. However, since the emigration defect is not 100%, even for conventional T cells, it is unclear if γδ NKT cells expand from a normal or reduced peripheral population in KLF2-deficient mice. Unfortunately, this population is numerically small in normal mice, making it difficult to study via an intrathymic labeling approach. Current studies are underway to define the mechanisms controlling γδ NKT cell development in KLF2-deficient mice.
Compared to secondary lymphoid organs, the trafficking requirements for T cells in non-lymphoid organs are quite distinct (33),(34),(35). Because we were studying γδ T cells, we focused on the small and large intestine, which are highly populated by γδ T cells in adult mice (36),(37). We first used Klf2GFP mice to determine the expression of KLF2 in T cell subsets in the gut. In accordance with data that sorted CD8αβ + and CD4+ cells from the large intestine expressed S1P1 but CD8αα+ IEL did not (8), we observed a 5–10 fold higher expression of KLF2 in CD8αβ + and CD4+ αβ IEL, in comparison to CD8αα + αβ IEL or γδ T cells in the gut. Although Kunisawa et al. did not measure S1P1 mRNA in γδ T cells, its absence was inferred from the lack of an effect with FTY720 treatment (which did influence CD8αβ + and CD4+ IEL).
We utilized both intact mice (data not shown) and mixed bone marrow chimeras to determine if KLF2 or S1P1 deficiency resulted in alterations in the intestinal compartment. Contrary to the S1P-independent model previously proposed (8), we detected a profound reduction of KLF2 and S1P1-deficient γδ T cells and CD8αα+ IELs in the gut, suggesting that KLF2 and S1P1 are important in the emigration and/or gut homing of these populations in the adult. Kunisawa et al. concluded that γδ + and CD8αα+ αβ + IEL homing were S1P-independent based on the lack of an effect of long term FTY720 treatment on the percent (or numbers) of γδ and CD8αα + αβ IEL in the gut. It is possible however, that T cells are retained in the gut environment in a KLF2/S1P1-independent fashion after initial entry in a KLF2/S1P1-dependent fashion. We previously reported that β7 integrin is expressed at lower levels on KLF2-deficient CD8SP thymocytes (4) and this could contribute to reduced IEL homing in knockout mice, since β7 integrin is critical for this process (35). However, we found no differences in β7 integrin mRNA levels on thymic γδ T cells in KLF2-deficient animals (data not shown).
Alternatively, it is possible that the low numbers of KLF2/S1P1-deficient γδ and CD8αα+ αβ IEL reflect a role for these molecules in the thymic emigration of γδ and CD8αα+ precursors into the circulation. This result is consistent with the requirement for KLF2 and S1P1 in γδ emigration to the spleen, and for the emigration of CD4+ and CD8αβ + cells to the gut. Again, Kunisawa et al. suggested that thymic emigration of γδ and CD8αα+ IEL precursors was S1P-independent because short term FTY720 treatment did not affect the appearance of FITC positive γδ and CD8αα+ “recent thymic emigrants” in the gut 24 hours after intrathymic injection of FITC. We do not have an explanation for this discrepancy at this time, except to note that the highly pleiotropic effects of FTY720 on immune and non-immune cells, including on endothelial cells (38) may contribute to their findings. Clearly, T cell intrinsic deficiency of S1P1 caused a profound reduction in the accumulation of γδ and CD8αα+ αβ T cells in the gut in the adult bone marrows chimeras. In this regard, our data support a thymic origin for gut IEL progenitors in adult animals (39).
Despite the requirement for KLF2 and S1P1 in localization of emigration or homing of γδ and CD8αα+ αβ T cells to the gut, once in that environment, such cells express very low levels of both molecules ((8) and Figure 5). These populations are noted for their CD69+ phenotype. CD69 is upregulated in T cells activated through TCR ligation, although it is unclear if this expression in IEL reflects recent antigen stimulation (40). Alternatively, CD69 induction on IEL may more generally reflect the T cells perception of microenvironmental cues, independent of TCR specificity (41). Interestingly, our data showed a clear inverse correlation between CD69 and KLF2 expression gut T cells. This same relationship is seen in T cells from all tissues in fact ((11)and data not shown). Since CD69 is upregulated in KLF2-deficient (4) and S1P1-deficient (1) T cells, it is possible that the initiating microenvironmental cues that T cells perceive in the gut result in KLF2 downregulation. This in turn results in S1P1 loss and CD69 expression (42). Such a process might be predicted to contribute to the long-term retention of T cells in the gut either by the known role of S1P1 for egress into the circulation (1), or the proposed role of CD69 in cellular retention (43, 44).
Our findings are of relevance to understanding normal and pathologic immune responses. In humans, γδ T cells have been implicated in control of airway (45),(46), (47), (48) and intestinal inflammation (49). In fact, γδ subset studies in mice suggest that Vγ1+ cells (i.e. γδ NKT) enhance airway hyperresponsiveness (AHR), while Vγ4+ cells suppress AHR (48). We here demonstrated that KLF2 and S1P1 are important for thymic emigration/trafficking of both conventional γδ T cells and gut homing γδ and CD8αα+ αβ T cells—therefore suggesting potential strategies to control specific subsets of γδ T cell migration to lymphoid and non-lymphoid organs. Additionally, our results indicate the need for future studies aimed at understanding the mechanisms controlling γδ NKT expansion.
Acknowledgments
The authors wish to thank Xiao-Jie Ding, Jason Vevea and Carolina Mora-Solano for technical support. We thank the members of the University of Minnesota Medical Scientist Training Program and FlowCore facilities. We also thank Dr. David Masopust and Dr. Kensuke Takada for reading the manuscript.
This work was supported by the National Institutes of Health: AI084524 to OAO, AI038903 to SCJ, and AI039560 to KAH, and a T32 Institutional Cancer Biology Training Grant-CA009138 to OAO.
Abbreviations used
- AHR
airway hyperresponsiveness
- DN
double-negative
- DP
double-positive
- IEL
intraepithelial T lymphocyte
- KLF2
Krüppel like factor 2
- NKT cell
natural killer T cell
- PLZF
promyelocytic leukemia zinc finger
- S1P
sphingosine 1-phosphate; S1P1 type 1 S1P receptor
- SP
single-positive
Footnotes
DISCLOSURES
The authors declare no competing financial interests.
References
- 1.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 2.Gallatin WM, I, Weissman L, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Wilson A, Van Ewijk W, Scollay R. Phenotype and localization of thymocytes expressing the homing receptor-associated antigen MEL-14: arguments for the view that most mature thymocytes are located in the medulla. J Immunol. 1987;138:342–351. [PubMed] [Google Scholar]
- 4.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 5.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 6.Dang X, Raffler NA, Ley K. Transcriptional regulation of mouse L-selectin. Biochimica et biophysica acta. 2009;1789:146–152. doi: 10.1016/j.bbagrm.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Kunisawa J, Kurashima Y, Higuchi M, Gohda M, Ishikawa I, Ogahara I, Kim N, Shimizu M, Kiyono H. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 10.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 13.Podd BS, Aberg C, Kudla KL, Keene L, Tobias E, Camerini V. MHC class I allele dosage alters CD8 expression by intestinal intraepithelial lymphocytes. J Immunol. 2001;167:2561–2568. doi: 10.4049/jimmunol.167.5.2561. [DOI] [PubMed] [Google Scholar]
- 14.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 17.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees RK, Ferrero I, MacDonald HR. Tissue-specific segregation of TCRgamma delta+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR alphabeta+NKT cells. Eur J Immunol. 2001;31:2901–2909. doi: 10.1002/1521-4141(2001010)31:10<2901::aid-immu2901>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nat Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Lingrel JB. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene. 2004;23:8088–8096. doi: 10.1038/sj.onc.1207996. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Bohanan CS, Neumann JC, Lingrel JB. KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem. 2008;283:3942–3950. doi: 10.1074/jbc.M707882200. [DOI] [PubMed] [Google Scholar]
- 23.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 24.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 25.Gerber DJ, Azuara V, Levraud JP, Huang SY, Lembezat MP, Pereira P. IL-4-producing gamma delta T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 26.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viney JL, Dianda L, Roberts SJ, Wen L, Mallick CA, Hayday AC, Owen MJ. Lymphocyte proliferation in mice congenitally deficient in T-cell receptor alpha beta + cells. Proc Natl Acad Sci U S A. 1994;91:11948–11952. doi: 10.1073/pnas.91.25.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zuniga-Pflucker JC, Wiest DL. Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Lee SK, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev. 2005;208:169–180. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 33.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 34.Staton TL, Habtezion A, Winslow MM, Sato T, Love PE, Butcher EC. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 35.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Critical reviews in immunology. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonneville M, Janeway CA, Jr, Ito K, Haser W, Ishida I, Nakanishi N, Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988;336:479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 37.Goodman T, Lefrancois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 38.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends in immunology. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20:185–191. doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 41.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 42.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama T, Kasprowicz DJ, Yamashita M, Schubert LA, Gillard G, Kimura M, Didierlaurent A, Koseki H, Ziegler SF. The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J Immunol. 2002;168:87–94. doi: 10.4049/jimmunol.168.1.87. [DOI] [PubMed] [Google Scholar]
- 44.Feng C, Woodside KJ, Vance BA, El-Khoury D, Canelles M, Lee J, Gress R, Fowlkes BJ, Shores EW, Love PE. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14:535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 45.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 46.Lahn M. The role of gammadelta T cells in the airways. Journal of molecular medicine (Berlin, Germany) 2000;78:409–425. doi: 10.1007/s001090000123. [DOI] [PubMed] [Google Scholar]
- 47.Svensson L, Lilliehook B, Larsson R, Bucht A. gammadelta T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 49.Meresse B, Cerf-Bensussan N. Innate T cell responses in human gut. Semin Immunol. 2009;21:121–129. doi: 10.1016/j.smim.2009.01.002. [DOI] [PubMed] [Google Scholar]