Abstract
Background
Adequate zinc is critical for immune function; however, zinc deficiency occurs in >50% of HIV-infected adults. We examined the safety and efficacy of long-term zinc supplementation on HIV disease progression.
Methods
A prospective randomized controlled clinical trial was conducted with 231 HIV+ adults with low plasma zinc levels (<0.75 μg/ml), randomly assigned into zinc (12 mg of elemental zinc for women and 15 mg for men) or placebo, for 18 months. The primary endpoint was immunological failure. HIV-viral load and CD4+ cell count were determined every 6 months. Questionnaires, pill-counts, plasma zinc and C-reactive protein (hsCRP) were used to monitor adherence with study supplements and ART. Intent-to-treat analysis utilized multiple-event analysis, treating CD4+ cell count <200 cells/mm3 as recurrent immunological failure event. Cox proportional-hazard models and the general-linear model were used to analyze morbidity and mortality data.
Results
Zinc supplementation for 18 months reduced four-fold the likelihood of immunological failure, controlling for age, gender, lack of food, baseline CD4+ cell count, viral load, and antiretroviral therapy (RR=0.24[95%CI:0.10,0.56],p<0.002). Viral load indicated poor control with ART but was not affected by zinc supplementation. Zinc supplementation also reduced the rate of diarrhea by more than half (OR=0.4[95%CI:0.183-0.981],p=0.019) compared to placebo. There was no significant difference in mortality between the two groups.
Conclusion
This study demonstrated that long-term (18-month) zinc supplementation at nutritional levels delayed immunological failure and decreased diarrhea over time. This evidence supports the use of zinc supplementation as an adjunct therapy in HIV+ adult cohorts with poor viral control.
Summary
This study demonstrated that long-term (18-month) zinc supplementation at nutritional levels delayed immunological failure and decreased diarrhea over time. This evidence supports the use of zinc supplementation as an adjunct therapy in HIV+ adult cohorts with poor viral control.
Keywords: zinc supplementation, immunological failure, diarrhea, HIV disease progression
INTRODUCTION
Adequate zinc status is critical for immune function.1 Zinc deficiency reduces generation of T-cells, depresses humoral and cell-mediated immunity, leads to lymphopenia, and thymic atrophy,2 and increases the frequency and number of infections.2 Low plasma zinc levels and inadequate zinc intake were found in up 50% of participants in several cohorts of HIV infected adults,3-5 and were independently associated with faster HIV disease progression6,7 and increased mortality,8 while increased plasma zinc levels were associated with improved relative risk of virological response,5 and slower HIV disease progression.9
Zinc supplementation delayed HIV-disease progression and decreased the rate of opportunistic infections with and without antiretroviral therapy (ART),10 and zinc supplementation along with multivitamins and selenium significantly reduced HIV-related mortality.11 Nutritional doses of zinc in children have shown beneficial effects on acute and persistent diarrhea, including lower stool and diarrheal frequency.12-15 Zinc in doses higher than the FDA Daily Values, however, may lead to faster HIV-disease progression.16
The effects of zinc deficiency and zinc supplementation on the development of immunological failure, morbidity and mortality have not been adequately explored. In patients not receiving ART, CD4+ count<200 cells/mm3 is considered immunological failure.17 In those receiving ART, immunological failure has been defined as inadequate increase in CD4+ cell count over a certain threshold, although the specific definitions vary in the amount of the increase in CD4+ T-cell counts expected.18 Immunological failure is associated with twice the relative risk of clinical progression and increased risk of mortality.19-;20,21
This study responded to a need created by convergence of the following factors: the role of zinc in maintaining the integrity of the immune system, the low zinc intake and inadequacy of zinc status among a large proportion of HIV-infected adults, the association between zinc deficiency, faster HIV disease progression and HIV-related mortality, and the need to develop an optimal zinc therapy for HIV-infected adults. Therefore, we investigated the benefits and safety of zinc supplementation in nutritional doses to prevent immunological failure, and decrease morbidity and mortality in HIV infected adults.
METHODS
Study design
A prospective, longitudinal randomized double-blind placebo-controlled clinical trial consisting of a pretreatment phase followed by an 18-month treatment protocol was conducted to test the safety and effectiveness of zinc supplementation. A cohort of 231 HIV-infected adults was recruited between March, 2002 and December, 2005. Participants were eligible for the study if HIV infection was documented, had low plasma zinc levels (<0.75 μg/ml), were ≥18 years, and did not have history of endocrine or psychiatric disorders; premenopausal women were excluded if pregnant or had intention to become pregnant. Participants with plasma zinc levels ≤0.35 μg/ml at any time during the study were excluded for scientific and ethical reasons. HIV-viral load, complete blood counts and chemistries, including parameters of renal and liver function were monitored at baseline and every 6 months and any abnormal value communicated to the primary care physician. The study protocol was approved by the Florida International University (FIU) Internal Review Board.
The pre-randomization phase included one screening and two run-in visits that were used to obtain confirmation of eligibility and to identify potential noncompliant individuals who were no longer interested in participating. Participants were counseled on strategies to overcome problems with supplement adherence. Demographics, plasma zinc levels, and urine toxicology were determined at the initial screening visit. Participants who attended all three pre-treatment visits, within a two-week time window, returned the supplement bottles and used >80% of the pills, were randomized into the study. In the last pre-randomization visit, HIV-viral load was obtained and used as a stratification variable for randomization (<1,000; 1,000-99,999; 100,000-500,000; and >500,000). For the treatment protocol, participants were assigned randomly into zinc supplementation or placebo, and were contacted monthly for 18 months for dispensation of study supplement (12 mg of zinc for women and 15 mg for men, or placebo). The Pharmacist bottled 35 zinc or placebo pills, indistinguishable in shape, size, and color, for the entire study and coded the bottles with the participants’ identification number following the randomization code. Only the Pharmacist and the Statistician were aware of randomization assignments during the trial. The bottles were dispensed to each participant monthly, and the remaining pills counted to assess adherence. A questionnaire was administered to collect data on acceptability of the supplement, adherence, adverse effects, and intercurrent morbidity. Clinical and study personnel, and participants were blinded to the assignment groups. The Monarch Company (Ogden, Utah) prepared the pills but was not involved in the study design, implementation, analysis, or reporting of findings. The pill doses and randomization scheme were independently validated by the Oscar E Olson Biochemistry Laboratory, Brookings, SD.
Assessments
At baseline and every six months, physical examination and medical history and urine toxicology were performed by a nurse practitioner, blood was drawn for assessment of CD4+ cell count, HIV viral load, high-sensitive C-reactive protein (hsCRP), plasma zinc and blood chemistry. History of alcohol and drug use in the preceding 6 months was obtained. Medical history included currently prescribed medications, past and current use of ART and adherence in the previous 6 months; a review of records was used to verify prescriptions and determine changes in ART. Adherence to the study regimen was determined with questionnaires, pill counts, plasma zinc levels, and plasma hsCRP using frozen plasma samples (−80°C) after the study was completed and the assignment was unblinded. Morbidity information was collected by questionnaires at screening, every monthly visit and confirmed by documentation in the medical chart. Cause of death was obtained through authorized contacts, medical records and death certificates from Miami-Dade Health Department of Vital Statistics.
Biochemical assays
Lymphocyte phenotype was determined with a four-color immunophenotyping panel of monoclonal antibodies. Differential counts were determined using a Coulter MaxM hematology instrument and corroborated with cytocentrifuge smears. HIV-viral load was determined using an in-vitro nucleic acid amplification test (Amplicor reagents and protocol, Roche-Diagnostics, Branchburg, NJ); hsCRP was determined using the CRP Ultra-Range Reagent Kit (Equal Diagnostics). Plasma zinc levels were measured by atomic-absorption spectrophotometry; quality assurance used standard reference material (National Institute of Standards and Technology, Gaithersburg, MD).
Statistical Analysis
The intent-to-treat principle was used for analyses. The primary endpoint of this trial was HIV-disease progression, specifically immunological failure. Multiple event analysis was performed with the use of a marginal approach,22 in which confirmed CD4+ counts <200 cells/mm3 were treated as a recurrent immunological failure event. The WLW model controlled for demographic factors, baseline CD4, ART and viral load.
Differences in CD4+ count and viral load between treatment groups were estimated with GEE models for repeated measurements.23 Point estimates of post-randomization change in values and 95% CIs directly modeled the difference between repeated measures. P-values were obtained through group analyses, and adjusted for baseline. Descriptive statistics were used to characterize the population. Continuous variables with non-normal distribution were analyzed using Mann-Whitney, and those with normal distribution with the Student’s t-test. All p-values reported are two-sided; statistical significance was defined as p<0.05.
Study outcomes and sample size
A sample size of 210 participants (105 in each arm of the study) was proposed to examine the zinc supplementation effect on immunological failure, defined as any CD4+ count <200 cells/mm3, which was the primary endpoint. HIV-viral load, morbidity, and mortality were secondary endpoints. With a proposed sample size of ≥210, we had 91% power to detect a 20-cell/mm3 change in CD4 cell count.24,25 With the permission of the FIU-IRB, and the Data and Safety Monitoring Board (DSMB) the sample size exceeded the proposed 210 (231 recruited).
At the initiation, and at six-month intervals, the DSMB reviewed the safety and efficacy of the supplement. The Peto stopping boundary was used for early stopping with nominal p=0.001 for efficacy endpoints and p=0.05 for safety endpoints.26
Role of the Funding Source
This study was funded by the National Institute on Drug Abuse (RO1-DA-14966) which had no role in study design, data collection, analysis, interpretation, writing or submission of this manuscript for publication.
RESULTS
a. HIV-Disease Progression (CD4+ cell count and Viral Load)
As shown in Figure 1, 115 participants were randomized into the supplementation group and 116 into the placebo group; 104 zinc supplemented and 96 participants on placebo completed the 18-month trial. There were no significant differences in demographics or any other characteristics including plasma zinc levels, disease stage, drug use, intermittent treatment, or adherence to ART at baseline between zinc supplementation and placebo groups.
figure1. Randomization Flow Chart.
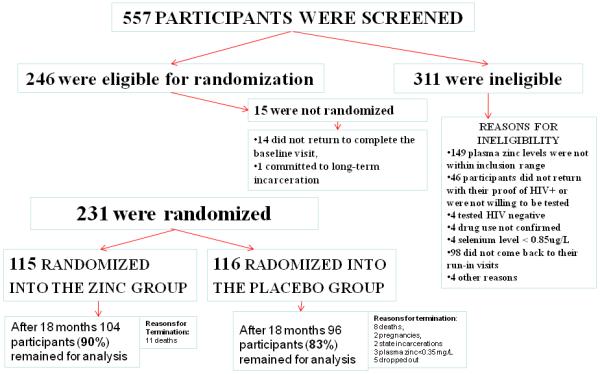
Flow chart depicts the study participant screening, randomization, disposition, and those included in the analyses.
Intervention with the study supplement did not result in any adverse effects. At the end of the trial, zinc-supplemented participants had significantly higher plasma zinc over time (β=0.04, p=0.047) than those on placebo, after controlling for hsCRP at baseline and over time. The mean hsCRP was 4.93±1.97mg/L and was used in the analyses to control for acute-phase reaction, and distinguish between those with low plasma zinc due to zinc deficiency, and those with an acute-phase reaction due to infectious diseases and/or inflammation, as presented elsewhere.27 The average study pill-return throughout the study was 3.65±0.31 out of possible 4 pills monthly for those coming to their visits on time. An average 89.7±4.17% came on time for each visit, with a drop-out rate of 8% annually. These rates did not differ between the study groups. Despite the majority of this cohort (62.3%) reporting the use of antiretroviral medications, of those on ART, more than two-thirds (70.14%) had detectable viral load (>400 copies/mL),28 and the rate was not different between the two study groups (Table 1). Viral load indicated poor control with ART but was not affected by zinc supplementation.
Table 1. Baseline Characteristics by Study Group.
| Variables | Total (N=231) |
Zinc Group (N=115) |
Placebo Group (N=116) |
p-Value |
|---|---|---|---|---|
| Age, mean ± SD, years | 42.7 (7.0) | 42.5(7.1) | 42.8(7.0) | p=0.73 |
| Ethnicity: | ||||
| Black | 77% | 78% | 76% | p=0.44 |
| White, Non-Hispanic | 13% | 12% | 13% | |
| White, Hispanic | 7% | 7% | 7% | |
| Other | 3% | 3% | 4% | |
|
| ||||
| Gender: | ||||
| Male | 73.2% | 72.2% | 74.1% | p=0.74 |
| Female | 26.8% | 27.8% | 25.9% | |
|
| ||||
|
Socioeconomic status
mean ± SD | ||||
| Family monthly income | $326.4(630.6) | $402.8(829.0) | $249.3(311.4) | p=0.066 |
| Highest grade attained, years | 11.1(3.0) | 11.4(2.7) | 10.8(3.3) | p=0.092 |
|
| ||||
|
HIV disease stage
classification | ||||
| Asymptomatic | 28.57% | 29.57% | 27.59% | p=0.87 |
| Symptomatic | 37.66% | 36.52% | 38.79% | |
| AIDS | 33.77% | 33.91% | 33.62% | |
|
| ||||
| CD4 cell count stratification | ||||
| 1. <200 cells/mm3 (%) | 33.8% | 33.9% | 33.6% | p=0.703 |
| 2. 200 to 350 cells/mm3 (%) | 21.6% | 19.1% | 24.1% | |
| 3. >350 cells/mm3 (%) | 44.6% | 47% | 42.3% | |
|
| ||||
|
Years since HIV disease was
diagnosed, mean ± SD |
10.1(8.7) | 9.5(6.0) | 10.8(10.9) | p=0.30 |
|
| ||||
| Antiretroviral (ART) Use | ||||
| On ART regimen | (144/231)=62.3% | (68/115)=59.1% | (76/116)=65.5% | p=0.104 |
| Not on ART | (87/231)=37.7% | (47/115)=40.9% | (40/116)=34.5% | |
|
| ||||
|
HIV ART with undetectable
viral load |
29.9% | 32.4% | 27.6% | p=0.54 |
|
| ||||
| HCV seropositive, % | 25.1% | 21.7% | 28.5% | p=0.24 |
|
| ||||
|
Total No. of Changes* and % changes in Antiretroviral treatment during the study |
N=181 | N=97 | N=84 | p=0.7 |
| 18.1% | 18.7% | 17.5% | ||
|
| ||||
|
Cocaine use, abuse or
dependence, % |
29.9% | 28.7% | 31.0% | p=0.70 |
|
| ||||
| Other drug abuse % | 69.7% | 69.6% | 69.8% | p=0.97 |
|
| ||||
| Alcohol use % | 54.6% | 51.3% | 57.8% | p=0.33 |
|
| ||||
| Cigarette use % | 82.6% | 80% | 85.2% | p=0.30 |
|
| ||||
|
CD4+ cell count,
mean ± SD, cells/uL |
373.1(279.7) | 385.3 (285.1) | 360.9 (275.0) | p=0.51 |
|
| ||||
|
Log10 HIV-1 viral load, mean ± SD |
4.0(1.0) | 4.0(1.0) | 4.0(1.1) | p=0.83 |
|
| ||||
|
Serum zinc,
mean ± SD, mg/L |
0.6 (0.1) | 0.6(0.1) | 0.7(0.2) | p=0.61 |
Changes include initiation, discontinuation, intermittent use and switches in antiretrovirals.
Intent-to-treat analyses of the effect of zinc supplementation compared to placebo on HIV-disease progression show that zinc supplementation for 18 months prevented immunological failure defined as a drop of CD4 count <200 cells/mm3. Using Multiple Event Modeling analyses (WLW Model), indicated that zinc supplementation for 18 months reduced four times the likelihood of immunological failure compared to placebo, controlling for age, gender, food insecurity, baseline CD4+ cell count, viral load, and antiretroviral therapy (RR = 0.24 [0.10,0.56], p<0.002) [Table 2]. This relationship remained significant after including hsCRP in the model.
Table 2.
Results of the Multiple Event Analysis (WLW Model)
| Multivariate RR(95% CI) |
p-value | |
|---|---|---|
| Reduction of Immunological Events in the zinc-supplemented group compared to placebo |
0.24 (0.10,0.56) |
0.002* |
Adjusted for age, gender, food insecurity, baseline CD4+ cell count, baseline HIV viral load (log scale), and antiretroviral therapy.
Significant at the level of p<0.05.
b. Morbidity
At baseline, 32% of the participants reported history of diarrhea within the last year, with 11.6% reporting moderate or severe diarrhea. Although there was no difference in the prevalence of diarrhea between the groups at baseline, the intent-to-treat analysis showed that zinc supplementation significantly reduced the rate of diarrhea over time by more than half (OR=0.4[CI:0.183-0.981], p=0.019) compared to placebo. This reduction was evident by 12 months and was maintained during the study period. Diarrhea was also significantly associated with lower mean plasma levels of zinc (0.59 ±0.11 vs. 0.68±0.21, p<0.001), and remained significant after controlling for ART, levels of hsCRP, CD4+ cell count, and viral load (p=0.006). There were no significant differences among lower or upper respiratory disease or other health events between the groups. Changes in weight, wasting or incidence of anemia also did not affect the findings. No symptoms of zinc toxicity were detected with the level of supplementation given during the study.
c. Mortality
Using the Cox proportional hazards models, no significant difference in the rate of mortality was found between the arms of the study. Eleven participants died in the supplemented group and 8 participants died in the placebo group. Death certificates were obtained to verify time and cause of death and whether the death was HIV-related.
d. Subset analyses
Analyses were conducted in participants who were on ART with suppressed viral load (N=40, 20 in each group); 7 were excluded from the Cox analyses because their CD4 was <200 cells/mm3 (immune failure) at baseline. There were four new events of immune failure during the 18-month follow-up, all in the placebo group, X2=4.4, p=0.043.
DISCUSSION
In this randomized, double-blind, placebo-controlled trial, nutritional levels of zinc supplementation given to HIV-infected adults resulted in a four-fold decrease of the likelihood of immunological failure defined as a drop of CD4+ cell count <200 cells/mm3, after 18 months of use, as compared to placebo. Viral load indicated poor control with ART but was not affected by zinc supplementation. Zinc supplementation also significantly reduced diarrhea compared to placebo. Respiratory diseases or HIV mortality were not affected by supplementation. There were no adverse effects associated with the dose of zinc supplementation used.
In this trial, we defined immunological failure as a drop of CD4+ count <200 cells/mm3 with or without ART, since this definition is predictive of HIV-related morbidity and mortality as well as non-AIDS-related morbidity.20,29,30 Moreover, patients with CD4+ counts <200 cells/mm3 remain susceptible to opportunistic infections despite ART and have an increased risk of mortality.19-30 Although this cohort was at risk for several factors associated with defective immune reconstitution,31 including poor access to therapy, previous therapeutic failure, intermittent duration, and low adherence to ART, a nutritional dose of zinc over 18 months prevented immunological failure as compared to placebo. In addition, zinc supplementation provided immunological and clinical benefits despite persisting detectable HIV-viral load in the majority of the participants. Similar findings were reported by Grabar et al.32 who found that improvement in immunological response is associated with favorable clinical outcomes regardless of virologic response. The adherence to our study regimen was higher than to ART, most likely due to a low pill burden, lack of adverse effects, and active case management with the study supplement.28 We used similar active case management strategies to refer participants to treatment and increase their adherence and compliance with ART.28 Because zinc is also obtained from the diet, zinc therapy might not require as strict adherence as that needed to maintain the benefits of antiretrovirals.
Due to early reports that intakes of zinc above nutritional levels were associated with disease progression,16 this study included only participants whose baseline plasma zinc levels were under 0.75 mg/L, which has been recognized in the literature as low levels of zinc with clinical implications.8,33 The use of plasma zinc levels to detect true zinc deficiency has been controversial, as zinc is an acute-phase reactant.34 To distinguish between true zinc deficiency and acute-phase reaction due to inflammation, hsCRP was used to control for acute-phase reaction.35 The cautious approach to zinc supplementation, by using nutritional levels of zinc to supplement HIV-seropositive individuals with low plasma zinc levels, might have producedthe a delayed (18-month) effect on preventing immunological failure and morbidity observed in this study.
Zinc supplementation may prevent immunological failure through its action on thymic function, expression of interleukin-2, T-cell proliferation, and potential reduction of mitochondrial toxicity and oxidative stress.36 Thymulin, a thymic peptide important for the maturation and differentiation of immature thymocytes,37 is active only when bound to zinc, and its activity is reduced in immune-suppressed and zinc-deficient conditions.38 Improved ability to reconstitute CD4+ cells, as shown in this clinical trial, may be related to the effects of supplementation on increasing the levels of the active zinc-bound thymulin, offering an additional mechanism for slowing HIV disease progression.
Zinc supplementation also affected morbidity in this population. Long-term zinc supplementation significantly decreased the rate and prevented new episodes of diarrhea over time, as compared to placebo. Findings in the literature have been contradictory, mostly due to the use of variable doses of zinc and variable duration of therapy, and whether zinc supplementation was used for prevention or as a treatment of diarrhea. High doses of zinc for short periods of time and during episodes of diarrhea have shown adverse outcomes or no effect,39,40 while preventive zinc supplementation in low doses over long periods had successful results.15 An in-vitro mechanism that supports these conclusions demonstrates that Tat (viral peptide essential for HIV replication) stimulates active fluid secretion from the serosal to the luminal side of enterocytes. Addition of zinc prevents the Tat-induced fluid secretion,41 which may explain the clinical benefits of zinc supplementation in preventing HIV-related diarrhea.
We did not observe significant differences in upper or lower respiratory infections, or in mortality between the zinc-supplemented and the placebo groups, although other studies have reported a reduction in rates of tuberculosis, pneumonia and mortality with zinc supplementation in HIV+ populations in developing countries.42,43 Our results may be limited by the small number of cases of pneumonia (N=4) and tuberculosis (N=1), and although we confirmed 19 HIV-related deaths, the lack of significant difference by study group might be due to the equal use of ART in both arms of the study.
Limitations
The study had a strong design, adequate power and modest drop-out rates. Our findings may be extended to other HIV-infected cohorts with high prevalence of zinc deficiency such as men-who-have-sex-with-men (MSM), children, and those in developing countries.5,8,15,43 However, because of the high prevalence of substance abuse and poor viral control with ART, these findings might not be generalizable to populations on ART with adequate viral control. Subset analyses of participants on stable ART with controlled viral load (N=40), however, indicate that those receiving zinc supplementation had no immunological failure compared to 4 events in the placebo group. While this subset analysis had a small sample size, it appears that it may broaden our findings to cohorts with adequate viral control. Further studies with adequate sample size and power are needed to confirm our findings in populations on stable ART with adequate viral control.
Conclusion
Nutritional levels of zinc supplementation given to HIV-infected adults resulted in a four-fold decrease of the likelihood of immunological failure defined as a drop of CD4+ count <200 cells/mm3 in this randomized, double-blind, placebo-controlled trial after 18 months of use, with no effect on viral load. Zinc supplementation also significantly reduced the morbidity associated with HIV-related diarrhea compared to placebo. Respiratory diseases or mortality were not affected by zinc supplementation. Viral load indicated poor control with ART but was not affected by zinc supplementation. The results of this study can be generalized primarily to HIV-infected populations with prevalent zinc deficiency such as drug users, children, MSM, and populations in developing countries.5,8,15,43 as well as those with poor viral control on ART. This evidence supports the recommendation of zinc therapy as a safe, simple and cost-effective tool to improve the immune response and reduce morbidity and should be considered as an adjunct therapy in HIV infection.
Acknowledgments
Supported by the National Institute on Drug Abuse, Grant No. 1R01-DA-14966.
This clinical trial was registered with clinicaltrials.gov
Footnotes
Appropriate informed consent was obtained and clinical research was conducted in accordance with guidelines for human experimentation as specified by the U.S. Department of Health and Human Services and/or authors’ institutions.
Presented in part at XVII International AIDS Conference in Mexico City, Mexico, August 3-8, 2008, and the 5th International AIDS Society Conference in Cape-Town, South Africa, July 19-22, 2009.
All authors: no conflicts
REFERENCES
- 1.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997 Aug;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 2.Endre L, Beck F, Prasad A. The role of zinc in human health. J Trace Element Exp Med. 1990;3:337–75. [Google Scholar]
- 3.Baum MK, Shor-Posner G, Zhang G, et al. HIV-1 infection in women is associated with severe nutritional deficiencies. J Acquire Immune Defic Syndr Hum Retroviral. 1997;16:272–278. doi: 10.1097/00042560-199712010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Beach RS, Mantero-Atienza E, Shor-Posner G, Javier JJ, Szapocznik J, Morgan R, Sauberlich HE, Cornwell PE, Eisdorfer C, Baum MK. Specific nutrient abnormalities in asymptomatic HIV-1 infection. AIDS. 1992;6:701–708. doi: 10.1097/00002030-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Jones CY, Tang AM, Forrester JE, Huang J, Hendricks KM, Knox TA, Spiegelman D, Semba RD, Woods MN. Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the Nutrition for Healthy Living cohort. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):475–82. doi: 10.1097/01.qai.0000243096.27029.fe. [DOI] [PubMed] [Google Scholar]
- 6.Falutz J, Tsoukas C, Gold P. Zinc as a cofactor in human immunodeficiency virus-induced immunosupression. JAMA. 1988;259:2850–2851. doi: 10.1001/jama.259.19.2850. [DOI] [PubMed] [Google Scholar]
- 7.Graham NMH, Sorenson D, Odaka N, Brookmayer R, Chan D, Willett WC, Morns JS, Saah AJ. Relationship of serum copper and zinc levels to HIV-1 seropositivity and progression to AIDS. J Acquir Immune Defic Syndr. 1991;4(10):976–980. [PubMed] [Google Scholar]
- 8.Baum MK, Campa A, Lai S, Lai H, Page JB. Zinc Status in Human Immunodeficiency Virus Type 1 Infection and Illicit Drug Use. Clin Infec Dis. 2003;37(Suppl 2):S117–23. doi: 10.1086/375875. [DOI] [PubMed] [Google Scholar]
- 9.Baum MK, Shor-Posner G, Lu Y, Rosner B, Sauberlich HE, Fletcher MA, Szapocznik J, Eisdorfer C, Buring JE, Hennekens CH. Micronutrients and HIV-1 disease progression. AIDS. 1995;9(9):1051–6. doi: 10.1097/00002030-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mocchegiani E, Veccia S, Ancarani F, Scalise G, Fabris N. Benefit of oral zinc supplementation as an adjunct to zidovudine (AZT) therapy against opportunistic infections in AIDS. Int J Immunopharmacol. 1995 Sep;17(9):719–27. doi: 10.1016/0192-0561(95)00060-f. [DOI] [PubMed] [Google Scholar]
- 11.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multivitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006;95(4):762–70. doi: 10.1079/bjn20051684. [DOI] [PubMed] [Google Scholar]
- 12.Sazawal S, Black RE, Bhan MK, Ghandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333:839–844. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- 13.Penny ME, Peerson JM, Marin RM, Duran A, Lanata CF, Lonnerdal B, Black RE, Brown KH. Randomized community-base trial of the effect of zinc supplementation, with or without other micronutrients, on the duration of persistent diarrhea in Lima, Peru. J Pediatr. 1999;135:208–217. doi: 10.1016/s0022-3476(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 14.Polat TB, Uysalol M, Cetinkaya F. Efficacy of zinc supplementation on the severity and duration of diarrhea in malnourished Turkish children. Pediatr Int. 2003;45(5):555–9. doi: 10.1046/j.1442-200x.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 15.Bobat R, Coovadia H, Stephen C, Naidoo KL, McKerrow N, Black RE, Moss WJ. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: a randomized double-blind placebo-controlled trial. Lancet. 2005;336:1862–67. doi: 10.1016/S0140-6736(05)67756-2. [DOI] [PubMed] [Google Scholar]
- 16.Tang AM, Graham NM, Kirby AJ, McCall LD, Willett WC, Saah AJ. Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol. 1993 Dec 1;138(11):937–51. doi: 10.1093/oxfordjournals.aje.a116814. [DOI] [PubMed] [Google Scholar]
- 17.CDC Revised Surveillance Case Definitions for HIV Infection Among Adults, Adolescents, and Children Aged <18 Months and for HIV Infection and AIDS Among Children Aged 18 Months to <13 Years --- United States. MMWR. 2008 December 5;57(RR10):1–8. Recommendations and Reports. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a1.htm?s_cid=rr5710a1_e. [PubMed]
- 18.Gazzola L, Tincati C. The absence of CD4+ T cell count recovery despite of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. CID. 2009;48:328–337. doi: 10.1086/595851. ACTG Guidelines. [DOI] [PubMed] [Google Scholar]
- 19.Nicastri E, Chiese A, Angeletti C, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–60. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 20.Gutierez F, Padilla S, Masia M, et al. Patients’ characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr HIV Res. 2008;6:100–7. doi: 10.2174/157016208783885038. [DOI] [PubMed] [Google Scholar]
- 21.Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, Montaner JSG. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–293. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 22.Metcalfe C, Thompson SG. Wei, Lin and Weissfeld’s marginal analysis of multivariate failure time data: should it be applied to a recurrent events outcome? Stat Methods Med Res. 2007 Apr;16(2):103–22. doi: 10.1177/0962280206071926. [DOI] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 24.Raboud JM, Montaner JS, Conway B, et al. Variation in plasma RNA levels, CD4 cell counts, and p24 antigen levels in clinically stable men with human immunodeficiency virus infection. J Infect Dis. 1996;174:191–4. doi: 10.1093/infdis/174.1.191. [DOI] [PubMed] [Google Scholar]
- 25.Paxton WB, Coombs RW, McElrath MJ, et al. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group Longitudinal analysis of quantitative virologic measures in human immunodeficiency virus-infected subjects with > or = 400 CD4 lymphocytes: implications for applying measurements to individual patients. J Infect Dis. 1997;175:247–54. doi: 10.1093/infdis/175.2.247. [DOI] [PubMed] [Google Scholar]
- 26.Hughes MD, Pocock SJ. Interim monitoring of clinical trials. In: Finkelstein DM, Schoenfeld DA, editors. AIDS Clinical Trials. Wiley & Sons; Hoboken, NJ: 1995. pp. 177–196. [Google Scholar]
- 27.Baum MK, Rafie C, Sales S, Lai S, Duan R, Jayaweera DT, Page JB, Campa A. C-reactive protein: a poor marker of cardiovascular disease risk in HIV+ populations with a high prevalence of elevated serum transaminases. Int J STD AIDS. 2008 Jun;19(6):410–3. doi: 10.1258/ijsa.2007.007207. [DOI] [PubMed] [Google Scholar]
- 28.Campa A, Jayaweera DT, Rafie C, Sales S, Page JB, Baum MK. When Access to Antiretroviral for All is Not Enough. Journal of Public Administration and Management. 2007;12(3):147–159. [Google Scholar]
- 29.Baker JV, Peng G, Rapking J, et al. Poor initial CD4 recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–6. doi: 10.1097/QAI.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, Cavassini M, Bernasconi E, Rickenbach M, Hirschel B, Battegay M. Swiss HIV Cohort Study. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41(3):361–72. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 31.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8(2):88–97. [PubMed] [Google Scholar]
- 32.Grabar S, Le Moing V, Goujard C, et al. Clinical outcome of patients with HIV infection according to immunologic and virologic response after 6 months of HAART. Ann Intern Med. 2000;133:401–10. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 33.Baum MK, Shor-Posner G, Campa A. Zinc status in human immunodeficiency virus infection. J Nutr. 2000;(130):1421S–1423S. doi: 10.1093/jn/130.5.1421S. [DOI] [PubMed] [Google Scholar]
- 34.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007 Sep;28(3 Suppl):S403–29. doi: 10.1177/15648265070283S303. [DOI] [PubMed] [Google Scholar]
- 35.Craig GM, Evans SJ, Brayshaw BJ. An inverse relationship between serum zinc and C-reactive protein levels in acutely ill elderly hospital patients. Postgrad Med J. 1990 Dec;66(782):1025–8. doi: 10.1136/pgmj.66.782.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazzola L, Tincati C, Bellistrì GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48(3):328–37. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 37.Safie-Garabedian B, Ahmed K, Khamashta MA, Taub NA, Hughes GRV. Thymulin modulates cytokine release by peripheral blood mononuclear cells: a comparison between healthy volunteers and patients with systemic lupus erythrematodes. Int. Arch. Allergy Immunol. 1993;101:126–131. doi: 10.1159/000236509. [DOI] [PubMed] [Google Scholar]
- 38.Mocchegiani E, Scalise G, Veccia S. Zinc-dependant thymic hormone failure in AIDS. Ann NY Acad Sci. 1992;650:94–98. doi: 10.1111/j.1749-6632.1992.tb49102.x. [DOI] [PubMed] [Google Scholar]
- 39.Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop-Clewes CA, Hoare S, Cole TJ, Hoaran SJ, Longman SC, Stirling D, Agget PJ. A trial of zinc supplementation in young rural Gambian children. Br J Nutr. 1993;69:243–55. doi: 10.1079/bjn19930026. [DOI] [PubMed] [Google Scholar]
- 40.Cárcamo C, Hooton T, Weiss NS, Gilman R, Wener MH, Chavez V, Meneses R, Echevarria J, Vidal M, Holmes KK. Randomized controlled trial of zinc supplementation for persistent diarrhea in adults with HIV-1 infection. J Acquir Immune Defic Syndr. 2006 Oct 1;43(2):197–201. doi: 10.1097/01.qai.0000242446.44285.b5. [DOI] [PubMed] [Google Scholar]
- 41.Canani RB, Ruotolo S, Buccigrossi V, Passariello A, Porcaro F, Siani MC, Guarino A. Zinc fights diarrhoea in HIV-1-infected children: in-vitro evidence to link clinical data and pathophysiological mechanism. AIDS. 2007 Jan 2;21(1):108–10. doi: 10.1097/QAD.0b013e328011849a. [DOI] [PubMed] [Google Scholar]
- 42.Zar HJ. Pneumonia in HIV-infected and HIV-uninfected children in developing countries: epidemiology, clinical features, and management. Curr Opin Pulm Med. 2004 May;10(3):176–82. doi: 10.1097/00063198-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multivitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006 Apr;95(4):762–70. doi: 10.1079/bjn20051684. [DOI] [PubMed] [Google Scholar]


