Abstract
Apoptosis is a critical process for the maintenance of tissue homeostasis and prevention of tumorigenesis. The members of the Bcl-2 family of proteins are the central regulators of the intrinsic apoptotic pathway. Within the Bcl-2 family, the BH3-only subfamily of proteins is tasked with sensing a broad range of apoptotic stimuli and transmitting this signal to other Bcl-2 proteins to initiate programmed cell death. This family of proteins is highly regulated at both transcriptional and post-translational levels, as well as by prominent protein-protein interactions among the family members. Bcl-2 family proteins are often deregulated in cancer, with overexpression of anti-apoptotic members, as well as mutations or defects in pro-apoptotic members. These proteins have been the subject of intensive study for many years and the complex relationships between their regulation and tumorigenesis have spawned a new thinking about cancer treatment. New generations of small molecule Bcl-2 family inhibitors and BH3 and SMAC mimetics have brought new optimism to the pursuit of more individualized and effective cancer therapies.
Keywords: apoptosis, Bcl-2 family, mitochondria, cancer, BH3 mimetics
Introduction
Apoptosis, also called programmed cell death, is a well-regulated, ordered process that occurs both in development and in response to stress to help maintain tissue homeostasis. Additionally, apoptosis induction helps prevent tumorigenesis by eliminating damaged cells. During oncogenic transformation, both genetic and epigenetic changes can lead to defects in apoptosis, as well as sub-sequent accumulation of mutations and alterations in cell proliferation, cell cycle regulation, cell-cell or cell-extra-cellular matrix (ECM) interactions, which can lead to malignancy.1 The two major pathways of apoptosis, extrinsic and intrinsic, are responsible for processing the stress signals and executing cellular demolition. These pathways have their own distinct regulatory processes, as well as significant cross-talk in many situations. Signaling to and from the extrinsic, death receptor pathway and the intrinsic, mitochondrial pathway under normal and abnormal conditions has a profound effect on the fate of the individual cell as well as the tissue and the entire organism it inhabits. Defects in apoptosis also contribute to therapeutic resistance.2 As understanding of these pathways increases, so do efforts to manipulate their function to improve the therapeutic efficacy of anti-cancer agents.3 This review will focus on the current knowledge of the signaling to and from the mitochondria in both normal and transformed cells, and how that can be exploited therapeutically. The emphasis is placed on the role of the Bcl-2 family of proteins and the intrinsic pathway. The regulation of the extrinsic pathway has been discussed elsewhere,4,5 and is not a subject of this review.
The Intrinsic Pathway in Normal Cells
The Bcl-2 family of proteins shares structural and functional characteristics of its patriarch, which was initially discovered to be genetically altered in B-cell lymphomas (Fig. 1).6,7 This protein family is characterized by the presence of at least one Bcl-2 homology (BH) domain. These proteins are further divided into three subfamilies based on their function and structure. The anti-apoptotic members include Bcl-2, Bcl-XL, Mcl-1, Bcl-w and A1/Bfl1. The second subfamily is the pro-apoptotic group, highlighted by Bax and Bak. Finally, the third subfamily is the BH3-only group, so named because their sequence homology with the rest of the family is restricted to the BH3 domain.8 The BH3-only proteins are largely responsible for sensing the apoptotic signals and then transmitting them to other Bcl-2 family members (Fig. 1A).6 A transmembrane (TM) domain in the carboxyl (C)-terminal in some family members is responsible for their membrane localization at the mitochondria, endoplasmic reticulum, or other membrane-bound organelles and for proper function in apoptosis regulation (Fig. 1B). The role of Bcl-2 family proteins in regulating apoptosis is extremely well conserved in metazoans, with a single family member in each of the two subgroups in the worm C. elegans9 and multiple members in each of the three subgroups in mice and human.6,7 Most notably, the number of BH3-only proteins identified in mice and humans has exceeded a dozen.8 Together with an extended caspase family, this perhaps endows the higher organisms with the ability to fine tune apoptotic responses when faced with greater levels of stress due to longer life spans and more complicated environments (Fig. 1).
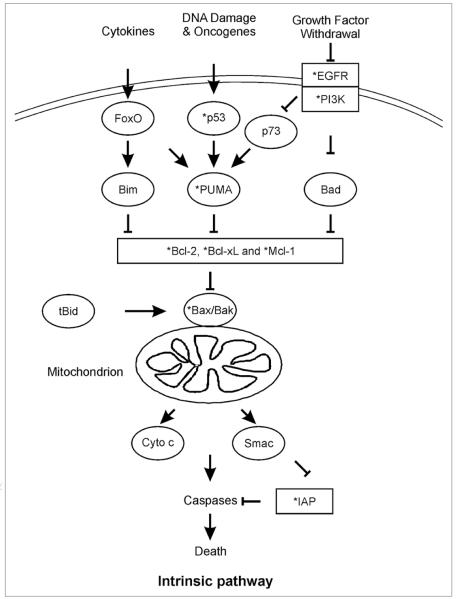
Figure 1.
The Bcl-2 family in apoptosis. (A) The conserved function of the Bcl-2 family in apoptosis regulation. A linear apoptotic pathway with a single gene at each step is genetically defined in C. elegans, with the Bcl-2 family of proteins as upstream regulators.9 EGL-1, the only BH3-only protein in C. elegans, is required for all developmental cell death in this organism. EGL-1 binds to the Bcl-2 homologue CED-9 to free ATPase and Apaf-1 homolog CED-4, leading to its assembly into the apoptosome to activate the caspase, CED-3. A similar pathway has been defined in mammalian cells. The Bcl-2 members have expanded greatly from 2 in C. elegans to 25, with over 10 BH3-only proteins, 5 Bcl-2 like proteins and 3 Bax-like proteins in mouse and human. (B) The Bcl-2 family is structurally and functionally divided into three groups in mammals. The Bax-like group is absent in C. elegans.
The BH3-only proteins have evolved highly specialized functions and do not all respond to every apoptotic stimulus (Fig. 2). Bid receives apoptotic signals from the extrinsic death receptor pathway and transmits them, via its cleavage product truncated Bid (tBid), to the mitochondria.10,11 Alternatively, Bad responds to growth factor deprivation, while PUMA and Noxa are transcriptionally induced by p53 following DNA damage.12-15 This degree of functional diversity allows for cell and tissue-specific regulation of apoptosis in response to a broad range of stress stimuli. An area with some controversy, however, is exactly how the stress signals received by the BH3-only proteins are translated into apoptotic responses. There is a wealth of experimental evidence that BH3-only proteins facilitate the apoptotic response by competitively binding to the anti-apoptotic Bcl-2 family members and displacing them from the pro-apoptotic members, Bax and Bak.16,17 However, there is emerging evidence that some BH3-only proteins can directly interact with Bax or Bak to activate them under certain circumstances. A strong case has been made for direct interaction between tBid and Bax in apoptosis induction,18,19 but a similarly compelling argument has also been made that this interaction is not necessary.20 This controversy also includes the role of PUMA in Bax activation. A great deal of evidence demonstrates that PUMA induces Bax activation by displacing it from anti-apoptotic Bcl-2 proteins.21 Some evidence also suggests that PUMA can bind directly to Bax and aid in its translocation to the mitochondria and oligomerization.22 The story of how BH3-only proteins activate apoptosis has yet to be completed.
Figure 2.
The intrinsic pathway is deregulated in cancer. A wide range of stresses activate the intrinsic pathway in mammalian cells, which is regulated by the Bcl-2 family. The death signals initiated by various stresses are first transmitted through the BH3-only proteins that are activated through transcriptional or posttranslational modifications as shown by several examples. Inhibition of the anti-apoptotic Bcl-2 family members by BH3-only proteins leads to activation of Bax/Bak, subsequent mitochondrial dysfunction, and cytosolic release of apoptogenic proteins such as cytochrome c (Cyto c) and SMAC/Diablo. Following disruption of mitochondrial integrity, a caspase activation cascade is triggered to execute cell death irreversibly. tBid links the extrinsic pathway to the intrinsic pathway and mitochondria. Common genetic or epigenetic alterations (*) in both anti-apoptotic proteins (rectangle) and pro-apoptotic proteins (ellipse) are found in cancer.
Regardless of the pathway to activation of Bax and/or Bak, the end result is mitochondrial outer membrane permeabilization (MOMP), leading to the release of several pro-apoptotic proteins, including cytochrome c, SMAC/DIABLO and AIF (Fig. 2).23,24 The release of these proteins into the cytosol leads to activation of caspases through either the cytochrome c/Apaf-1/pro-caspase-9 apoptosome complex or through inhibition of IAPs by SMAC.23-25 Caspases then carry out the controlled demise of the cell, through cleavage of structural proteins, kinases, transcriptional proteins and others, all leading to cell death (Fig. 2).26 It is interesting to note that this aspect of apoptosis has also recently experienced some controversy, as some new evidence suggests that the effector caspases -3 and -7, long thought to be downstream players in apoptosis, may also play an important role in pathway initiation.27
A hierarchy exists within the Bcl-2 family to promote apoptosis (Fig. 2). The BH3-only proteins provide a versatile system for the initiation of the intrinsic pathway via several mechanisms. Each BH3-only protein is capable of interacting with some or all of the Bcl-2 family members with differing affinities, allowing for cell- and tissue-specific responses to various stress stimuli.28,29 For example, PUMA and Bim are able to bind to all five known anti-apoptotic Bcl-2 family members, giving them broad ability to induce apoptosis across tissues, while Bad only interacts with three out of five (Bcl-2, Bcl-XL, Bcl-w) and Noxa only binds to two (Mcl-1, A1/Bfl1). As discussed above, direct binding to Bax and Bak by selective BH3-only proteins might provide another level of control within the family.18,19,22 The molecular interactions contributing to this specificity remain to be fully elucidated.28
In addition to binding specificity and affinity, post-translational modifications of BH3-only and other apoptotic proteins can have a profound effect on their activity.10 Caspase-mediated proteolysis and subsequent N-myristylation of Bid leads to the active form, tBid,30 while Bcl-XL activity is modulated by deamidation of two asparagine residues.31 In some cells, tBid serves as an amplification loop of apoptotic signaling from the extrinsic pathway to engage the intrinsic pathway and mitochondria for more effective killing (Fig. 2).5 The caspases themselves are also regulated by proteolysis, as they each exist as an inactive pro-enzyme until cleaved. Caspases can also be deactivated by proteolysis, as IAPs can facilitate their ubiquitylation and proteasomal degradation.32 Another significant post-translational regulatory mechanism is phosphorylation. Phosphorylation of Bad by the PI3K/Akt pathway leads to its inactivation and cytosolic sequestration by 14-3-3.33 In contrast, phosphorylation of Bcl-2 enhances its pro-survival function by aiding in its ability to resist cell cycle entry.34
Transcriptional regulation of the mitochondrial pathway of apoptosis is also a significant component, as a variety of transcription factors including p53, NFκB, and the forkhead transcription factor O (FoxO) family can directly regulate the expression of various Bcl-2 family members (Fig. 2). p53 promotes apoptosis by both induction of pro-apoptotic genes and repression of pro-survival genes.35 NFκB is generally thought to be anti-apoptotic, as it promotes transcription of several pro-survival genes, including Bcl-2, Bcl-XL, A1/Bfl1 and IAPs.36 However, convincing evidence suggests that induction of PUMA,37 as well as the DR4 and DR5 members of the death receptor pathway can also be mediated by NFκB.38 The FoxO proteins can promote apoptosis by directly enhancing expression of PUMA and Bim,39,40 as well as indirectly through the repression of Bcl-XL.41
Deregulation of the Intrinsic Pathway in Cancer
While additional levels of complexity and regulation are certain to be discovered, what is currently known about the function of the intrinsic apoptotic pathway has allowed researchers to characterize a variety of aberrations present in cancer (Fig. 2).42 These losses of regulation can occur due to inactivating mutations or deletions of tumor suppressor genes, such as p53, which is mutated in over half of all cancers.43 For example, compromised p53 function can lead to defects in apoptosis because of failure to induce the expression of downstream apoptotic targets, like PUMA and Noxa.35 By contrast, many cancers overexpress NFκB, and subsequently overexpress prosurvival genes to resist apoptosis.44 Additionally, a comprehensive study of the human transcriptome revealed that the IAP family protein member survivin had elevated expression in virtually all human cancers, suggesting the importance of upregulation of pro-survival genes in tumorigenesis.45
The Bcl-2 family of proteins is a common target for deregulation in cancer, with several anti-apoptotic members being overexpressed and pro-apoptotic members being mutated or silenced in a variety of tumor types. Bcl-2 itself was the first such example. BCL-2 was discovered to be overexpressed in human B-cell lymphomas as it is located near chromosomal translocation break points frequently found in those cancers.46 Further studies demonstrated that Bcl-2 protein levels can be elevated due to loss of promoter methylation, loss of microRNA expression and gene amplifications, indicating that elevated Bcl-2 expression is a significant factor in a number of cancers.47 Additionally, Bcl-XL and Mcl-1 overexpression is also common in human cancers,6 particularly in acute lymphoblastic leukemia where elevated Bcl-XL expression appears to occur as a compounding factor in p53-deficient cancers.48 Overexpression of anti-apoptotic proteins not only inhibits the normal cell death process, but also can lead to therapeutic resistance. Bcl-XL and others have been implicated in therapeutic resistance because their overexpression blocks the pathways that the treatments are designed to exploit.49,50
On the other side of the coin, the pro-apoptotic Bcl-2 family members can be downregulated to suppress apoptosis. Spontaneous deletions or mutations of BAX have been observed in colorectal tumors, leading to significant impairment of apoptosis in response to nonsteroidal anti-inflammatory drugs (NSAIDs) and partial impairment in response to other anti-cancer agents.51,52 The BH3-only protein PUMA is downregulated or silenced in a subset of cutaneous melanoma and Burkitt lymphomas, respectively.53,54 In addition to Bcl-2 family proteins being affected by gene alterations or mutations, there are also post-translational modifications that can affect their activity. For example, some cancers have hyperactive kinases in growth factor responsive pathways, such as Akt, which can phosphorylate and inactivate Bad through cytosolic sequestration.33
In summary, genetic and epigenetic alterations are rampant in the components that lie before or at the arbitration step of apoptosis at the mitochondria (Fig. 2). Studies using genetically engineered mice have demonstrated that deregulation of the Bcl-2 family can enhance tumorigenesis. Overexpression of Bcl-2, or loss of Bim, Bid or Bad can lead to hematological malignancy, while loss of Bax and PUMA accelerate solid tumors.6,55 Virtually all tumor suppressor genes and oncogenes are either directly or indirectly linked to apoptosis regulation, and lie before the mitochondrial step,42,56 suggesting that blocking parallel and redundant apoptotic pathways at these nodal points such as p53 or PTEN/PI3K is favored and therefore selected for during malignant transformation.
Therapeutic Exploitation of the Intrinsic Pathway
Chemotherapy and radiation therapy are major cancer treatments that rely largely on induction of apoptosis for efficacy, and can be rendered ineffective by blockade of the intrinsic pathway.2 p53, Bax and PUMA deficiencies have all been implicated in resistance to chemotherapies or radiation,52,57,58 while increased levels of anti-apoptotic Bcl-2 proteins cause similar problems even when the normal pro-apoptotic components are all present.49,50 These challenges, along with the gradual unwinding of the intrinsic pathway in greater detail, have led researchers to devise several strategies to try to restore apoptosis in cancer cells. Protein-based treatments largely utilize the extrinsic pathway to induce apoptotic signaling from the cell surface, but small molecule inhibitors that readily enter cells allow for direct engagement of the components of the intrinsic pathway, as well as potentially having more desirable pharmacological properties.
This engagement is primarily focused on inhibiting anti-apoptotic Bcl-2 family members located upstream of MOMP, since evidence suggests that these proteins are largely responsible for the apoptotic defects found in a wide range of cancers, while the principle downstream proteins are generally left intact (Fig. 2).6,7 With this in mind, several strategies have been employed to target this family of proteins. One strategy involves using peptides designed to target anti-apoptotic Bcl-2 proteins in the same way that BH3-only proteins would, but this method seems to suffer from practical problems as the peptides generally have poor pharmacological characteristics to enter the cells or stay active.59,60 Another interesting strategy has been employed utilizing antisense oligonucleotides designed to target the mRNA of Bcl-2, Bcl-XL and Mcl-1. These targets have all been explored in preclinical studies, and the Bcl-2 antisense oligonucleotide, oblimersen sodium, has advanced to Phase III clinical studies.61,62
However, the most promising approach at the moment appears to be small molecules designed to inhibit anti-apoptotic Bcl-2 proteins by mimicking the function of BH3 domains, commonly termed BH3 mimetics. HA14-1, antimycin A and analogs, BH3Is, gossypol and analogs, and GX015-070 have all been designed to antagonize Bcl-2 proteins, with varying degrees of success.63 ABT-737, a molecule specifically designed to mimic the BH3 domain of Bad, has shown a great deal of promise as a Bcl-2 and Bcl-XL inhibitor.64 When used on its own, ABT-737 is very effective against small cell lung carcinomas, as well as some lymphomas and leukemias in preclinical studies. When used in combination with conventional chemotherapeutics, a powerful synergy was observed in a variety of cases.64 A variant of ABT-737, ABT-263, has advanced from pre-clinical studies into clinical trials. Some recent evidence suggests that elevated Mcl-1 expression may lead to resistance to either ABT compound, consistent with its mechanism of action.63,65
Another emerging area is the development of small molecules targeting IAPs by mimicking the action of SMAC, which relieves the inhibition of caspases through binding and facilitating the ubiquitylation and degradation of the IAPs. The pro-apoptotic properties of SMAC mimetics have been demonstrated by their ability to enhance the apoptotic effects of different classes of anti-cancer drugs.50,66 A number of SMAC mimetic compounds have been developed, with some having shown promise in vitro in combination with chemotherapy.67,68 In some cases, SMAC mimetics may be effective at inducing apoptosis on their own through a TNF-α autocrine loop engaging the death receptor pathway.69 It is expected that some of these compounds will advance into clinical trials in the near future.
Other highly selective anticancer drugs can induce apoptosis through the intrinsic pathway by targeting oncogenic alterations commonly found in cancer such as those in the receptor tyrosine kinase (RTK) EGFR and non-receptor kinases PI3K or AKT. In particular, the regulation of BH3-only proteins has recently emerged as a common mechanism underlying the effectiveness of several kinase inhibitors. An excellent example is provided by the tyrosine kinase inhibitors (TKIs) preferentially targeting mutant EGFR proteins. These TKIs induce Bim or Bad-dependent apoptosis through the intrinsic pathway in lung cancer,70-72 although secondary mutations in EGFR can lead to resistance.73 Another recent study reported that induction of PUMA via p73-dependent transcription is correlated with the sensitivity of head and neck cancer cells to EGFR targeting agents.74 In both cases, suppressing the PI3K/AKT pathway appears to be a major mechanism.70-72,74
As discussed above, most of the effort in cancer therapy in recent years has focused on strategies for killing cancer cells. However, effectively managing or controlling the side effects caused by conventional chemotherapy and radiation in the clinic might be very beneficial if the acute damage and apoptosis in normal tissues can be selectively inhibited. Given the prominent roles of several BH3-only proteins such as PUMA in regulating DNA damage-induced apoptosis and injury in the small intestine and bone marrow,75-77 it might be useful to develop small molecule inhibitors of BH3-only proteins to control the side effects caused by conventional anticancer therapies relying heavily on the induction of DNA damage.
Concluding Remarks
Studying the intrinsic pathway in both normal and cancerous cells has and will continue to offer insights into how a cell makes fundamental decisions about life and death, as well as myriad targets for intervention. Better understanding of critical node points in this pathway will provide greater refinements to drug targeting efforts. This will allow for small-molecule inhibitors with increased selectivity, reduced off-target effects, and greater efficacy that can be used either as single agents or in combination with preexisting cytotoxic agents. It is anticipated that a better understanding of apoptotic signaling will lead to greater utilization of the ever-expanding arsenal of selective agents, and bring the hope for truly individualized cancer therapy closer to reality.
Acknowledgements
We would like to thank Drs. Lin Zhang and Crissy Dudgeon for critical reading. Work in the Yu lab is supported in part by grants from NIH (RO1CA129829, UO1DK085570, U19A1068021) and FAMRI.
Abbreviations
- BH3
Bcl-2 homology 3
- PUMA
p53 upregulated modulator of apoptosis
- SMAC
second mitochondria-derived activator of caspase
- AIF
apoptosis-inducing factor
- IAPs
inhibitors of apoptosis
- NFκB
nuclear factor kappaB
- NSAID
non-steroidal anti-inflammatory drug
- EGFR
epidermal growth factor receptor
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 3.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–98. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 4.El-Deiry WS. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001;8:1066–75. doi: 10.1038/sj.cdd.4400943. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danial NN, Korsmeyer SJ. Cell death. Critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–42. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 9.Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701–6. [PubMed] [Google Scholar]
- 10.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–12. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 11.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–6. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 13.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 14.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–99. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Xing D, Liu L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-XL during UV-induced apoptosis. Mol Biol Cell. 2009;20:3077–87. doi: 10.1091/mbc.E08-11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 25.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 27.Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–51. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–5. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 31.Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosova I, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111:51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001;98:8662–7. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 34.Deng X, Gao F, Flagg T, May WS., Jr. Mono- and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA. 2004;101:153–8. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 36.Karin M, Lin A. NFkappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, et al. PUMA is directly activated by NFkappaB and contributes to TNFalpha-induced apoptosis. Cell Death Differ. 2009;16:1192–202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, et al. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NFkappaB. Nat Cell Biol. 2001;3:409–16. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 39.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 40.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–65. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- 42.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 43.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 44.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NFkappaB. Nat Med. 1999;5:412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 45.Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–8. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 46.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 47.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 48.Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of Bcl-2, Bcl-xl and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89:2986–93. [PubMed] [Google Scholar]
- 49.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–10. [PubMed] [Google Scholar]
- 50.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 51.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–9. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–92. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 53.Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol. 2008;28:5391–402. doi: 10.1128/MCB.00907-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–6. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 55.Qiu W, Carson-Walter EB, Kuan SF, Zhang L, Yu J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009;69:4999–5006. doi: 10.1158/0008-5472.CAN-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 57.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denicourt C, Dowdy SF. Medicine. Targeting apoptotic pathways in cancer cells. Science. 2004;305:1411–3. doi: 10.1126/science.1102974. [DOI] [PubMed] [Google Scholar]
- 60.Shangary S, Oliver CL, Tillman TS, Cascio M, Johnson DE. Sequence and helicity requirements for the proapoptotic activity of Bax BH3 peptides. Mol Cancer Ther. 2004;3:1343–54. [PubMed] [Google Scholar]
- 61.O’Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki A, Koziner B, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–20. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 62.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–18. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–17. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 65.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 66.Arnt CR, Kaufmann SH. The saintly side of Smac/DIABLO: giving anticancer drug-induced apoptosis a boost. Cell Death Differ. 2003;10:1118–20. doi: 10.1038/sj.cdd.4401294. [DOI] [PubMed] [Google Scholar]
- 67.Bank A, Wang P, Du C, Yu J, Zhang L. SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced apoptosis by promoting caspase-3-mediated cytochrome c release. Cancer Res. 2008;68:276–84. doi: 10.1158/0008-5472.CAN-07-5242. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 69.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 74.Sun Q, Ming L, Thomas SM, Wang Y, Chen ZG, Ferris RL, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;18:2348–57. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastroin-testinal syndrome. Cell Stem Cell. 2008;2:576–83. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2009 doi: 10.1038/onc.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–53. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]