Abstract
Angiotensin II (AngII) acts at central type I (AT1) receptors to increase intake of water and saline. In vitro studies demonstrated rapid desensitization of the AT1 receptor after AngII exposure and behavioural studies in rats suggest that exposure to AngII decreases the dipsogenic potency of subsequent AngII. Nevertheless, the effect of repeated AngII injections on saline intake remains untested and a reliable paradigm for examining this purported behavioural desensitization has not emerged from the literature. To address these issues, we established a reliable approach to study AngII-induced dipsetic desensitization and used this approach to test the requirement of central AT1 receptors and the specificity of the effect for water intake. Rats given a treatment regimen of three injections of AngII (300 ng, icv), each separated by 20 min, drank less water than control rats after a subsequent test injection of AngII. The effect was relatively short lasting, dependent on the dose and timing of AngII, and was almost completely blocked by the AT1 receptor antagonist losartan. In further testing, when rats were given access to both water and 1.5% saline, animals that received an AngII treatment regimen drank less water than controls, but saline intake was unaffected. These data support previous suggestions that AngII-induced water and saline intakes are separable. Given the role of G protein uncoupling in desensitization of the AT1 receptor, these data are consistent with the emerging hypothesis that AT1 receptor G protein-dependent intracellular signalling pathways are more relevant for water, but not saline intake.
Keywords: thirst, desensitization, fluid homeostasis, salt intake
Introduction
Angiotensin II (AngII) plays an important role in the maintenance of body fluid homeostasis through coordinated actions at the kidney, heart, vascular smooth muscle, and brain. Within the brain, AngII acts at several sites to stimulate intake of water and NaCl (McKinley et al., 1996; McKinley & Johnson, 2004; Daniels & Fluharty, 2009). Although the intakes of water and saline generally occur together, there are several examples of these ingestive behaviours being dissociated (Daniels et al., 2005; Samson et al., 2008; Daniels et al., 2009). The separability of water and saline intakes, taken together with the rich literature in the field, make the dipsogenic and natriorexigenic actions of AngII powerful models of endocrine coordination of physiology and behaviour.
The ingestive responses to AngII are mediated primarily by the AngII type I (AT1) receptor (Kirby et al., 1992; Sakai et al., 1994; Sakai et al., 1995). Previous studies demonstrated a rapid desensitization of the AT1 receptor that may be important in the regulation of systems that respond to AngII (Thomas et al., 1996; Thomas, 1999; Guo et al., 2001). Indeed, a C-terminal deletion of the AT1 receptor prevents receptor desensitization, thereby generating a receptor that is hypersensitive to AngII in vitro (Conchon et al., 1998). When combined with an N111S substitution that produces mild constitutive activation in vitro (Miserey-Lenkei et al., 2002) and expressed in vivo using a knockin approach, the gain-of-function mutations produce a moderately hypertensive phenotype and the development of cardiovascular fibrosis (Billet et al., 2007). Perhaps more relevant to the present studies, wild-type mice displayed acute hypertensive responses to AngII (returning to baseline within 3 minutes), but the transgenic mice, with disrupted receptor desensitization, showed a more sustained response that stayed elevated for at least 30 min (Billet et al., 2007). Thus, the desensitization of the receptor may serve an important role in both normal and pathophysiological states, offering precise regulation in one and protection in the other.
In spite of the compelling evidence for a role of receptor desensitization in some of the physiological responses to AngII, much less is known about its role in ingestive responses. High doses of AngII desensitized calcium transients in primary cultures of subfornical organ (SFO) neurons (Gebke et al., 1998) and AngII reduced the dipsogenic potency of subsequent administration of AngII in rats (Quirk et al., 1988; Torsoni et al., 2004). Nevertheless, a reliable paradigm for AngII-induced behavioural desensitization appears to be lacking in the literature. In the present studies, we sought to establish a reliable paradigm to produce behavioural desensitization of the response to AngII with the primary goal of testing for separability of the effect on water and saline intakes. In our initial studies we found an optimal means of producing behavioural desensitization, determined that this desensitization was dependent on the timing and dose of AngII, found that the effect was relatively short-lasting, and showed that it was attenuated by an AT1 receptor antagonist. With respect to our more salient goal of evaluating the separability of the effect on water and saline intakes, we found that repeated AngII decreased the water intake after a subsequent AngII challenge, but we failed to detect any effect of repeated AngII on saline intake. These data add to a growing body of research demonstrating divergence of AngII-induced water and saline intakes. Moreover, these findings suggest that the observed behavioural desensitization provides a useful model system to elucidate mechanisms underlying receptor desensitization and the separability of water and salt intakes.
Materials and Methods
Experimental Animals
The handling and care of laboratory animals conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo. Adult male Sprague Dawley rats (175–199 g, 196 total; individual experimental group numbers are provided in the relevant sections of results) were obtained from Charles River Laboratories (Wilmington, MA, USA) or Harlan Laboratories Incorporated (Indianapolis, IN, USA). Rats were individually housed in hanging steel wire mesh cages in a temperature and humidity controlled colony room and maintained on a 12:12 hr light:dark schedule. All animals were allowed access to food (2018 global rodent diet, Harlan Teklad, Madison, WI) and tap water ad libitum, unless otherwise stated.
Drugs
Angiotensin II (AngII) was purchased from Bachem Bioscience Inc. (King of Prussia, PA, USA) and diluted in tris-buffered saline (TBS). Losartan potassium (losartan) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and diluted in normal saline (0.9%).
Lateral ventricle cannula implantation
Rats were anesthetized by a combination of ketamine (70 mg/kg) and xylazine (5 mg/kg) before being fixed in a stereotaxic frame. A chronic indwelling cannula (26 GA; Plastics One Inc., Roanoke, VA, USA) aimed at the lateral ventricle was implanted 1.4 mm lateral to midline; 0.9 mm posterior to bregma; and 1.8 mm ventral to dura. Cannulae were affixed with dental cement and bone screws and rats were allowed to recover for no fewer than 5 days.
Verification of proper cannula placement
Proper cannula placement and AngII responsiveness were verified one week before and three days after each complete experiment by lateral intracerebroventricular (icv) injection of 10 ng AngII in 1μl tris-buffered saline (TBS) using the injection procedure described below. A lower dose of AngII was selected for the verification procedures because it is less likely than a high dose to stimulate drinking if a cannula is near, but not precisely positioned in the ventricle. Data from animals that drank at least 6 ml of water in 30 min were included in the analysis (approximately 7% of rats drank less than 6 ml in the verification procedures and were, therefore, removed from subsequent analysis). To reduce the number of experimental animals required during the initial experiments in which we attempted to optimize the protocol, some rats were used in more than one experiment. Reuse of rats was minimal and, almost exclusively in the early studies that are not included in the figures presented. Whenever rats were used in a second experiment, we first verified cannula patency again, as described above, and care was taken to ensure that the previous treatment status and ratio of new rats to previously used rats was balanced between experimental groups in the new experiment.
Injection protocol and intake measures
Injections were performed using a Hamilton syringe attached to water-filled PE 50 tubing and were made through a 33 ga injection cannula fabricated to extend beyond the guide cannula into the lateral ventricle. All injections were 1μl and the injection cannula was left in place for approximately 30 seconds after each injection. Fluid intake was measured using a 100 ml bottle with 1 ml gradations. Total intake was calculated as the volume of fluid remaining at a given time less the initial starting volume. All injections were performed early in the lights-on portion of the light cycle. For the experiment in which both water and 1.5% NaCl intakes were measured, animals were given access to water and 1.5% NaCl overnight prior to the day of testing in order to allow for habituation to the two-bottle paradigm. The concentration of saline (1.5%) was selected to maintain consistency with previous experiments (Daniels et al., 2005; Daniels et al., 2009) and to help ensure a modest salt ingestion following AngII administration.
In each experiment, multiple injections were administered at varying intervals and concentrations. To differentiate experimental and challenge injections, we refer to the experimental injection(s) as the “treatment regimen” and use the term “test injection” to describe the challenge dose, which was given immediately before intake measures began and does not vary between animals in any given experiment. Food and water were removed at the onset of the first injection and subsequently replaced after the test injection in every experiment. For clarity, the design of each experiment is described in the corresponding portion of the results section and a timeline is provided when appropriate in each corresponding figure.
Data analysis
To avoid statistical errors related to measures of cumulative intake data (Fitts, 2006), we analyzed and report non-cumulative intake, except where noted. All data were analyzed using SPSS software (version 16.0; SPSS, Chicago, IL, USA). In most cases, mixed design ANOVAs using between-subjects effects of treatment groups and within-subjects effects of time were used and statistically significant main or interaction effects (p<0.05) were further analyzed by posthoc tests using simple-effects probes. When reported, analysis of total (30 min) intake was performed using a Student’s t-test.
Results
A single injection of AngII failed to reliably affect the drinking response to subsequent AngII
In order to establish a reliable protocol for producing AngII-induced desensitization, we first sought to replicate a previous finding (Torsoni et al., 2004) using a treatment regimen consisting of a single injection of AngII (300 ng) followed by a test injection of AngII (300 ng or 10 ng) given 2 hr later. Unlike the previous study, we failed to detect a statistically significant effect of this treatment regimen on water intake stimulated by a test dose of 300 ng AngII (F1,8=0.622, p=0.453, n=5 per group; data not shown) or 10 ng AngII (F1,16=1.442, p=0.247, n=9 per group; data not shown). We also performed the experiment using a lower dose of AngII (10 ng) as the treatment regimen, but still observed no effect of this treatment on intake after a subsequent test injection of 10 ng AngII (F1,8=0.647, p=0.444, n=5 per group; data not shown).
Repeated injections of AngII decreased the drinking response to subsequent AngII
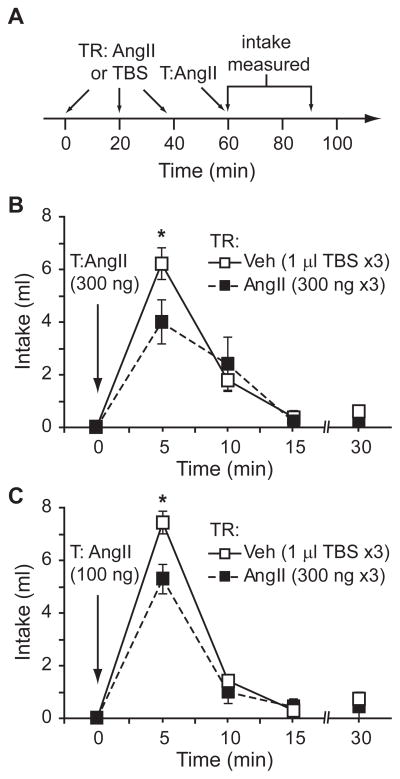
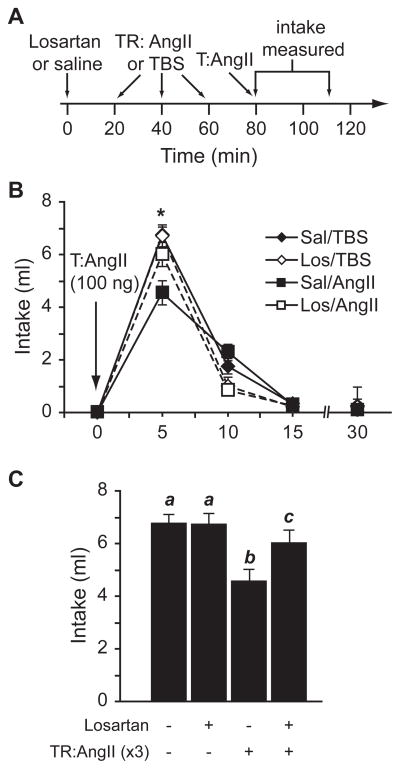
In light of the finding that 2 successive injections of AngII at varying concentrations, administered 2 hr apart, did not reliably attenuate water intake in our laboratory, we tested the effects of greater exposure to AngII over a longer period of time. Accordingly, we used a treatment regimen that comprised 3 injections of AngII (300 ng) or vehicle (TBS), each given 20 min apart (a timeline of the design is shown in Figure 1A). Rats that received a treatment regimen of AngII drank less water than controls after control and experimental groups received a subsequent test injection of 300 ng AngII (F1,8=5.762, p=0.043, n=5 per group; Figure 1B) or 100 ng AngII (F1,12=7.078, p=0.021, n=7 per group; Figure 1C) 20 min after the treatment regimen. When we used a 100 ng test injection, we found a statistically significant time × treatment interaction (F3,36=3.521, p=0.025), but failed to observe a statistically significant interaction when a 300 ng test injection was used (F3,24=1.879, p=0.160). Posthoc tests on the statistically significant interaction indicated that the majority of water intake occurred during the first 5 min and that the desensitizing effect of AngII was most prominent during this period. The desensitizing effect of the AngII treatment regimen approximated but did not reach statistical significance when a test dose of 10 ng AngII was used (F1,8=4.348, p=0.071, n=5 per group; data not shown).
Figure 1.
A treatment regimen (TR) comprising 3 injections of AngII decreased the drinking response to a subsequent test injection (T) of AngII. A timeline displaying the injection protocol is shown in panel A. Rats given a treatment regimen of AngII (300 ng) drank less water than those in the vehicle treatment regimen group after a test injection of 300 ng AngII (*p=0.043, n=5 per group; panel B) or 100 ng AngII (*p=0.021, n=7 per group; panel C).
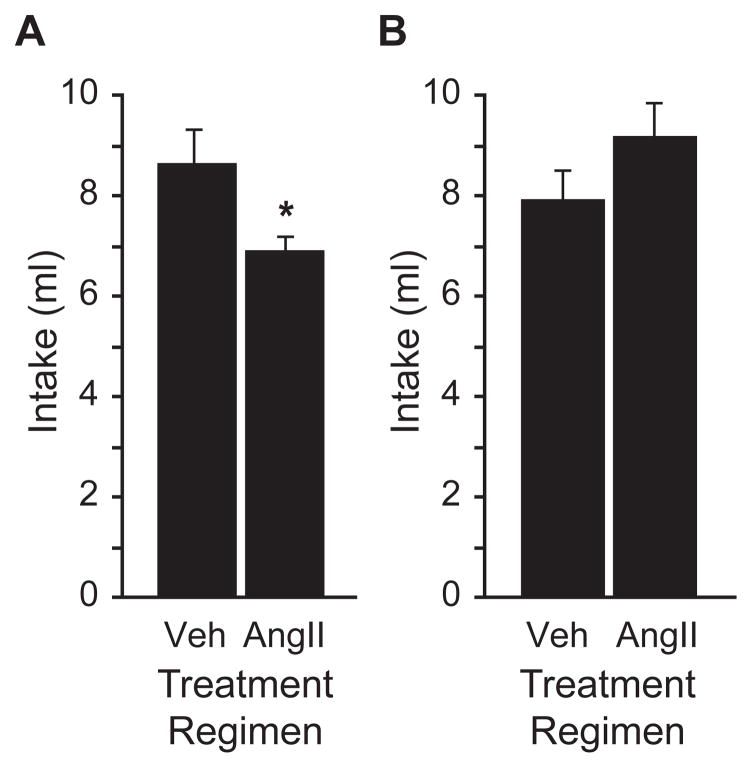
To determine the duration of the desensitizing effect of repeated AngII, we replicated the above experiment but administered an additional test injection of 100 ng AngII to all animals 24 hr after the final test injection and measured water intake over the following 30 min. In replication of our earlier finding, animals that had received a treatment regimen comprising 3 injections of AngII (300 ng) drank less water in response to the subsequent test injection of 100 ng AngII (F1,14=4.935, p=0.043, n=8 per group; Figure 2A). After a second test injection of 100 ng AngII given 24 hr later, we did not observe any differences in water intake between animals that had previously received an AngII or a vehicle treatment regimen (t=1.210, p=0.246; Figure 2B). Food and water were available ad libitum for the 24 hr between test injections.
Figure 2.
The experiment illustrated in Figure 1 was repeated using new groups of rats and the total water intake over a thirty minute testing period is shown in panel A. Again, rats in the AngII treatment regimen group (300 ng AngII ×3) drank less after a test injection of AngII (100 ng) than rats in the vehicle treatment regimen group (1 μl TBS ×3; *p=0.043, n=8 per group). There were no differences in thirty minute water intake after a second test injection of AngII (100 ng) was given to all rats 24 h after the first test injection (p=0.246; panel B).
The AT1 receptor antagonist losartan attenuated the desensitizing effect of AngII
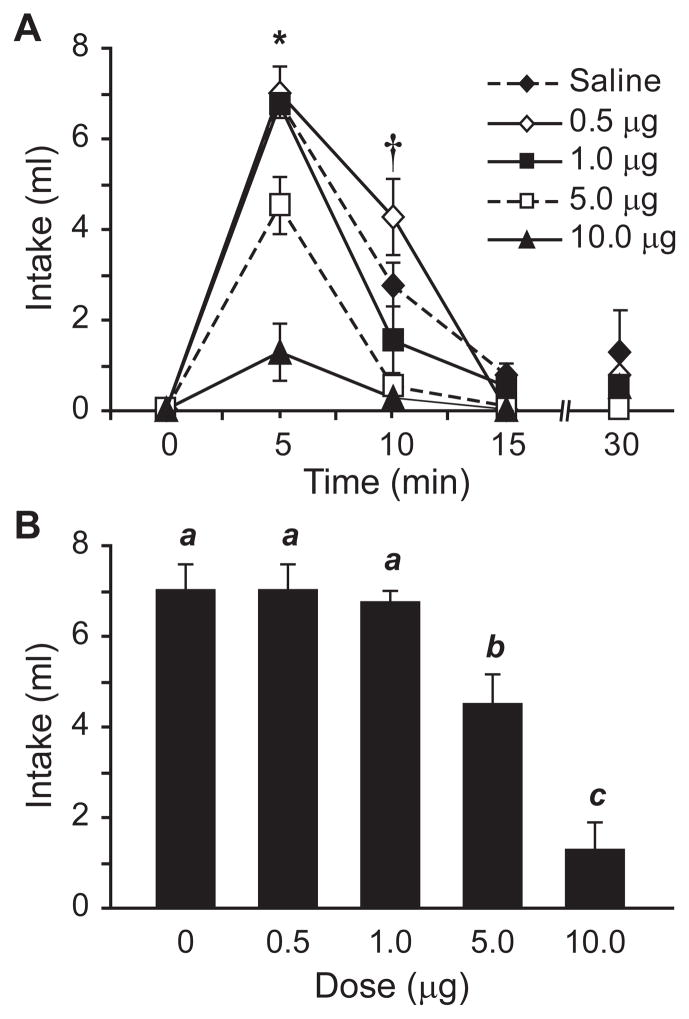
To confirm previous studies (e.g., Toney & Porter, 1993a, b) and determine the optimal dose of losartan for our experiments, we administered losartan (0.5, 1.0, 5.0, or 10 μg) or saline 20 min before an injection of AngII (100 ng) and measured the resultant water intake. As shown in Figure 3, increasing losartan concentration was associated with less water intake after injection of AngII (main effect of losartan, F4,15=25.261, p<0.001, n=4 per dose group). A statistically significant time x dose interaction (F12,45=5.179, p<0.001 ) and subsequent posthoc tests found significant effects of the 5 and 10 μg doses at both 5 (p<0.01; Figure 3B) and 10 min (p<0.01); the times at which most of the water intake occurred in the saline-pretreated rats.
Figure 3.
Injection of the AT1-receptor antagonist losartan decreased the drinking response to AngII in a dose-dependent manner. Non-cumulative intakes by rats given various doses of losartan, administered by icv injection 20 min before 100 ng AngII, are shown in panel A (*p<0.001, †p<0.01, n=4 per group). The effects of losartan on intake were most prominent during the first 5 min of testing, when most water consumption occurred (bars with different letters differed from each other statistically, p<0.05; panel B).
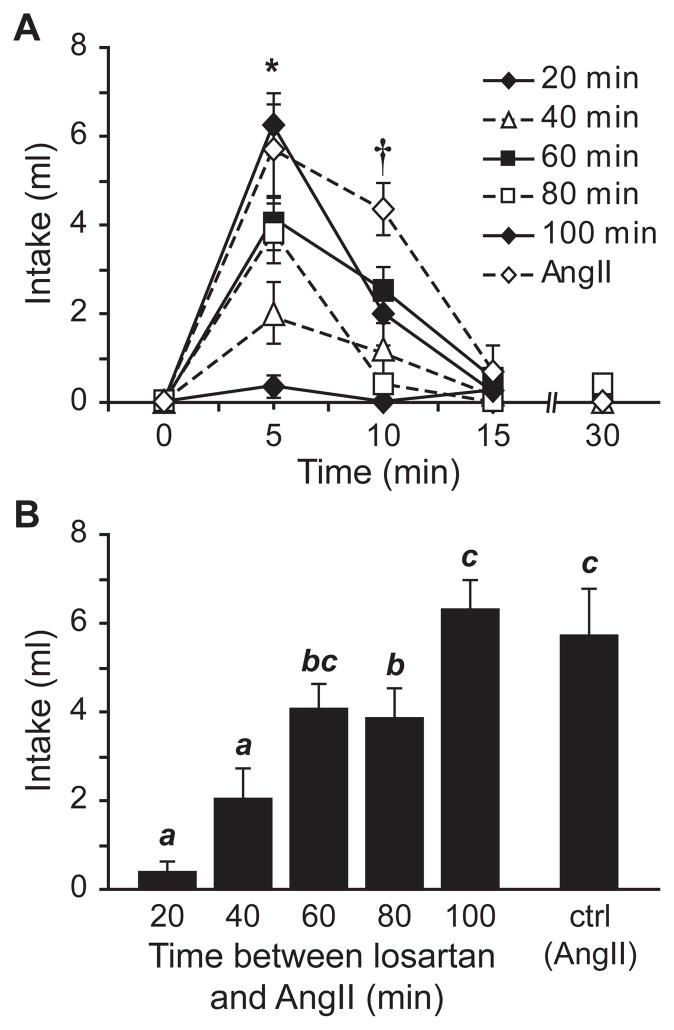
Additional investigation also was important to determine the duration of the effect of losartan, because the goal of the studies was to minimize any effect of losartan on the test injection while maximizing the blockade of AT1 receptors during the treatment regimen. To this end, we performed a time-course analysis using 10 μg of losartan given 20, 40, 60, 80, or 100 min before injection of 100 ng AngII. We then compared water intake from rats exposed to losartan and AngII with intake by rats given AngII alone. As shown in Figure 4, there was a significant main effect of losartan on water intake (F5,28=10.681, p<0.001, n=3–6 per group). Posthoc tests indicated that this effect was markedly diminished or completely absent when losartan was given 60 min or more before AngII. A statistically significant time x group interaction (F15,84=3.982, p<0.001 ) and additional posthoc tests on the time course of fluid intake indicated that the effect of losartan was most prominent when the greatest amount of water intake occurred in control rats; specifically, during the first 5 (p<0.01; Figure 4B) and 10 min (p<0.01) after AngII was given.
Figure 4.
Time-course of the antagonistic effect of losartan on AngII-induced drinking. Non- cumulative water intake by rats given icv injections of losartan 20, 40, 60, 80, or 100 min before 100 ng AngII is shown in panel A. Analysis of the data revealed a main effect of losartan on water intake (p<0.001, n=3–6 per group) and posthoc tests confirmed that the effect was statistically significant at 5 and 10 min (*p<0.001, †p<0.01). Data from the 5 min bin are shown in the histogram in panel B. Bars with different letters differed from each other statistically (p<0.05).
Using the dose-response and time-course analyses as guides, we examined the effects of losartan pretreatment on the desensitizing properties of the repeated AngII treatment regimen. As illustrated in Figure 5, losartan attenuated the desensitizing effects of an AngII treatment regimen (300 ng ×3) on water intake after a test injection of 100 ng AngII (F3,123=3.731, p=0.013 in a three-way interaction of within-subject effects of time and between-subjects effects of pretreatment and treatment regimen, n=10–12 per group). Posthoc tests were used to probe data from individual times and, as was true in the studies described above, we found that the effects of losartan occurred during the first 5 min after the test injection, when the greatest amount of water intake occurred in the control groups. Consistent with the studies above, our posthoc tests showed that, in the absence of losartan, animals receiving a treatment regimen of AngII drank less water than those receiving a vehicle treatment regimen (p<0.001). We did not detect an effect of losartan pretreatment on intake in the vehicle treatment regimen groups (p=0.898). On the other hand, losartan almost completely blocked the desensitizing effect of the AngII treatment regimen (p<0.001), although animals in the losartan/AngII group drank slightly less than controls (mean intakes: 6.0 ml and 6.7 ml, respectively, p<0.05). As was true for the other experiments, the effects were prominent in the first 5 min (p<0.01) after the test injection, when the greatest amount of water intake occurred in control rats. We found no statistically significant differences in intake at any other time.
Figure 5.
Losartan markedly attenuated the desensitizing effect of a repeated AngII treatment regimen (TR) on water intake after a test injection (T) of AngII. A timeline indicating the injection protocol is illustrated in panel A. We found a three-way interaction between a within-subject effect of time and between-subjects effects of pretreatment and treatment regimen (p=0.013, n=10–12 per group). Posthoc probes indicated that the effect of losartan occurred at the 5 min bin (*p<0.01), which is shown in the histogram in panel C. Bars with different letters differed from each other statistically (p<0.05).
The desensitizing properties of the AngII treatment regimen did not affect AngII-induced intake of 1.5% NaCl solution
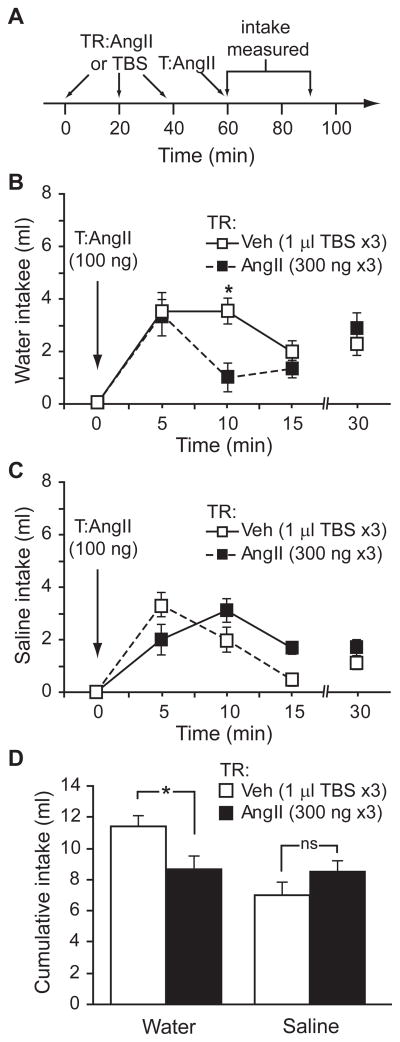
Although the studies described above measured AngII-induced water intake, it is important to note that AngII also stimulates intake of saline solutions (Buggy & Fisher, 1974; Daniels et al., 2005; Daniels et al., 2009) and recent studies suggest that these intakes are governed by separable cellular signalling mechanisms (Daniels et al., 2005; Daniels et al., 2009). To determine if the attenuation of intake observed above affected both water and saline intakes, we repeated our experiments using a two-bottle test (water vs. 1.5% NaCl). Using the paradigm established above in which rats received a treatment regimen comprising 3 injections of vehicle (TBS) or AngII (300 ng), followed by a test injection of AngII (100 ng), we again found that rats given an AngII treatment regimen drank less water than controls (F1,16=7.102, p=0.017, n=9 per group; Figure 6B). The intake pattern appeared to differ from that observed in our previous experiments, but posthoc probes were not justified because we did not find a statistically significant group x time interaction (F3,48=2.611, p=0.062). Nevertheless, a direct comparison of this experiment with the earlier experiments is difficult because of clear differences in the context. More relevant, however, is the finding that total water intake over the 30 min testing period was different between animals that had received the AngII treatment regimen and those that had received vehicle (t=2.665, p=0.017; Figure 6D), but no similar differences in saline intake were found (F1,16=2.00, p=0.176; Figures 6C and 6D).
Figure 6.
Repeated injections of AngII affected subsequent water, but not 1.5% NaCl intake, in a two-bottle test. Rats were given a treatment regimen (TR) comprising 3 injections of AngII or vehicle, before a test injection (T) of AngII. A timeline of the experimental design is shown in panel A. Consistent with the findings above, rats given multiple injections of AngII drank less water than control rats (*p=0.017, n=9 per group; panel B). Intake of 1.5% NaCl did not differ between the groups (p=0.176; panel C). Total intake during the course of the testing period is shown in panel D (*p=0.017).
Discussion
In the present studies, we used supraphysiological doses of AngII to produce a behavioural desensitization of the water intake normally occurring after central injection of AngII. Although a rat will drink in response to very low doses of AngII, we found that larger doses were needed to observe behavioural desensitization. Nevertheless, the finding that animals given multiple injections of AngII summing to as much as 1 μg drank less than animals given only 100 ng of AngII is remarkable, especially considering very early studies on AngII suggesting a sigmoidal dose-response curve with doses as high as 4 μg (Epstein et al., 1970). Given the timing of the injections, it is reasonable to conclude that the observed behavioural desensitization reflects receptor desensitization that has been observed in a number of in vitro studies (Thomas et al., 1996; Thomas, 1999; Guo et al., 2001).
It is important to note that the magnitude of the difference in water intake may be less important than the fact that a difference was found. Indeed, drinking by rats given multiple injections of AngII may reflect the dipsogenic effect of any or all of the injections. Accordingly, any desensitization occurring after multiple injections of AngII actually may be underestimated. The extent to which this occurred is likely minimal, however, because Fregly and Rowland (1986) showed that when water was withheld for 15 min after central application of AngII, drinking was only 40% of that observed when rats were given immediate access after the injection of AngII. When the delay was 30 min, rats drank no more than vehicle controls. As such, any residual drinking from earlier injections, given no sooner than 20 min before the test injection, would likely have only a modest, if any, impact on water intake after the test injection. Similarly, differences in the timing of water and saline intakes could selectively mask desensitization of saline intake, but leave an effect on water intake observable. Specifically, if there were a delayed saline intake resulting from one or more of the earlier injections of AngII, it could negate any desensitization that would become observable later. We believe this is unlikely, however, especially because of our previous findings that showed only a slight difference in time between water and 1.5% NaCl intakes (no more than 5 min) after injection of AngII (Daniels et al., 2009). It is also notable that after isoproterenol injection, the dipsogenic effects of which require intact AT1 receptors in the SFO (Krause et al., 2008), delaying access to fluid attenuated saline intake, but had no effect on water intake (Chiaraviglio, 1979). Thus, it seems that any delayed response would more likely mask desensitization of water intake than of saline intake. These considerations make it clear that the magnitude of the observed desensitization may be less meaningful than the actual existence of the effect.
Although it was important to establish a working protocol for detecting the behavioural desensitization, the primary goal of the present work was to better our understanding of AngII-induced desensitization and determine if the observed response was specific to water intake or if it extended to saline intake. These experiments found a notable difference in the desensitizing effect of AngII on water and saline intakes. This effect was clear even though the pattern of intake differed slightly from the single-bottle studies that measured only water intake. Specifically, most fluid intake occurred during the first 5 min in the single-bottle tests, but intake was more distributed across the first 10 min in the two-bottle test; however, this difference in intake pattern is not surprising. Indeed, numerous intake measures have been shown to change when rats are given fewer or a greater number of choices (Sclafani & Springer, 1976; Tordoff & Bachmanov, 2003). In spite of the different intake patterns, rats given the AngII treatment regimen drank less water than the controls, but saline intakes were similar.
Exploring the possible explanations for why the effect was limited to water intake without extending to saline intake is important and potentially informative. One possibility is that separate sub-populations of receptors, with different susceptibility to desensitization, mediate the respective effects of AngII on water and saline intakes. A second possibility is that differences in the dose-response curves for the two ingestive behaviours make one less sensitive to perturbation than the other, especially if the mechanism underlying the desensitization involves receptor availability. Any such differences in receptor availability could develop through a number of mechanisms, including receptor occupancy with or without receptor internalization. Although receptor occupancy is a feasible means by which the receptor may have a limited response to additional AngII, it is unlikely that such occupancy would not involve other meaningful changes. Indeed, receptor internalization has been identified as an important component of desensitization (Thomas et al., 1996; Hunyady et al., 2000). This internalization, or even simply receptor occupancy, could make the population of receptors less sensitive and this could become behaviourally relevant given that we found previously that 1.5% saline intake occurred after lower doses of AngII than those needed to stimulate water intake (Daniels et al., 2009). A third, and perhaps more integrative, possibility is that the differences in behaviour reflect differential regulation of intracellular signalling pathways.
It has been hypothesized that AT1 receptor signalling pathways play separable roles in water and saline intakes stimulated by AngII (Daniels et al., 2005; Daniels et al., 2007). More recent experiments provide support for this hypothesis by demonstrating that G protein-dependent pathways appear to be more important for water intake stimulated by AngII, whereas G protein-independent pathways may be more relevant for AngII-stimulated saline intake (Daniels et al., 2009). This alone cannot explain the present results, but additional studies have demonstrated that AT1 receptors, like other members of the G protein-coupled receptor (GPCR) superfamily, are regulated by GPCR kinases (GRKs) that reduce receptor-G protein interactions and contribute to receptor desensitization (Gainetdinov et al., 2004; Penela et al., 2006; Mehta & Griendling, 2007). Given that previous studies from our lab have shown a greater role for G protein-mediated pathways in water intake than in saline intake and agonist binding is known to affect G protein coupling, it is tempting to speculate that the behavioural differences observed resulted from agonist-induced G protein uncoupling. If further research supported this, it would provide additional support for the hypothesis that water and saline intakes are regulated by separable intracellular signalling pathways.
In spite of the clear difference in water intake after repeated injections of AngII and the plausible underlying mechanisms involved, it is important to consider some caveats and limitations of the present studies before drawing any conclusions. In addition to the observed fluid intake, injection of AngII causes increased blood pressure, which we did not measure. This is an important consideration because elevations in blood pressure inhibit drinking through actions involving arterial baroreceptors (Stocker et al., 2002). With respect to the present findings, however, it is important to note that the vast majority of studies investigating AngII-induced pressor responses use chronic infusions of AngII, rather than a single injection or a series of acute injections. Nevertheless, the informative time-course of the pressor response after central or peripheral AngII infusion is found in the studies of Hutchinson et al. (1976). These experiments show that the pressor response to peripheral infusion of AngII is far more sensitive than the response to central infusions. Perhaps more relevant to the present studies, blood pressure returned to baseline by 10 min after administration of AngII ended, regardless of the route of AngII administration. This was true even when a total of 20 μg AngII was infused over a 10 min period (most of the present studies injected a total of 1 μg AngII over 60 min). Thus, the rapid recovery of blood pressure previously observed makes it unlikely that any hypertensive effects persisted for the entire inter-injection interval in the present studies. This is not to say that there was no pressor response to the test injection, but simply that there is no reason to believe that the response would differ between the experimental groups (which all received the same test injection in any given experiment). Accordingly, it seems unlikely that group differences in blood pressure account for the different ingestive responses observed.
In addition to affecting blood pressure, AngII increases natriuresis and vasopressin secretion, which combine to reduce plasma sodium concentration and osmolality (Coghlan et al., 1981; Fluharty & Manaker, 1983). It is imaginable that decreased plasma sodium would selectively increase sodium intake, thereby masking any desensitization of sodium intake by the treatment regimen. We believe this is unlikely, however, because previous reports in sheep (Coghlan et al., 1981) and rats (Fluharty & Manaker, 1983) indicate that the loss of sodium is delayed, and therefore not likely to have occurred until after our intake measures are complete. Indeed, it is important to note that the saline intake measured in the present study and our previous studies (Daniels et al., 2005; Daniels et al., 2009) likely occurs during what Fluharty and Manaker (1983) called the “early phase” of sodium intake, which precedes sodium loss. This rapid, “early phase” intake is observable when rats are offered more concentrated saline (e.g., 3%), but even more so when rats are provided more dilute, yet still hypertonic, concentrations of saline such as the 1.5%–1.8% saline used here and previously (Buggy & Fisher, 1974; Daniels et al., 2005; Daniels et al., 2009). Nevertheless, the more dramatic phase of intake, that traditionally considered a salt appetite, occurs after a delay of 8–12 hr that likely results from the increased natriuresis and vasopressin secretion previously reported (Coghlan et al., 1981; Fluharty & Manaker, 1983). As described above, our earlier studies suggest that lower doses of AngII are needed to generate “early phase” intake of 1.5% saline than are needed to stimulate water intake (Daniels et al., 2009). Accordingly, it seems unlikely that the effect of AngII on sodium loss affected intake of either water or saline in the present studies because these intakes both occurred in the timeframe of the “early phase” noted by Fluharty and Manaker (1983).
A responsible interpretation of any decrease in behaviour resulting from a pharmacological treatment must consider the possibility that the decrease is a secondary effect of the treatment, potentially resulting from an unforeseen deleterious consequence of the treatment such as malaise. The potential for AngII-induced malaise is suggested by a previous report showing taste aversion learning associated with AngII (Grupp & Chow, 1993). On the other hand, a comparison of taste aversion learning in rats and cats showed a robust taste aversion in cats, but not rats, after pairing novel flavour with AngII (Rabin et al., 1986). The copious water intake after central administration of AngII at doses as high as 4 μg, taken together with the indication that the dose-response curve levelled off after 100 ng (Epstein et al., 1970), further argues against any malaise stimulated by the doses of AngII given in the present studies. Moreover, we failed to detect an effect of repeated AngII on saline intake, which would likely be altered if the observed treatment effect was due to a general malaise. Therefore, it seems unlikely that malaise accounts for the observed differences. Even in the absence of malaise, it is still possible that the observed response was not specific to drinking stimulated by AngII, but also would affect the responses to other dipsogenic stimuli. In spite of the differences in approaches used here and previously, it is notable and relevant that the behavioural desensitization produced by repeated AngII, as reported in Quirk et al. (1988), did not affect drinking after central injection of carbachol or neurotensin. Thus, it is reasonable to predict that the response observed here would be similarly specific.
Although additional research is needed to clarify the many issues raised by the present studies, this report describes a useful model for understanding more about the effects of AngII and, more specifically, the mechanisms underlying receptor desensitization. Indeed, receptor desensitization appears to be a critical feature of the renin-angiotensin system and may play a role in various human health issues. Moreover, determining how the behavioural desensitization remains specific for one AT1 receptor-mediated behaviour (water intake) and not another (saline intake) could provide valuable information about the mechanism underlying desensitization to AngII and, perhaps, other receptor systems.
Acknowledgments
Helpful technical support was provided by Elizabeth Mietlicki, Kimberly Plyler, & Naomi McKay.
Grants. Support provided by NIH awards DK-73800 and HL-91911
Footnotes
Disclosures. The authors have nothing to disclose.
References
- Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–1925. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy J, Fisher AE. Evidence for a dual central role for angiotensin in water and sodium intake. Nature. 1974;250:733–735. doi: 10.1038/250733a0. [DOI] [PubMed] [Google Scholar]
- Chiaraviglio E. Drinking behaviour in rats treated with isoprenaline, angiotensin II or angiotensin antagonists. J Physiol. 1979;296:193–202. doi: 10.1113/jphysiol.1979.sp012999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan JP, Considine PJ, Denton DA, Fei DT, Leksell LG, McKinley MJ, Muller AF, Tarjan E, Weisinger RS, Bradshaw RA. Sodium appetite in sheep induced by cerebral ventricular infusion of angiotensin: comparison with sodium deficiency. Science. 1981;214:195–197. doi: 10.1126/science.6169149. [DOI] [PubMed] [Google Scholar]
- Conchon S, Peltier N, Corvol P, Clauser E. A noninternalized nondesensitized truncated AT1A receptor transduces an amplified ANG II signal. Am J Physiol. 1998;274:E336–345. doi: 10.1152/ajpendo.1998.274.2.E336. [DOI] [PubMed] [Google Scholar]
- Daniels D, Fluharty SJ. Neuroendocrinology of Body Fluid Homeostasis. In: Pfaff Dw, Arnold Ap, Fahrbach Se, Etgen Am, Rubin Rt., editors. Hormones, Brain and Behavior. 2. Academic Press; San Diego: 2009. pp. 259–288. [Google Scholar]
- Daniels D, Mietlicki EG, Nowak EL, Fluharty SJ. Angiotensin II stimulates water and NaCl intake through separate cell signalling pathways in rats. Exp Physiol. 2009;94:130–137. doi: 10.1113/expphysiol.2008.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D, Yee DK, Faulconbridge LF, Fluharty SJ. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology. 2005;146:5552–5560. doi: 10.1210/en.2005-0774. [DOI] [PubMed] [Google Scholar]
- Daniels D, Yee DK, Fluharty SJ. Angiotensin II receptor signalling. Exp Physiol. 2007;92:523–527. doi: 10.1113/expphysiol.2006.036897. [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970;210:457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts DA. Misuse of ANOVA with cumulative intakes. Appetite. 2006;46:100–102. doi: 10.1016/j.appet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Fluharty SJ, Manaker S. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: I. Relation to urinary sodium excretion. Behav Neurosci. 1983;97:738–745. doi: 10.1037//0735-7044.97.5.738. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, Rowland NE. Do peripheral and cerebroventricular injections of angiotensin II act at the same site? Studies on additivity of drinking. Brain Res Bull. 1986;16:249–257. doi: 10.1016/0361-9230(86)90039-0. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gebke E, Muller AR, Jurzak M, Gerstberger R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience. 1998;85:509–520. doi: 10.1016/s0306-4522(97)00601-5. [DOI] [PubMed] [Google Scholar]
- Grupp LA, Chow SY. Angiotensin II mediates a conditioned taste aversion in water-replete rats. Pharmacol Biochem Behav. 1993;44:985–988. doi: 10.1016/0091-3057(93)90036-s. [DOI] [PubMed] [Google Scholar]
- Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ, Clark AJ, Gaborik Z. Mechanisms and functions of AT(1) angiotensin receptor internalization. Regul Pept. 2000;91:29–44. doi: 10.1016/s0167-0115(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Hutchinson JS, Schelling P, Mohring J, Ganten D. Pressor action of centrally perfused angiotensin II in rats with hereditary hypothalamic diabetes insipidus. Endocrinology. 1976;99:819–823. doi: 10.1210/endo-99-3-819. [DOI] [PubMed] [Google Scholar]
- Kirby RF, Thunhorst RL, Johnson AK. Effects of a non-peptide angiotensin receptor antagonist on drinking and blood pressure responses to centrally administered angiotensins in the rat. Brain Res. 1992;576:348–350. doi: 10.1016/0006-8993(92)90703-c. [DOI] [PubMed] [Google Scholar]
- Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl. 1996;3:S99–104. [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Parnot C, Bardin S, Corvol P, Clauser E. Constitutive internalization of constitutively active agiotensin II AT(1A) receptor mutants is blocked by inverse agonists. J Biol Chem. 2002;277:5891–5901. doi: 10.1074/jbc.M108398200. [DOI] [PubMed] [Google Scholar]
- Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Wright JW, Harding JW. Tachyphylaxis of dipsogenic activity to intracerebroventricular administration of angiotensins. Brain Res. 1988;452:73–78. doi: 10.1016/0006-8993(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Bakarich AC, Chedester AL, Lee J. Angiotensin II-induced taste aversion learning in cats and rats and the role of the area postrema. Physiol Behav. 1986;36:1173–1178. doi: 10.1016/0031-9384(86)90496-8. [DOI] [PubMed] [Google Scholar]
- Sakai RR, He PF, Yang XD, Ma LY, Guo YF, Reilly JJ, Moga CN, Fluharty SJ. Intracerebroventricular administration of AT1 receptor antisense oligonucleotides inhibits the behavioral actions of angiotensin II. J Neurochem. 1994;62:2053–2056. doi: 10.1046/j.1471-4159.1994.62052053.x. [DOI] [PubMed] [Google Scholar]
- Sakai RR, Ma LY, He PF, Fluharty SJ. Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept. 1995;59:183–192. doi: 10.1016/0167-0115(95)00111-n. [DOI] [PubMed] [Google Scholar]
- Samson WK, Yosten GL, Chang JK, Ferguson AV, White MM. Obestatin inhibits vasopressin secretion: evidence for a physiological action in the control of fluid homeostasis. J Endocrinol. 2008;196:559–564. doi: 10.1677/JOE-07-0364. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Stricker EM, Sved AF. Arterial baroreceptors mediate the inhibitory effect of acute increases in arterial blood pressure on thirst. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1718–1729. doi: 10.1152/ajpregu.00651.2001. [DOI] [PubMed] [Google Scholar]
- Thomas WG. Regulation of angiotensin II type 1 (AT1) receptor function. Regul Pept. 1999;79:9–23. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Thekkumkara TJ, Baker KM. Cardiac effects of AII. AT1A receptor signaling, desensitization, and internalization. Adv Exp Med Biol. 1996;396:59–69. [PubMed] [Google Scholar]
- Toney GM, Porter JP. Effects of blockade of AT1 and AT2 receptors in brain on the central angiotensin II pressor response in conscious spontaneously hypertensive rats. Neuropharmacology. 1993a;32:581–589. doi: 10.1016/0028-3908(93)90054-7. [DOI] [PubMed] [Google Scholar]
- Toney GM, Porter JP. Functional roles of brain AT1 and AT2 receptors in the central angiotensin II pressor response in conscious young spontaneously hypertensive rats. Brain Res Dev Brain Res. 1993b;71:193–199. doi: 10.1016/0165-3806(93)90171-6. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsoni MA, Carvalheira JB, Calegari VC, Bezerra RM, Saad MJ, Gontijo JA, Velloso LA. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–128. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]