The administration of the anti-VEGF agent, bevacizumab, decreases tube formation and migration of human ocular melanoma cells, cultured alone or with HUVECs in vitro. The authors found that systemic bevacizumab decreased the size of ocular melanoma and the number of micrometastases in a mouse model of metastatic ocular melanoma. These findings are important with regard to a potential systemic therapy for patients with ocular melanoma with micrometastasis to the liver, as there is currently no effective treatment.
Abstract
Purpose.
This study was undertaken to determine whether anti-vascular endothelial growth factor (VEGF) therapy inhibits growth of primary uveal melanoma and spread of its hepatic micrometastases.
Methods.
The human uveal melanoma cell lines Mel290 and Mel 270, HUVECs, mouse B16LS9 melanoma cells, and mouse vascular endothelial cells were separately cultured or co-cultured and incubated with bevacizumab or IgG1. The level of VEGF protein in the culture medium was measured by ELISA. In vitro angiogenesis and invasion assays were performed under bevacizumab or IgG1 treatment. Mel290 or B16LS9 cells were inoculated into NU/NU or C57Bl/6 mouse eyes which were enucleated after 7 days. The sizes of the intraocular tumors were determined. Time and dosage experiments were performed by using 50 or 250 μg bevacizumab starting at day 1 or 4 after inoculation. Hepatic micrometastases were enumerated. Proliferation, apoptosis, and angiogenesis markers were detected in the ocular tumor by immunofluorescence staining.
Results.
Bevacizumab significantly reduced the level of VEGF in the culture media from human uveal melanoma cells, mouse melanoma cells, and co-cultured cells. It also inhibited cell tube formation and decreased in vitro invasion of tumor cells. In the mouse model, bevacizumab suppressed primary ocular melanoma growth and the formation of hepatic micrometastases in a dose-dependent manner. Furthermore, immunohistochemical staining showed decreased Ki67 and unchanged caspase 3 expression after treatment with bevacizumab.
Conclusions.
Treatment with bevacizumab suppressed in vitro growth and in vivo hepatic micrometastasis of ocular melanoma cells. Bevacizumab is a potential therapeutic agent for the treatment of uveal melanoma micrometastases.
Brachytherapy, local resection, and enucleation are current treatments for primary uveal melanoma. The goals of treatment of primary uveal melanoma are to prevent further growth of the tumor, preserve vision, achieve a cosmetically acceptable result, and, if possible, reduce the risk of metastasis. Although these therapies have achieved many of these goals, mortality from metastatic disease has remained unchanged for decades.1,2
The formation of new blood vessels (angiogenesis) is a feature of growth and invasion in primary neoplasms and their metastases. Angiogenesis occurs in uveal melanoma and its metastases.3,4 Vascular endothelial growth factor (VEGF) plays a central role in tumor development including potentiating cell proliferation, survival, migration, and angiogenesis.5–7 VEGF is a member of the platelet-derived growth factor family of structurally related mitogens and has four isoforms: VEGF-A, -B, -C, and -D.8,9 VEGF-A is the most important isoform with regard to angiogenesis. VEGF is a homodimeric glycoprotein with a molecular mass of approximately 45 kDa. VEGF-A induces angiogenesis via a direct effect on endothelial cells.9–12 In vitro experiments using microvascular endothelial cells grown on the surface of three-dimensional collagen gels have shown that VEGF induces cellular invasion of the underlying matrix with formation of capillary-like tubules.13
VEGF-mediated angiogenesis is essential for tumor growth. Without an adequate vascular supply, solid tumors are only able to grow to approximately 1- to 2-mm diameter spheres (∼106 cells), primarily because of a lack of oxygen and nutrients.14,15 Increasing concentrations of VEGF in a tumor results in blood vessels that are structurally different from normal, mature blood vessels.16 These disordered, abnormal tumor vessels are critical for intravasation and extravasation of tumor cells during the metastatic process. The vascular endothelium marker CD31 is used to identify the vascular density of the primary tumor and that value correlates with metastasis. Previous studies in mouse models of breast carcinoma and fibrosarcoma have shown that a neutralizing anti-VEGF monoclonal antibody blocks the interaction of VEGF-A with its receptor, VEGF-R1, resulting in a reduction in the size of the primary tumor and the number of micrometastases.17,18
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that contains human framework regions, ∼93% human and 7% murine protein sequence,19 and the complementarity-determining regions of a murine antibody that binds to VEGF (Genentech, San Francisco, CA). Bevacizumab binds to and inactivates all isoforms of VEGF, thus inhibiting angiogenesis, tumor proliferation, and growth.19,20 Since tumor cell lines often overexpress VEGF, bevacizumab's activity reflects a key targeting strategy for cancer therapy.19–22 Recently, bevacizumab has been shown to control metastatic colorectal carcinoma, metastatic breast carcinoma, and non–small cell lung carcinoma.23–26 However, a potential role of bevacizumab therapy in uveal melanoma has not been demonstrated. Uveal melanoma becomes vascularized, with metastasis that is virtually exclusively hematogenous, thus making it a potential target for antiangiogenic therapy. The purpose of this study was to determine whether anti-VEGF therapy inhibits the growth of primary uveal melanoma and its micrometastases.
Materials and Methods
Cells and Treatment
The following cell lines were used: human uveal melanoma Mel 290 and Mel 270 cells (courtesy of Bruce Ksander, Schepens Eye Institute, Boston, MA), mouse melanoma B16LS9 cells (courtesy of Dario Rusciano, Friedrich Miescher Institute, Basel Switzerland); HUVECs and mouse vascular endothelial cells (Lifeline Cell Technology, Walkersville, MD); and human uveal melanocytes (courtesy of Dan-Ning Hu, The New York Eye and Ear Infirmary). The tumor cells were cultured at 37°C in a 5% CO2 incubator in complete culture medium (RPMI-1640 with HEPES and l-glutamine, 10% fetal bovine serum 1% nonessential amino acids, 1% sodium pyruvate solution, 1% MEM vitamin solution [Invitrogen-Gibco, Grand Island, NY], 1% antibiotic–antimycotic solution containing 100 U/mL penicillin G, 250 ng/mL amphotericin B, and 100 μg/mL streptomycin solution [Invitrogen-Gibco]). The culture medium for vascular endothelium was obtained from Lifeline Cell Technology. The culture methods for human uveal melanocytes and generation of Mel290-GFP have been published.27,28
VEGF ELISA Assay
A portion (1 × 105) of Mel 290, Mel 270, B16LS9, HUVECs, and mouse vascular endothelial cells were separately plated in a six-well plate. Human uveal melanoma and mouse melanoma cells were also co-cultured with the HUVECs and mouse vascular endothelial cells, respectively. All cultures were performed in triplicate. When the cells reached 90% confluence at the third day after they were seeded, the media were changed to complete culture media with 10 or 100 μg/mL bevacizumab, or an equal amount of IgG1. The cell culture media were collected at 72 hours after treatment in complete culture medium in 5% CO2 at 37°C. The concentration of VEGF in the supernatants was measured with an ELISA kit (Quantikine; R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
In Vitro Angiogenesis Assay
HUVECs or Mel290-GFP cells were suspended in complete culture medium with 100 μg/mL bevacizumab or an equal amount of IgG1 and were seeded on growth factor-containing gel (ECMatrix; Millipore, Billerica, MA) in a 24-well-plate, incubated in 5% CO2 at 37°C for 10 hours, and inspected for tube formation with an inverted light microscope (Olympus, Tokyo, Japan). The pattern/value association criteria for tube formation are: 0, individual cells, well separated; 1, cells beginning to migrate and align themselves; 2, capillary tubes visible without sprouting; 3, sprouting of new capillary tubes; 4, closed polygons beginning to form; and 5 complex meshlike structures developing. Three random fields per well were examined at 40× magnification, and the values were averaged.29
Invasion Assay
The in vitro invasion assay was performed with a 96-well collagen-based cell-invasion assay kit (Chemicon, Temecula, CA) and read on a spectrophotometer at 480/520 nm (μQuant Spectra MAX 190; Bio-Tek Instruments, Inc. Winooski, VT). After the Mel 290 and B16LS9 cells were treated with 10 or 100 μg/mL bevacizumab or an equal amount of IgG1 for 24 hours, 2 × 105 cells were placed into an invasion chamber consisting of a 96-well, collagen-based plate. The cells were incubated for 24 hours at 37°C in a 5% CO2 incubator. The cells and media were discarded from the top of the insert, and the invasion chamber plate was placed onto the new 96-well feeder tray containing 150 μL of warm cell-detachment solution in the wells and incubated for 30 minutes at 37°C. Dye solution (50 μL) was added to each well of the feeder tray containing the detachment solution and the cells that invaded through the collagen-coated membrane and incubated for 15 minutes at room temperature. The mixture (100 μL) was transferred to a new 96-well plate for measurement of fluorescence.
Animal Experiments
A mouse uveal melanoma model was used as previously described. Ten-week-old female C57BL/6 or NU/NU mice were used (Charles River, Wilmington, MA). All experiments were conducted in accordance with the Guiding Principles in the Care and Use of Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Aliquots of 1 × 106 of Mel290 or 5 × 105 cells of B16LS9/2.5 μL were inoculated into the choroid of the right murine eye with a transscleral technique that allows the inoculated cells to remain in the eye. The mice were anesthetized, and a tunnel was prepared from the limbus within the sclera to the choroid with a 30 ½-gauge needle under the guidance of a dissection microscope. The tip of a 10-μL glass syringe with a blunt metal needle (Hamilton, Reno, NV) was introduced into the choroid through the needle track; no cells were inoculated until the needle tip was inside the eye. A 2.5-μL suspension of cells was inoculated. No tumor cell reflux occurred, and the subconjunctival space remained free of tumor cells. The inoculated eyes were enucleated after 1 week. Livers were collected 4 weeks after inoculation.30 Bevacizumab (50 or 250 μg/100 μL) was administered by intraperitoneal (IP) injection starting at day 1 or 4 after inoculation, and the dose was repeated twice per week for 4 or 6 weeks. Mice in a dosage experiment were divided into three groups, with the groups receiving 50 or 250 μg IP bevacizumab or an equal volume of PBS starting on day 4. Mice in a timing experiment were divided into four groups, with two groups separately receiving 250 μg IP bevacizumab starting at day 1 or 4, and the other two groups receiving an equal volume of PBS starting at day 1 or 4. In a longitudinal experiment, the mice were divided into 10 groups, with half of them receiving 250 μg IP bevacizumab twice per week and half receiving PBS. All were killed, with hepatic tissue collection at 1, 2, 3, 4, or 6 weeks after the intraocular inoculation of melanoma cells. There were 10 mice in each group. Nine eyes and all livers were fixed in 10% formalin and embedded in paraffin. The eyes were cut into 7-μm serial sections and stained with hematoxylin/eosin. Ten sections with the largest tumor area in each eye were photographed at 40× magnification (DP10; Olympus). The sizes of the intraocular tumors were determined with Image J software (Image Processing Analysis in Java, ver. 1.42; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Hepatic micrometastases were microscopically determined in the three central sections of the liver and enumerated as previously described.30 The animal experiments are outlined in Table 1.
Table 1.
Outline of Animal Experiments
| Experiment/Groups | Day 1 Ocular Inoculation | Day 4* Intraperitoneal Injection | Day 8 Enucleation | Day 29† Necropsy |
|---|---|---|---|---|
| Dosage experiment | ||||
| Group 1 | BI6LS9 | Bevacizumab 250 μg/100 μL | Yes | Yes |
| Group 2 | BI6LS9 | Bevacizumab 50 μg/100 μL | Yes | Yes |
| Group 3 | BI6LS9 | PBS 100 μL | Yes | Yes |
| Timing experiment | ||||
| Group 1 | Mel 290 | Bevacizumab 250 μg/100 μL (day 1) | Yes | Yes |
| Group 2 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes |
| Group 3 | Mel 290 | PBS 100 μL (day 1) | Yes | Yes |
| Group 4 | Mel 290 | PBS 100 μL | Yes | Yes |
| Longitudinal experiment‡ | ||||
| Group 1 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes (day 8) |
| Group 2 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes (day 15) |
| Group 3 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes (day 21) |
| Group 4 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes |
| Group 5 | Mel 290 | Bevacizumab 250 μg/100 μL | Yes | Yes (day 43) |
n = 10 mice in each group.
Intraperitoneal injection was performed on day 4 unless otherwise indicated.
Hepatic tissue was obtained on day 29 unless otherwise indicated.
All treated groups in this experiment had a PBS control group.
Immunofluorescence Staining
Frozen sections of one enucleated mouse eye from each group were obtained from the in vivo experiments. Immunofluorescence staining for intratumoral vessels with anti-CD31 conjugated with FITC (BD-PharMingen, San Jose, CA), cellular proliferation with anti-Ki67 (Novocastra Laboratories, Newcastle, UK), and apoptosis with anti-caspase 3 (Abcam, Cambridge, MA), was followed by incubation with a secondary antibody, counterstaining with propidium iodide (Vector Laboratories, Burlingame, CA), mounting with mounting medium, and coverslipping. In the in vitro experiment, after treatment of Mel 290 cells with 100 μg/mL bevacizumab, IgG1 (negative treatment control), or staurosporine (positive treatment control) for 48 hours, Mel 290 cells were stained with anti-Ki67 or anti-caspase 3. In the in vivo experiment, after treatment with bevacizumab or PBS (negative treatment control), tumors in the enucleated eyes were stained with anti-Ki67 or anti-caspase 3. The quantitative assay for proliferation and apoptosis was performed with an image-analysis program (Image-Pro Plus 6; Media Cybernetics, Bethesda, MD). The software automatically counted the stained nuclei. The mean vascular density was determined by measuring the total vessel length or counting the number of CD31-positive capillaries per high-power field using 40× magnification and the image analysis program.
Results
VEGF in Human Uveal Melanoma and Mouse Melanoma Cell Lines
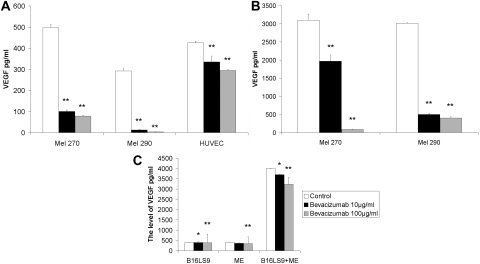
Since uveal melanoma becomes vascularized in the eye and its metastases become vascularized in the liver, we studied VEGF expression and suppression with bevacizumab in melanoma and vascular endothelial cells lines cultured separately and co-cultured. Both 10 and 100 μg/mL bevacizumab decreased the level of VEGF secreted by human uveal melanoma cell lines and HUVECs, compared with IgG1 control cells. There were significant differences in the VEGF levels between the 10 or 100 μg/mL bevacizumab and IgG1 control cells, and between the 10 and 100 μg/mL bevacizumab treatment groups in both uveal melanoma cell lines and HUVECs (t-test, P < 0.01). Treatment with 100 μg/mL bevacizumab caused a more pronounced decrease in VEGF than treatment with 10 μg/mL bevacizumab (Fig. 1A). Analysis of ELISA results showed that bevacizumab decreased VEGF 84.30%, 95.52%, and 30.91% in the culture media of Mel270 and Mel290 cells and HUVECs, respectively. Mel290 was the most sensitive cell line to bevacizumab. The levels of VEGF were significantly increased when tumor cells were co-cultured with vascular endothelium. The levels of VEGF in co-culture media were 6.22 and 10.39 times greater than those in medium from tumor cells only, for Mel270 and Mel290, respectively. VEGF was reduced in Mel270 and Mel290 to 1128.81 and 2512.66 pg/mL, respectively, after treatment with 10 μg/mL bevacizumab and 3008.76 and 2611.66 pg/mL after treatment with 100 μg/mL bevacizumab in Mel270 and Mel290 cells co-cultured with HUVECs. There were significant differences in the VEGF levels between the 10 and 100 μg/mL bevacizumab treatment groups and control cells and between the 10 and 100 μg/mL bevacizumab treatment groups for both uveal melanoma cell line co-cultured with HUVECs (t-test, P < 0.01, Fig. 1B). A high concentration of bevacizumab was more effective than a low concentration on reducing VEGF in tumor cells and HUVECs. VEGF secretion was slightly suppressed with 10 μg/mL bevacizumab (P = 0.0046) and greatly reduced with 100 μg/mL (P = 0.0091) in B16LS9 cells, compared with the IgG1 control treatment. Treatment with 10 and 100 μg/mL bevacizumab resulted in 8.51 and 15.99 pg/mL of VEGF, respectively. The VEGF level of 3591.57 pg/mL of VEGF was increased in the medium of the B16LS9 cells co-cultured with vascular endothelium and was 9.75 times greater than medium of B16LS9 cells cultured alone. Treatment with 10 or 100 μg/mL bevacizumab resulted in 7.44% and 19.18% decreases, respectively, in VEGF in the co-cultured medium (P < 0.05 and P < 0.01, Fig. 1C).
Figure 1.
VEGF expression after in vitro treatment with bevacizumab. (A) Human uveal melanoma cell lines and HUVECs expressed from 150 to 500 pg/mL VEGF, with a significant decrease after treatment with 10 or 100 μg/mL bevacizumab. (B) Human uveal melanoma cells co-cultured with HUVECs expressed higher levels of VEGF (1750–3000 pg/mL) than did individually cultured cells, which significantly decreased after treatment with 10 or 100 μg/mL bevacizumab. (C) Mouse B16LS9 melanoma cells expressed VEGF, which was significantly decreased after treatment with 10 or 100 μg/mL bevacizumab. Mouse B16LS9 melanoma cells co-cultured with mouse vascular endothelium expressed higher levels of VEGF did individually cultured cells, with a significant decrease in VEGF expression after treatment with 10 or 100 μg/mL bevacizumab.
Bevacizumab Suppression of Angiogenesis In Vitro
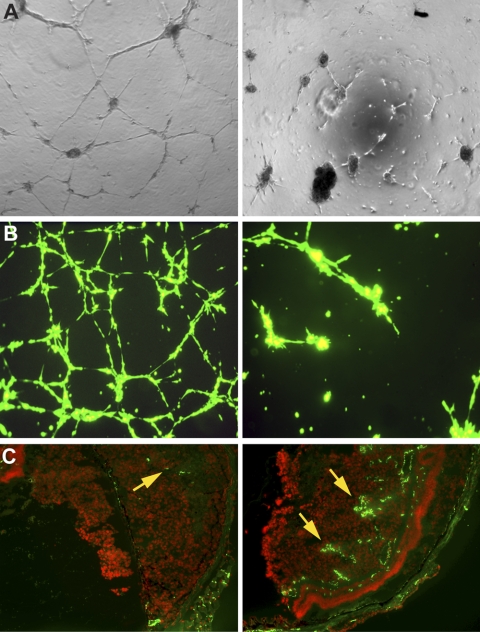
HUVECs developed complex meshlike structure patterns (grade 5) when grown in a growth-factor gel matrix (ECMatrix; Millipore; Fig. 2A, left). After treatment with 10 μg/mL bevacizumab, the HUVECs formed new sprouting capillary tube patterns (grade 3) and after treatment with 100 μg/mL bevacizumab, the cells showed a migration/alignment pattern (grade 1; Fig. 2A, right). The average total capillary tube length in HUVECs with IgG1, 10 or 100 μg/mL bevacizumab, respectively, was 2076 ± 183, 888.9 ± 77, and 633 ± 28 μm. When Mel290-GFP and HUVECs were plated on the gel, fluorescent, complex, meshlike structure (grade 5) patterns formed. After treatment with 100 μg/mL bevacizumab, the cells formed sprouting new capillary patterns (grade 3; Fig. 2B). The average total capillary tube lengths in HUVECs treated with IgG1 or 100 μg/mL bevacizumab, were 5097.26 ± 386.52 and 1182.67 ± 70.61 μm, respectively (P < 0.01).
Figure 2.
Bevacizumab decreased angiogenesis in vitro and in vivo. Bevacizumab decreased tube formation: IgG1-treated HUVECs (A, left) and Mel290-GFP co-cultured with HUVECs (B, left) exhibited meshlike patterns of tube formation (grade 5). Corresponding cells treated with bevacizumab showed a migration/alignment pattern (grade 1) in HUVECs (A, right) and sprouting new capillary tubes (grade 3) in the melanoma-GFP co-cultured cells (B, right). Bevacizumab decreased the vascular density in our mouse model of ocular melanoma (C). CD31+ vascular channels constitute 1% of the area of the tumor in mice treated with bevacizumab 250 μg/mL (C, left, arrow), compared with 26% of the area of the tumor in control mice treated with PBS (C, right, arrows).
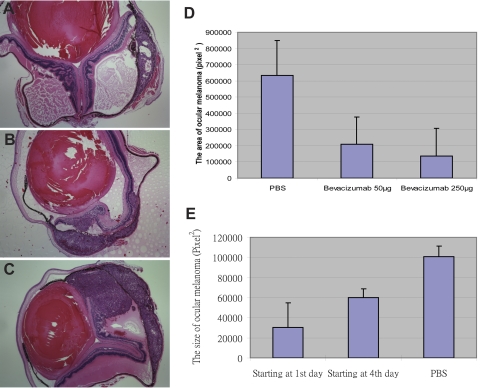
Effect of Bevacizumab on Tumor Growth
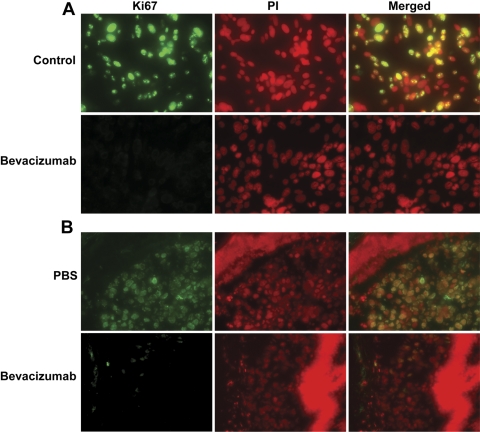
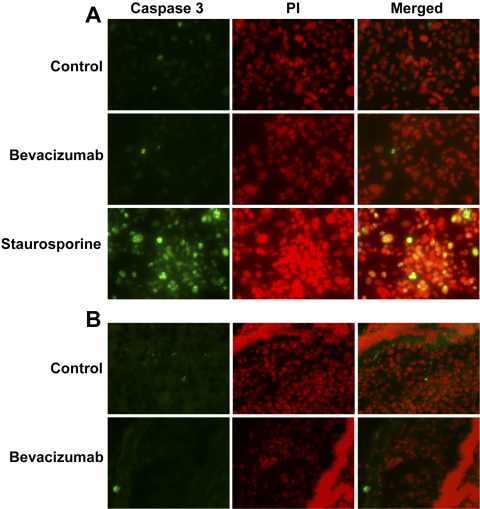
Systemic treatment with bevacizumab reduced the size of the intraocular tumor in our mouse model (Fig. 3). In the dosage experiment, the size of the intraocular melanoma in the mice treated with 250 μg bevacizumab was smaller than that in the mice treated with 50 μg, with significant differences between either group and the PBS-treated control mice (P < 0.01) and between the 250- and 50-μg treated groups (P < 0.01, Figs. 3A 3B). In the timing experiment, the size of the intraocular tumor was smaller in the group where treatment had started on day 1 versus the group that was started on treatment on day 4 after intraocular inoculation of melanoma cells (Fig. 3C). Both in vitro and in vivo treatment with bevacizumab led to decreased nuclear expression of Ki67 (Fig. 4). Image analysis (Image-Pro Plus 6; Media Cybernetics) showed that in the in vitro experiment, 53% and 0% of nuclei stained for Ki67 after treatment with IgG1 and bevacizumab, respectively. In the in vivo experiment, 98% and 11% of nuclei stained for Ki67 in the PBS control and bevacizumab treatment groups, respectively. Bevacizumab did not change the expression of the apoptotic enzyme caspase 3 in uveal melanoma cells in vitro or in vivo (Fig. 5). Image analysis of the in vitro experiment showed that 6%, 4%, and 74% of nuclei stained for caspase 3 in the IgG1, bevacizumab, and staurosporine groups, respectively. In the in vivo experiment, there was no staining for caspase 3 in the tumors in both the PBS control and bevacizumab-treated groups.
Figure 3.
Bevacizumab inhibited intraocular melanoma growth. Groups with IP injections of (A) 250 or (B) 50 μg bevacizumab starting at day 4 after inoculation had smaller intraocular melanomas than did the PBS-treated control group (C). The 50- and 250-μg IP bevacizumab groups, both starting on day 4 after inoculation, exhibited decreased intraocular tumor size compared with that in the PBS-treated control (D). The size of the intraocular melanoma was significantly smaller in the day-1 and -4 groups compared with the control, and the tumors in the day-1 groups were significantly smaller than those in the day-4 groups (E). Each group contained 10 mice.
Figure 4.
Bevacizumab decreased Ki67 in human uveal melanoma and mouse melanoma cells. Melanoma cells grown in vitro and treated with 100 μg/mL bevacizumab for 48 hours showed inhibition of nuclear Ki67. (A) Top: IgG1-treated Mel290 cells showed 53% of nuclei stained. Bottom: bevacizumab-treated Mel 290 cells showed no stained nuclei. (B) Top: PBS-treated B16LS9 tumor showed 98% of nuclei stained. Bottom: bevacizumab-treated B16LS9 tumor showed 11% of nuclei stained.
Figure 5.
Bevacizumab did not cause apoptosis in melanoma cells. (A) Treatment of Mel 290 cells with IgG1 (top, 6% nuclear staining) or bevacizumab (middle, 4% nuclear staining) did not result in increased expression of caspase 3, compared with staurosporine-treated positive control cells (bottom, 74% nuclear staining). (B) Similarly, intraocular melanoma in the PBS control (top) and the bevacizumab-treated mice (bottom) both showed a lack of expression of caspase 3, with no nuclear staining detected in either group.
Effect of Bevacizumab on Angiogenesis In Vivo
In our mouse model, the intraocular melanomas in mice treated with 50 or 250 μg IP bevacizumab showed a decreased CD31-vascular density compared with those in the control animals (12%, 1%, and 26%, respectively; Fig. 2C).
Effect of Bevacizumab on Micrometastases
The in vitro invasion assay showed significantly less invasion of both Mel 290 cells and B16LS9 cells treated with bevacizumab, with 100 μg/mL bevacizumab being more effective than 10 μg/mL. Invasion of Mel290 cells decreased by 56% and 91% optical density (OD) units after treatment with 10 and 100 μg/mL bevacizumab (0.1063 ± 0.0142 and 0.0877 ± 0.0053), respectively, compared with IgG treatment (0.2399 ± 0.0184, P < 0.001). In B16LS9 cells compared with the IgG control (0.3604 ± 0.0198), 10 and 100 μg/mL bevacizumab reduced invasion by 15% and 27% (0.3064 ± 0.0166, 0.2626 ± 0.0112, P < 0.01), respectively. The human uveal melanoma cell line Mel290 was much more sensitive to bevacizumab than was the mouse melanoma cell line B16LS9. The number of hepatic micrometastases in the control group and the 50-μg and 250-μg bevacizumab groups were 194 ± 86, 108 ± 27, and 63 ± 23, respectively. There were significantly fewer micrometastases in our mouse ocular melanoma model after treatment with either 50 or 250 μg bevacizumab, (P = 0.03 and P = 0.01, respectively), compared with the number in the control group. There was a significant decrease in the number of micrometastases in the 250-μg/mL– compared with the 50-μg/mL–treated groups (P = 0.002). In the longitudinal experiment, treatment with 250 μg bevacizumab resulted in control of the number of micrometastases at all intervals (1, 2, 3, 4, and 6 weeks) after inoculation. The treatment effect continued longitudinally, with significantly fewer micrometastases in mice treated with 250 μg/mL bevacizumab than in the control animals at 1, 2, 3, 4, and 6 weeks after inoculation.
Discussion
VEGF is overexpressed in many human neoplasms, including uveal melanoma.6,31,32 This expression is associated with tumor size, necrosis and intraocular angiogenesis.6,31,32 New blood vessels that grow within the tumor secondary to VEGF expression are structurally and functionally irregular, as they exhibit dead ends, disordered blood flow, and increased permeability. These irregularities in blood flow lead to further tumor hypoxia and subsequent increases in VEGF production.33 Our study confirms previous studies showing that uveal melanoma cells overexpress VEGF mRNA.3,4
Angiogenesis is a critical process in tumor growth and development. VEGF is a specific stimulator of vascular endothelial cell proliferation and tumor angiogenesis. VEGF is produced in response to various cellular and environmental stimuli. We found that human uveal melanoma cells and human vascular endothelial cells spontaneously secrete VEGF when placed in culture. When melanoma cells were cocultured with vascular endothelium, VEGF production significantly increased 5- to 10-fold. The results of our in vitro angiogenesis assay showed that the total length of tubes formed from uveal melanoma cells co-cultured with vascular endothelium was two times longer than when using vascular endothelium alone. High serum levels of VEGF may be a marker in metastatic disease.34,35 Melanoma cell lines exhibit variable sensitivities to anti-VEGF antibodies (i.e., bevacizumab), with Mel 290 being the most sensitive cell line we tested.
VEGF exhibits trophic effects on the growth and progression of neoplasia. Several studies have shown a correlation between increased VEGF expression and tumor growth.19,20,36,37 Recent studies have indicated that bevacizumab treatment results in a 25% to 95% dose-dependent inhibition of tumor growth in mouse and rat models.19,38 In our study, bevacizumab gave a dose-dependent reduction of the size of the murine intraocular melanoma. Our study also showed that this was not due to a primary effect on melanoma cells, supporting the results of a prior study.39 Our results showed that bevacizumab caused decreased proliferation of vascular endothelium in vivo. We found that bevacizumab had no effect on inducing apoptosis in the melanoma cells and that the reduction in tumor size was probably due to reduced intratumoral endothelial cell proliferation and angiogenesis, leading to a shrinking effect on tumor volume. The in vivo timing experiment in this study identified that the early use of bevacizumab was more effective than later use in controlling ocular tumor growth. Our study suggests that if anti-VEGF treatment is initiated concomitantly with inoculation of tumor cells, there is a greater than 95% inhibition of tumor growth and a significant decrease in micrometastasis. This conclusion suggests that bevacizumab interferes with the establishment of individual or groups of melanoma cells to become established in the eye and decreases the ability of circulating melanoma cells to become adherent and established in hepatic sinusoids. However, if the tumor reaches a critical size before initiation of treatment, the inhibition decreases, indicating that the hepatic micrometastases have already become established. A previous study has shown that VEGF expression is associated with areas of necrosis in the primary intraocular melanoma and does not correlate with metastasis.31 That study did not address the possibility that VEGF is expressed by the micrometastatic melanoma and may correlate with metastasis.31 Our laboratory has recently found a correlation between VEGF expression by the micrometastatic melanoma, and the number and location of the micrometastasis (Crosby MB, et al., unpublished data, 2009). We were unable to test the effect of bevacizumab in primary tumors that were fully established since according to our protocol, the eyes were enucleated at 7 days after inoculation.
Angiogenesis is eventually necessary for growth and invasion of primary neoplasms and plays an important role in the establishment of metastases. VEGF has been shown to facilitate survival of existing blood vessels, to contribute to vascular abnormalities that may inhibit effective delivery of antitumor compounds, and to stimulate new blood vessel and lymphatic growth. Expression of VEGF also correlates with invasiveness of many types of cancer cells, vascular density in different tumors, appearance of metastasis, tumor recurrence, and poor prognosis. The vascular endothelium marker CD31 is used to determine the vascular density of the primary tumor and that value correlates with metastasis. In our study, we have found that the level of VEGF was increased many fold when melanoma cells were co-cultured with vascular endothelium. In addition, melanoma cells co-cultured with vascular endothelium formed 2.5 times longer tubes than did vascular endothelium cultured separately. Bevacizumab reduced the length of the tubes formed from the uveal melanoma cells co-cultured with vascular endothelium in vitro and in vivo and bevacizumab decreased CD31 expression in the ocular melanoma. We are unable to ascertain whether the increased VEGF was from the melanoma cells, vascular endothelium, or both. Our results do indicate that when growth of both melanoma and vascular endothelium occur together, there is increased VEGF production and that when this occurs, bevacizumab decreases the level of VEGF.
Several experimental studies have examined the extent to which VEGF inhibitors prevent tumor cell growth at metastatic sites.19,40,41 Warren et al.42 showed that administration of an anti-VEGF antibody in a colorectal cancer xenograft model caused a 90% reduction in the mass of the primary tumor and a 10- to 18-fold reduction in the number of liver metastases compared with the control. In addition, they did not find the presence of blood vessels or expression of VEFR-2 in the liver metastases of the mice treated with the anti-VEGF antibody. A similar reduction in the number and size of tumor lung metastases was observed after anti-VEGF antibody treatment 3 days after implantation of human prostate cancer cells in mice. When treatment was delayed until the primary tumors were well established, primary tumor growth and metastases were still inhibited by the anti-VEGF antibody. Our experiments showed similar results, with decreased size of primary intraocular melanoma and a decreased number of hepatic micrometastases after treatment with bevacizumab, although the greatest effect occurred with early administration of the bevacizumab. This result indicates that early treatment with bevacizumab is important for preventing the establishment of micrometastases.
In summary, our experimental findings show that VEGF inhibition causes the reduction of ocular melanoma proliferation, angiogenesis, and invasion, resulting in suppression of establishment of micrometastases. Anti-VEGF therapy is a potential strategy for the treatment of metastatic uveal melanoma.
Footnotes
Supported by National Institutes of Health, National Cancer Institute Grant R01 CA126447 and National Eye Institute Grant R24EY017045. HEG is a recipient of a Research to Prevent Blindness Senior Scientific Investigator Award.
Disclosure: H. Yang, None; M.J. Jager, None; H.E. Grossniklaus, None
References
- 1.Economou MA. Introduction: uveal melanoma. Acta Ophthalmol 2008;83:7–19 [DOI] [PubMed] [Google Scholar]
- 2.Pyrhonen S. The treatment of metastatic uveal melanoma. Eur J Cancer 1998;34(suppl 3):27–30 [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Xu Z, Iuvone PM, Grossniklaus HE. Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model. Mol Vis 2006;12:511–517 [PubMed] [Google Scholar]
- 4.Yang H, Akor C, Dithmar S, Grossniklaus HE. Low dose adjuvant angiostatin decreases hepatic micrometastasis in murine ocular melanoma model. Mol Vis 2004;10:987–995 [PubMed] [Google Scholar]
- 5.Brychtova S, Bezdekova M, Brychta T, et al. The role of vascular endothelial growth factors and their receptors in malignant melanomas. Neoplasia 2008;55:273–279 [PubMed] [Google Scholar]
- 6.Missotten G, Notting I, Schlingemann R, et al. Vascular endothelial growth factor A eyes with uveal melanoma. Arch Ophthalmol 2006;124:1428–1434 [DOI] [PubMed] [Google Scholar]
- 7.Stitt A, Simpson D, Boocock C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumors. J Pathol 1998;186:306–312 [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25 [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69(suppl 3):4–10 [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001;280:C1358–C366 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676 [DOI] [PubMed] [Google Scholar]
- 12.Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994;269:26988–26995 [PubMed] [Google Scholar]
- 13.Pepper MS, Ferrara N, Orci L, et al. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 1992;189:824–831 [DOI] [PubMed] [Google Scholar]
- 14.Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999;5:1041–1056 [PubMed] [Google Scholar]
- 15.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003;3:401–410 [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001;7:987–989 [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Ran S, Sambade M, et al. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/ Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis 2002;5:35–44 [DOI] [PubMed] [Google Scholar]
- 18.Brekken R, Overholser J, Stastny V, et al. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-2) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res 2000;60:5117–5124 [PubMed] [Google Scholar]
- 19.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671–680 [PubMed] [Google Scholar]
- 20.Zondor SD, Medina PJ. Bevacizumab: An angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother 2004;38:1258–1264 [DOI] [PubMed] [Google Scholar]
- 21.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–1027 [DOI] [PubMed] [Google Scholar]
- 22.Rosen IS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control 2002;9(suppl 2):36–44 [DOI] [PubMed] [Google Scholar]
- 23.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563–572 [DOI] [PubMed] [Google Scholar]
- 24.Patel DK. Clinical use of anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer. Pharmacotherapy 2008;28:31–41 [DOI] [PubMed] [Google Scholar]
- 25.Amselem L, Cervera E, Dßaz-Llopis M, et al. Intravitreal bevacizumab (Avastin) for choroidal metastasis secondary to breast carcinoma: short-term follow-up. Eye 2007;21:566–567 [DOI] [PubMed] [Google Scholar]
- 26.de Gramont A, Van Cutsem E. Investigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology 2005;69(suppl 3):46–56 [DOI] [PubMed] [Google Scholar]
- 27.Hu DH, McCormick SA, Ritch R. Studies of human uveal melanocytes in vitro: growth regulation of cultured human uveal melanocytes. Invest Ophthalmol Vis Sci 1993;34:2220–2227 [PubMed] [Google Scholar]
- 28.Yang H, Fang G, Huang X, Yu J, Hsieh CL, Grossniklaus HE. In-vivo xenograft murine human uveal melanoma model develops hepatic micrometastases. Melanoma Res 2008;18:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Grossniklaus HE. Constitutive overexpression of pigment epithelium-derived factor (PEDF) inhibits ocular melanoma growth and metastasis Invest Ophthalmol Vis Sci 2010;51:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dithmar S, Rusciano D, Grossniklaus HE. A new technique for implantation of tissue culture melanoma cells in a murine model of metastatic ocular melanoma. Melanoma Res 2000;10:2–8 [PubMed] [Google Scholar]
- 31.Sheidow TG, Hooper PL, Crukley C, et al. Expression of vascular endothelial growth factor in uveal melanoma and its correlation with metastasis. Br J Ophthalmol 2000;84:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol 2002;86:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–845 [DOI] [PubMed] [Google Scholar]
- 34.Alabi AA, Suppiah A, Madden LA, Monson JR, Greenman J. Preoperative serum vascular endothelial growth factor-a is a marker for subsequent recurrence in colorectal cancer patients. Dis Colon Rectum 2009;52:993–999 [DOI] [PubMed] [Google Scholar]
- 35.Alabi AA, Suppiah A, Madden LA, Monson JR, Greenman J. Preoperative serum levels of serum VEGF-C is associated with distant metastasis in colorectal cancer patients. Int J Colorectal Dis 2009;24:269–274 [DOI] [PubMed] [Google Scholar]
- 36.Zhang HT, Craft P, Scott PA, et al. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst 1995;87:213–219 [DOI] [PubMed] [Google Scholar]
- 37.Claffey KP, Robinson GS. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev 1996;15:165–176 [DOI] [PubMed] [Google Scholar]
- 38.Verheul HM, Hammers H, van Erp K, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res 2007;13:4201–4208 [DOI] [PubMed] [Google Scholar]
- 39.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993;362:841–844 [DOI] [PubMed] [Google Scholar]
- 40.Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res 2000;60:6253–6258 [PubMed] [Google Scholar]
- 41.Melnyk O, Zimmerman M, Kim KJ, Shuman M. Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol 1999;161:960–963 [PubMed] [Google Scholar]
- 42.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995;95:1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]