Abstract
In this study, composite scaffolds consisting of both synthetic and natural components with controllable properties were generated by incorporating mineralized extracellular matrix (ECM) and electrospun poly(ε-caprolactone) (PCL) microfiber scaffolds. Mesenchymal stem cells (MSCs) were cultured on PCL scaffolds under flow perfusion conditions with culture medium supplemented with dexamethasone to investigate the effect of culture duration on mineralized extracellular matrix deposition. MSCs differentiated down the osteogenic lineage and produced extracellular matrix with different compositions of mineral, collagen, and glycosaminoglycan with distinct morphologies at various stages of osteogenesis. To determine whether the presence and maturity of mineralized extracellular matrix influences osteogenic differentiation in vitro, PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds that were subsequently seeded with MSCs and cultured in the absence of dexamethasone. The presence of mineralized matrix reduced cellular proliferation while stimulating alkaline phosphatase activity with increasing amounts of calcium deposition over time. PCL/ECM composite scaffolds containing the most mature mineralized matrix resulted in the most rapid increase and highest levels of alkaline phosphatase activity and calcium deposition compared to all other scaffold groups. Therefore, we demonstrate that mineralized extracellular matrix generated under controlled flow perfusion conditions can impart osteogenic properties to an osteoconductive polymer scaffold, and that the maturity of this matrix influences osteogenic differentiation in vitro, even in the absence of dexamethasone.
Keywords: electrospun polymer scaffold, bone tissue engineering, mesenchymal stem cell, cell-matrix signals, microenvironment
Introduction
Bone has an innate ability to heal due to its vasculature and access to stem and progenitor cell populations. Although this innate healing response may repair bone fractures, large defects often require the aid of some scaffolding material to bridge the void space and facilitate bone regeneration. Currently, the most successful treatment for bone defects is autologous bone graft, which integrates well with the surrounding bone tissue and can be remodeled to restore structure and function. The success of autologous bone graft as a scaffold for bone regeneration is due to its osteoconductivity and osteoinductivity, as it not only supports stem and progenitor cell attachment, but also stimulates their osteogenic differentiation and bone formation. However, since autologous bone graft is harvested from healthy donor sites, drawbacks associated with its use include donor site morbidity and limited tissue availability [1]. Therefore, the need for alternative scaffolding materials with both osteoconductive and osteoinductive properties has launched the development of diverse biomaterials for bone regeneration applications.
An ideal scaffold to facilitate bone regeneration should be biocompatible, provide structural support to the repair region, allow cell attachment and infiltration, induce osteogenic differentiation of stem and progenitor cells, stimulate bone formation, and be degradable over time, ultimately leaving bone tissue with native structure and function. The three main classes of scaffolding materials that have been investigated for bone regeneration include metals, ceramics, and polymers [2]. Of these materials, metals do not degrade (with very few exceptions) and most ceramics are quite brittle. Polymer scaffolds on the other hand, can be synthesized with a wide variety of chemical and physical properties through tailored processing conditions. In particular, electrospun polymer scaffolds with a nonwoven fiber mesh structure are promising candidates for bone regeneration applications due to their large surface-to-volume ratio for cell attachment and high interconnected porosity for cell and tissue infiltration. Here, we explore the application of electrospun poly(ε-caprolactone) (PCL) microfiber scaffolds for bone regeneration, since PCL is a clinically applicable material regulated by the Food and Drug Administration that is both biocompatible and biodegradable. Electrospun PCL nanofiber scaffolds have been shown to support osteogenesis when seeded with mesenchymal stem cells (MSCs) and cultured in osteogenic cell culture medium containing dexamethasone [3]. As with many other porous scaffolds, electrospun PCL scaffolds are only osteoconductive, as they lack osteoinductive properties to stimulate osteogenesis on their own, and thus require the presence of exogenous induction agents such as dexamethasone or growth factors.
Drawing from the success of bone matrix, whose osteoinductivity is attributed to the presence and association of native organic and inorganic components, we seek to impart osteogenic properties to electrospun PCL microfiber scaffolds by incorporating mineralized extracellular matrix generated by differentiating bone marrow derived MSCs under engineered conditions in vitro. Previously, we have successfully differentiated MSCs down the osteogenic lineage and demonstrated the deposition of bone-like extracellular matrix (ECM) on titanium (Ti) fiber mesh scaffolds in a flow perfusion bioreactor system [4, 5]. After decellularization, Ti/ECM composite scaffolds were shown to support osteogenic differentiation with enhanced calcium deposition [6, 7]. Although these studies with titanium scaffolds demonstrate osteogenic differentiation in vitro with either the application of fluid shear stresses or the delivery of dexamethasone, and have also shown promising results in vivo when implanted with cells, titanium is not degradable and will remain in the defect even after bone has regenerated [8, 9].
Ultimately, we envision creating a biodegradable osteoinductive scaffold that, when implanted, would recruit infiltrating host cells and induce their osteogenic differentiation and bone formation, either as a stand alone bone scaffold or as a vehicle for cell transplantation. Since MSCs are self-renewing multipotent stem cells that can be easily isolated from bone marrow, we stimulate their differentiation down the osteogenic lineage under flow perfusion culture conditions to where they deposit increasing amounts of mineralized extracellular matrix on electrospun PCL microfiber scaffolds. In this study, we capture the state of mineralized matrix at various stages of osteogenesis in generating PCL/ECM (PE) composite scaffolds of various maturities, in order to evaluate how the presence and maturity of mineralized matrix influences the osteogenic differentiation of MSCs in vitro without the osteogenic cell culture supplement dexamethasone. For the fabrication of PCL/ECM composite scaffolds, we hypothesized that exposing MSCs to dexamethasone and fluid shear stresses for various culture durations would stimulate the deposition of ECM containing various quantities of minerals and signaling molecules. To evaluate the osteogenic properties of PCL/ECM composite scaffolds, we hypothesized that the presence of mineralized matrix would induce MSC differentiation down the osteogenic lineage even without the addition of dexamethasone, by providing cells with a more biological microenvironment compared to plain PCL scaffolds. Furthermore, we hypothesized that the maturity of this mineralized matrix would modulate osteogenic differentiation through physical interaction with various compositions of matrix signals. To investigate our hypotheses, rat MSCs were seeded on electrospun PCL microfiber scaffolds and cultured in medium containing dexamethasone in the flow perfusion bioreactor to characterize the effect of culture duration on mineralized matrix composition and morphology. Resulting PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds, which along with plain PCL scaffolds, were seeded with rat MSCs and cultured in medium without dexamethasone to determine how mineralized matrix maturity influences osteogenic differentiation in vitro as assessed through cellular proliferation, alkaline phosphatase activity, and calcium deposition.
Materials and Methods
Electrospinning
Nonwoven PCL microfiber mats were fabricated using a horizontal electrospinning setup previously described, consisting of a 10 mL syringe fitted with a blunt tip needle and set on a syringe pump, an 18 gauge copper ring 19 cm in diameter placed 6 cm in front of the needle tip, a power supply with the positive lead split and connected to both the needle and copper ring, and a 0.3 cm thick grounded copper plate covered with a glass collector plate [10]. Mats were electrospun to a targeted fiber diameter of 10 µm using PCL (Sigma-Aldrich, St. Louis, MO) with Mn = 73,000 ± 9,000 and Mw = 154,000 ± 26,000 from three samples relative to polystyrene as determined by gel permeation chromatography (Waters, Milford, MA) using a Phenogel 50 mm column (Phenomenex, Torrance, CA). Polymer was dissolved at 14 wt % in a solution with 5:1 volume ratio of chloroform to methanol. The polymer solution was pumped through a 16 gauge blunt tip needle at a flow rate of 18 mL/h while charged with an applied voltage of 25.5 kV. The copper ring served to stabilize the electric field as the charged polymer jet whipped through the air toward the grounded copper plate positioned 33 cm away from the needle tip. The resulting PCL mat was then removed from the glass collector plate and dried in a desiccator. Prior to use, mats were inspected through scanning electron microscopy to visualize microfiber morphology and to confirm the average fiber diameter.
Scaffold preparation
PCL scaffolds were die-punched from electrospun mats into 8 mm diameter disks with thicknesses between 0.95 and 1.05 mm. As previously characterized through mercury porosimetry, these scaffolds have a porosity of 87% with an average pore size of 45 µm [10]. PCL scaffolds were prepared for cell culture by first sterilizing with ethylene oxide gas for 14 h, then aerating overnight to remove residual fumes. Scaffolds were then pre-wetted through a gradient series of ethanol from 100% to 70%, followed by three rinses in phosphate buffered saline (PBS), and incubated in cell culture medium overnight. To ensure complete wetting in each solution, scaffolds were centrifuged at each step of the pre-wetting process. Finally, PCL scaffolds were press-fitted into cassettes designed to confine the cell suspension during seeding and to be used in the flow perfusion bioreactor to generate PCL/ECM composite scaffolds [11]. Cassettes holding the press-fitted scaffolds were placed in 6-well plates in preparation for seeding.
Mesenchymal stem cell isolation and seeding
MSCs were harvested and pooled from the tibiae and femora of male Fischer 344 rats (Charles River, Wilmington, MA) weighing 150–175 g according to previously established methods [5, 12]. Rats were anesthetized with 4% isoflurane in oxygen then euthanized by carbon dioxide asphyxiation. The tibiae and femora were excised, cleared of soft tissue, then cut and flushed using an 18 gauge needle with 5 mL of complete osteogenic medium consisting of α-MEM, supplemented with 10% fetal bovine serum (Cambrex, Walkersville, MD), 10−8 M dexamethasone, 10 mM β-glycerophosphate, and 50 mg/L ascorbic acid, also with the addition of 1.25 mg/L amphotericin-B, 50 mg/L gentamicin, and 100 mg/L ampicillin. Marrow pellets were triturated and plated in tissue culture flasks. Non-adherent cells were washed away after 24 h, and adherent cells were cultured for 7 days in complete osteogenic medium with medium changes every 2 days. After this primary culture period, MSCs were lifted with 2 mL of a 0.25% trypsin solution and suspended in culture medium for seeding onto press-fitted scaffolds at a seeding density of 250,000 cells in 200 µL of medium within each cassette. Scaffolds were incubated with the seeding solution for 2 h, after which 10 mL of medium was added to each well of the 6-well plates to fill the cassettes in which the scaffolds were held.
PCL/ECM composite scaffold generation
PCL/ECM constructs containing mineralized extracellular matrix of various maturities were generated by culturing MSCs on electrospun PCL scaffolds for 4, 8, 12, or 16 days under flow perfusion conditions with complete osteogenic medium containing dexamethasone. PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds designated as PE4, PE8, PE12, and PE16 experimental groups corresponding to their matrix maturities. To generate each batch of PCL/ECM constructs, MSCs were first harvested and pooled from five rats as described above and expanded through primary culture in complete osteogenic medium. Cells were then seeded onto PCL scaffolds and allowed to attach for 24 h, after which constructs were kept in their cassettes and transferred directly into the flow perfusion bioreactor, whose design and operation has been previously described in detail [11]. Medium was perfused through the press-fitted constructs at a flow rate of 0.7 mL/min with medium changes every 2 days. This flow rate was chosen to match the fluid shear stress applied in our previous study using titanium fiber mesh scaffolds [7]. At the end of each culture period, constructs were rinsed with PBS (without calcium and magnesium) and stored in 1.5 mL ddH2O at −80 °C. In addition to those PCL/ECM constructs generated to assess osteogenic differentiation, three constructs from each culture period were prepared for calcium, collagen, and glycosaminoglycan assays to characterize matrix composition and thus maturity, and two constructs from each culture period were prepared for x-ray imaging, histology, and scanning electron microscopy to visualize mineralized matrix morphology.
PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds via three consecutive cycles of a freeze and thaw process, in which constructs were frozen for 10 min in liquid nitrogen then thawed for 10 min in a 37 °C water bath [13]. This decellularization process has been shown to yield acellular constructs [6]. The resulting PCL/ECM composite scaffolds were then air-dried overnight, press-fitted into cassettes, sterilized with ethylene oxide gas for 14 h and aerated overnight in preparation for seeding.
Osteogenic differentiation using culture medium without dexamethasone
To assess osteogenic differentiation on PCL/ECM composite scaffolds of various maturities, MSCs were first harvested and pooled from eight rats as described above and expanded through primary culture in complete osteogenic medium. Cells were then seeded onto press-fitted experimental scaffolds PE4, PE8, PE12, and PE16, and also plain PCL control scaffolds at a seeding density of 250,000 cells in 200 µL of medium without dexamethasone within each cassette. Scaffolds were incubated for 2 h with the seeding solution, after which 10 mL of medium without dexamethasone was added to each well of the 6-well plates to fill the cassettes in which the scaffolds were held. After allowing 24 h for cell attachment, constructs were removed from their cassettes and transferred into 12-well plates with 3 mL of medium without dexamethasone and cultured static conditions for 4, 8, or 16 days with medium changes every 2 days. Five samples were cultured for each scaffold group (PCL, PE4, PE8, PE12, PE16) for each culture time (4, 8, 16 days), at the end of which, samples were rinsed with PBS (without calcium and magnesium) and stored for later analysis. Four samples were prepared for assessing construct cellularity, alkaline phosphatase activity, and calcium content, and one sample was prepared for scanning electron microscopy.
PCL/ECM matrix characterization assays
After culturing MSCs on electrospun PCL microfiber scaffolds for 4, 8, 12, or 16 days under flow perfusion conditions, the resulting PCL/ECM constructs were characterized for their mineralized extracellular matrix composition and morphology. Calcium content was determined by extracting calcium in an acetic acid solution then measuring free calcium ions using the Calcium assay, further described in the following section.
Total collagen content was determined by measuring hydroxyproline in a colorimetric assay [14]. Samples taken from culture and rinsed with PBS were placed in 0.75 mL of a proteinase K solution and digested in a 56 °C water bath for 16 h. The proteinase K solution consisted of 1 mg/mL proteinase K, 0.01 mg/mL pepstatin A, and 0.185 mg/mL iodoacetamide, in a tris-EDTA buffer made by dissolving 6.055 mg/mL tris(hydroxymethyl aminomethane) and 0.372 mg/mL EDTA with pH adjusted to 7.6. Digested matrix components were extracted via three repetitions of a freeze, thaw, and sonication cycle, where samples were frozen for 30 min at −80 °C, thawed at room temperature for 30 min, and sonicated for 30 min to allow matrix components into the solution. Hydroxyproline was quantified using a hydroxyproline assay as previously described [7]. After incubation for 30 min at 60 °C, absorbance at 570 nm corresponding to hydroxyproline concentration was measured on a plate reader (BioTek PowerWave ×340, Winooski, VT) and compared to a standard curve generated from known concentrations of hydroxyproline standards. Resulting hydroxyproline measurements in µg were finally converted to collagen contents for each construct following a 1:10 ratio of hydroxyproline to collagen [15].
Glycosaminoglycan content was determined by measuring glycosaminoglycan in a colorimetric assay [16]. Glycosaminoglycan was quantified in the supernatant previously obtained via proteinase K digestion using the dimethylmethylene blue assay (Sigma-Aldrich) as previously described [17]. After incubation for 10 min at room temperature, absorbance at 520 nm corresponding to glycosaminoglycan concentration, was measured on a plate reader (BioTek PowerWave ×340) and compared to a standard curve generated from known concentrations of chondroitin sulfate standards. Resulting glycosaminoglycan measurements in µg were finally determined for each construct.
Samples for x-ray imaging and histology were fixed in 10% neutral buffered formalin then cut in half and rinsed with 70% ethanol. Sample halves for x-ray imaging were air-dried overnight and imaged via x-ray (SkyScan 1172, Kontich, Belgium) according to the manufacturer’s recommended voltage of 40 kV with a current of 250 µA as previously described [18]. Sample halves for histology were cryo-embedded in HistoPrep freezing medium (Fisher Scientific, Pittsburgh, PA) and stored at −80 °C. Frozen sections 5 µm thick were cut using a cryostat (Microm HM 500, Ramsey, MN), mounted onto Superfrost Excell glass slides, and placed on a 37 °C slide warmer to facilitate adhesion. Sections were stained with hematoxylin and eosin to visualize the distribution of cells and extracellular matrix proteins. After mounting with Permount (Fisher Scientific), images were obtained using a light microscope (Nikon Eclipse E600, Melville, NY) with a video camera attachment (Sony DXC950P, New York, NY). Samples for scanning electron microscopy were fixed in glutaraldehyde and prepared for imaging as described in the scanning electron microscopy section.
Osteogenic differentiation assays
Construct cellularity was determined by measuring double-stranded DNA in a fluorometric assay [19]. Samples taken from culture and rinsed with PBS were placed in 1 mL of ddH2O, where DNA was extracted by lysing cells via three repetitions of a freeze and thaw cycle, in which samples were frozen for 10 min in liquid nitrogen then thawed for 10 min in a 37 °C water bath, and finally sonicated for 10 min to allow DNA into the solution. DNA was quantified using the PicoGreen assay (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions as previously described [6]. After incubation for 10 min room temperature, fluorescence at 520 nm corresponding to DNA concentration was measured on a plate reader (BioTek FL x800, Winooski, VT) and compared to a standard curve generated from known concentrations of DNA standards. Resulting DNA measurements in µg were finally converted to cell numbers by correlating to DNA extracted from a known number of MSCs in order to assess cellular proliferation.
Alkaline phosphatase activity was determined by measuring the enzyme-mediated conversion of the substrate p-nitrophenyl phosphate to p-nitrophenol in a colorimetric assay [20]. ALP enzymatic activity was quantified in the supernatant previously obtained via freeze, thaw, and sonication, using the Alkaline Phosphatase assay (Sigma-Aldrich) as previously described [5]. After allowing the reaction to progress for 1 h at 37 °C, absorbance at 405 nm corresponding to p-nitrophenol concentration was measured on a plate reader (BioTek PowerWave ×340) and compared to a standard curve generated from known concentrations of p-nitrophenol standards. Concentrated samples were diluted as needed to ensure readings within the linear range of the assay. Resulting ALP enzymatic activities as measured in pmol/hr corresponding to p-nitrophenol production were finally normalized to cell numbers in order to assess ALP enzymatic activity per cell as an early stage marker for osteogenic differentiation.
Calcium content was determined by measuring free calcium ions in a colorimetric assay. After completing both DNA and ALP assays, samples were transferred into 1 mL of a 1 N acetic acid solution and placed on a shaker table at 37 °C overnight to dissolve calcium deposited in the constructs. Calcium was quantified in the acetic acid solution using the Calcium assay (Genzyme, Cambridge, MA) as previously described [5]. After incubation for 10 min at room temperature, absorbance at 650 nm corresponding to calcium concentration was measured on a plate reader (BioTek PowerWave ×340) and compared to a standard curve generated from known concentrations of calcium chloride standards. Concentrated samples were diluted as needed to ensure readings within the linear range of the assay. Resulting calcium measurements in µg were finally determined for each construct in order to assess calcium deposition as a late stage marker for osteogenic differentiation.
Scanning electron microscopy
Samples for scanning electron microscopy were fixed in 2.5% glutaraldehyde for 1 h, dehydrated through a gradient series of ethanol from 70% to 100%, air-dried overnight then cut and mounted on aluminum stubs to visualize the top surface of the constructs. Samples were sputter coated with gold for 1 min prior to imaging via SEM (FEI Quanta 400, Hillsboro, OR).
Statistical analysis
Characterization results for the composition of mineralized extracellular matrix contained within PCL/ECM constructs following flow perfusion culture are reported as mean ± standard deviation for n = 3. A one-factor ANOVA was performed to determine whether culture duration (4, 8, 12, 16 days) had a significant effect. Comparisons were then made using the Tukey procedure to determine significant differences.
Biochemical assay results to assess osteogenic differentiation following static culture without dexamethasone are reported as mean ± standard deviation for n = 4. A two-factor ANOVA was first performed to determine significant main effects or interaction between scaffold group (PCL, PE4, PE8, PE12, PE16) and culture time (4, 8, 16 days). Multiple pairwise comparisons were then made using the Tukey procedure to determine significant differences. All statistical analyses were performed at a significance level of 5%.
Results
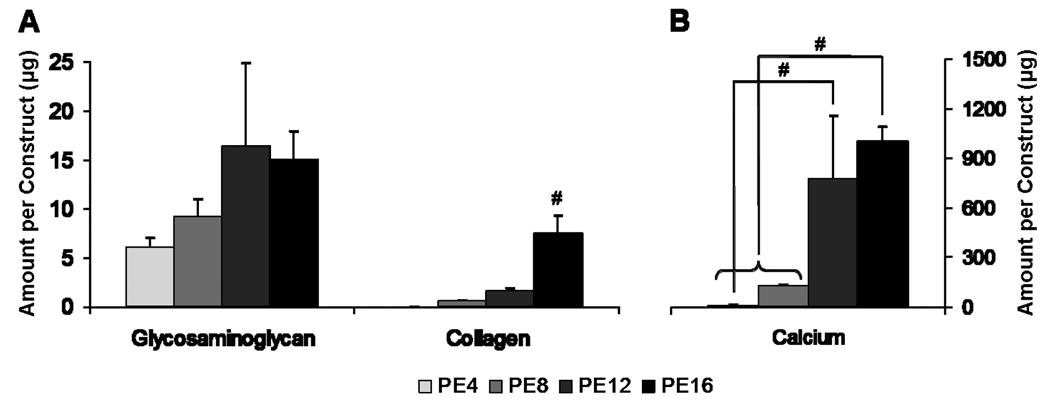
Mineralized extracellular matrix deposited on electrospun PCL microfiber scaffolds (fiber diameter 9.86 ± 0.56 µm) in generating PCL/ECM (PE) constructs were characterized for their composition and morphology prior to decellularization. Calcium content, collagen content, and glycosaminoglycan content following flow perfusion culture for 4, 8, 12, and 16 days to generate PE4, PE8, PE12, and PE16 constructs, respectively, are shown in Figure 1. Calcium content significantly increased over time, with PE16 constructs containing the most calcium as compared to both PE4 and PE8 constructs. Although PE12 constructs contained more calcium than PE4 constructs, the calcium content of PE12 constructs was not statistically different from PE8 or PE16 constructs. In terms of extracellular matrix protein composition, the amount of collagen in PE16 constructs was significantly higher than all other PCL/ECM constructs, while glycosaminoglycan content was not significantly different among the PCL/ECM constructs. Taking mineral content and glycosaminoglycan and collagen contents together, there was no significant difference between PE4 and PE8 constructs or between PE8 and PE12 constructs. However, there was a significant difference between PE12 and PE16 constructs in that PE16 constructs contained more collagen.
Figure 1.
Matrix composition of PCL/ECM (PE) constructs generated in flow perfusion culture of increasing durations (4, 8, 12, and 16 days) for PE4, PE8, PE12, and PE16 constructs. Plots show (A) glycosaminoglycan and collagen contents and (B) calcium content as mean ± standard deviation for n = 3. Significant difference (p < 0.05) between time points is noted with (#).
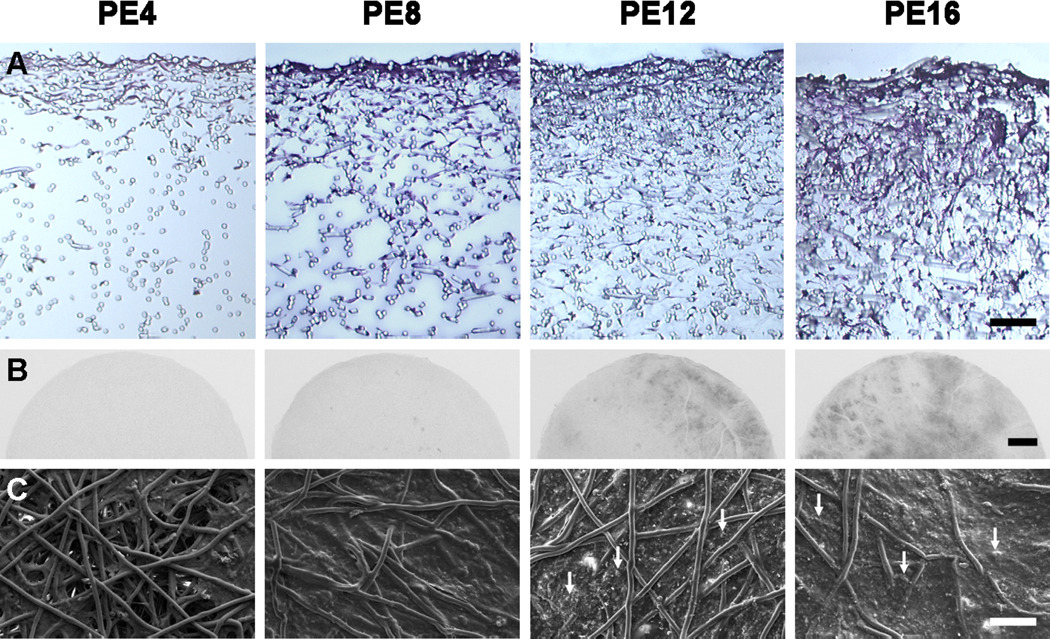
Flow perfusion culture enhanced the distribution of cells and extracellular matrix proteins over time, as seen in the histological sections stained with hematoxylin and eosin shown in Figure 2A. Radiopaque regions of mineralized matrix increased over time, with PE16 constructs demonstrating the most minerals visible through x-ray imaging, as shown in Figure 2B. The surface morphology of PCL/ECM constructs was visualized through scanning electron microscopy, as shown in Figure 2C. Extracellular matrix developed sparsely on PE4 constructs into a smooth surface seen on PE8 constructs. Mineral nodules on PE12 constructs eventually incorporated into a rough textured matrix on PE16 constructs.
Figure 2.
Matrix morphology of PCL/ECM (PE) constructs generated in flow perfusion culture of increasing durations (4, 8, 12, and 16 days) for PE4, PE8, PE12, and PE16 constructs. Histological sections stained with hematoxylin and eosin to visualize the distribution of cells and extracellular matrix proteins are shown in (A) with the scale bar representing 100 µm. X-ray images depicting radiopaque regions of mineralized matrix are shown in (B) with the scale bar representing 1 mm. Scanning electron micrographs of the top surface illustrating surface characteristics are shown in (C) with arrows indicating mineral nodules and the scale bar representing 100 µm.
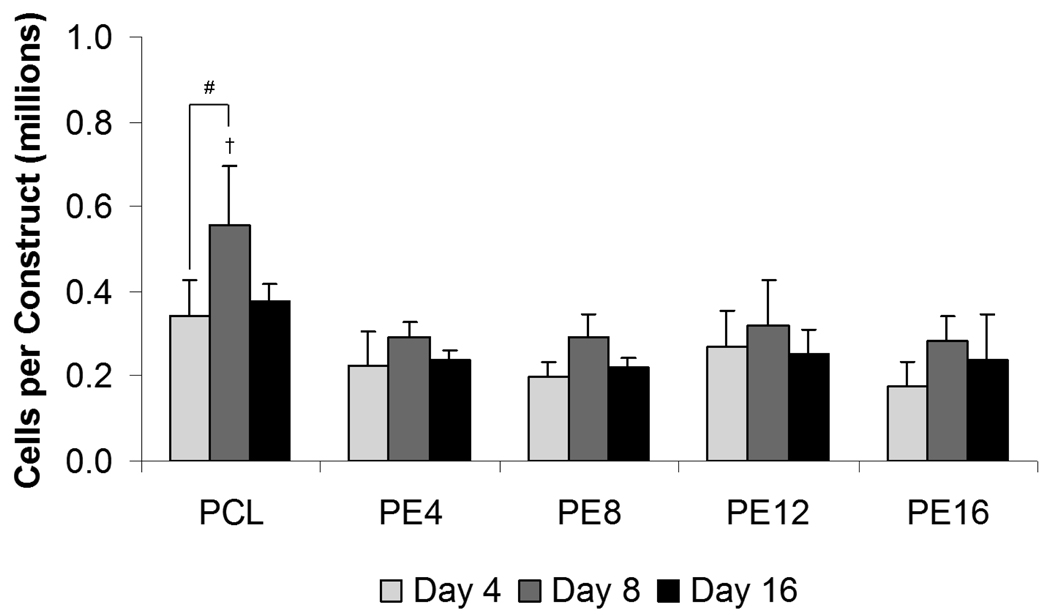
After PCL/ECM constructs were decellularized, the resulting PCL/ECM composite scaffolds along with plain PCL control scaffolds were seeded with MSCs to investigate how mineralized matrix maturity influences osteogenic differentiation in the absence of dexamethasone. Cellularity results showed that cell numbers remained constant over time at approximately the initial seeding density on all PCL/ECM composite scaffolds, whereas an increase in cellularity was observed on PCL scaffolds from 4 to 8 days of culture, as shown in Figure 3. Although cellularity peaked at 8 days on PCL scaffolds (0.56×106 ± 0.14×106 cells/construct) and was the highest compared to all PCL/ECM composite scaffolds, there was no statistical difference in cellularity among all scaffold groups after 16 days of culture.
Figure 3.
Cellularity of plain PCL scaffolds and PCL/ECM (PE) composite scaffolds seeded with MSCs and cultured in static conditions without dexamethasone for 16 days. PE4, PE8, PE12, and PE16 composite scaffolds contain mineralized matrix of various maturities generated in flow perfusion culture of increasing durations. Data are presented as mean ± standard deviation for n = 4. Within a specific scaffold group, significant difference (p < 0.05) between time points is noted with (#). At a specific time point, significant difference (p < 0.05) compared to all other scaffold groups is noted with (†).
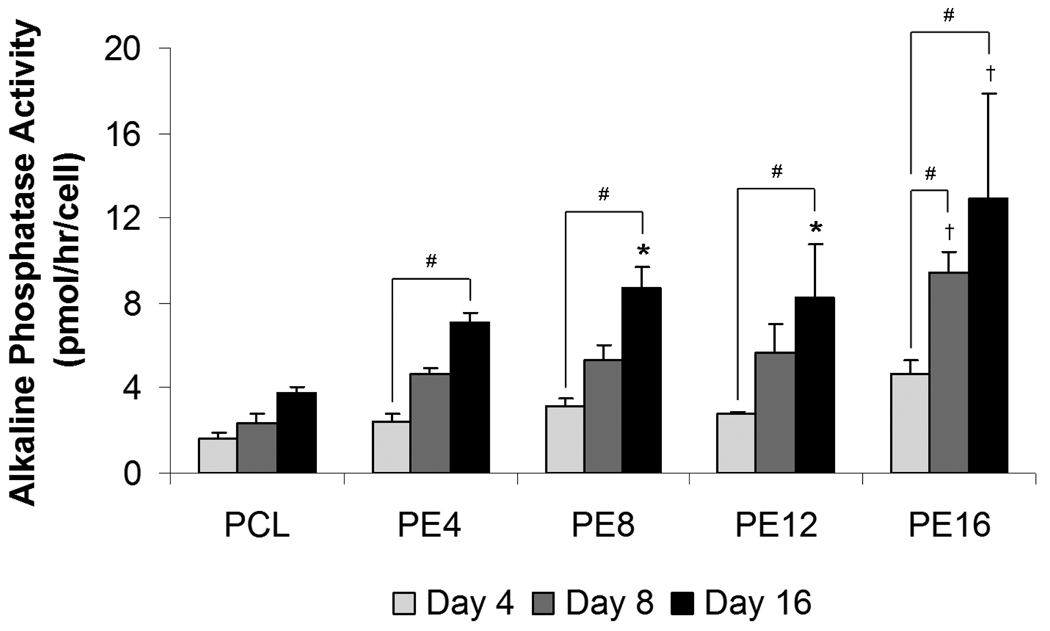
Alkaline phosphatase (ALP) activity per cell, as shown in Figure 4, was used as an early stage marker for osteogenic differentiation. Cells cultured on PCL scaffolds did not show a statistically significant increase in ALP activity over time, whereas those cultured on all PCL/ECM composite scaffolds demonstrated a significant increase in ALP activity from 4 to 16 days of culture with no observable peak. Furthermore, cells cultured on PE16 composite scaffolds also displayed a significant increase in ALP activity from 4 to 8 days of culture. Statistically significant differences in ALP levels compared to plain PCL controls were seen beginning at 8 days for PE16 and at 16 days for PE4, PE8, and PE12 composite scaffolds. Cells cultured on PE16 composite scaffolds exhibited the highest ALP activity compared to all other scaffold groups at 8 days (9.4 ± 1.0 pmol/hr/cell) and 16 days (12.9 ± 5.0 pmol/hr/cell).
Figure 4.
Alkaline phosphatase activity of plain PCL scaffolds and PCL/ECM (PE) composite scaffolds seeded with MSCs and cultured in static conditions without dexamethasone for 16 days. PE4, PE8, PE12, and PE16 composite scaffolds contain mineralized matrix of various maturities generated in flow perfusion culture of increasing durations. Data are presented as mean ± standard deviation for n = 4. Within a specific scaffold group, significant difference (p < 0.05) between time points is noted with (#). At a specific time point, significant difference (p < 0.05) compared to plain PCL controls is noted with (*), with significant difference (p < 0.05) compared to all other scaffold groups noted with (†).
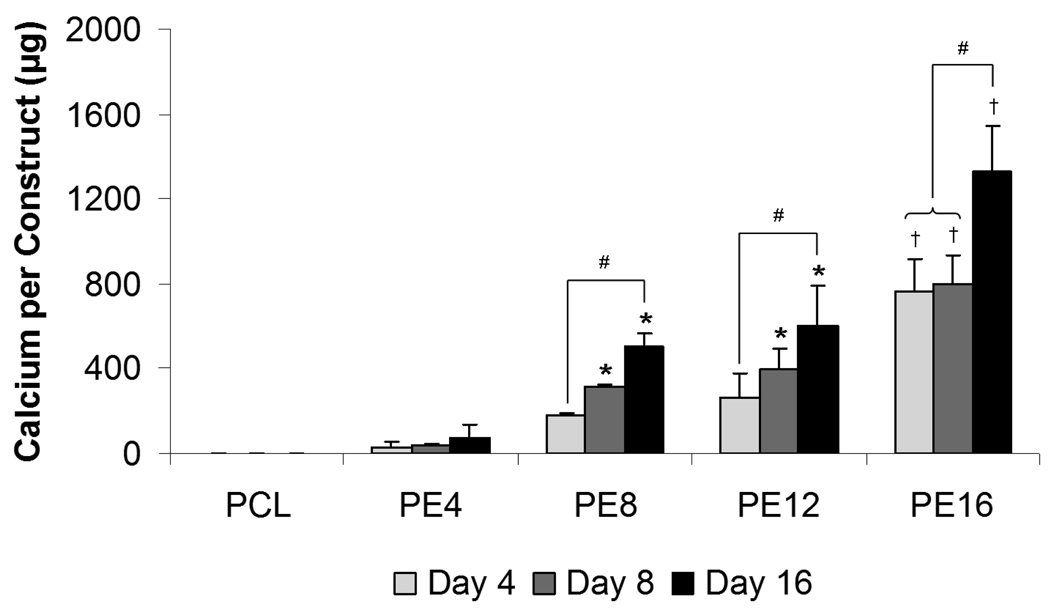
Calcium deposition per construct, as shown in Figure 5, was used as a late stage marker for osteogenic differentiation. Cells cultured on PCL scaffolds and PE4 composite scaffolds did not show a statistical increase in calcium deposition over time, whereas those cultured on PE8, PE12 and PE16 composite scaffolds demonstrated a significant increase in calcium deposition from 4 to 16 days of culture. Statistically significant differences in calcium content compared to plain PCL controls were seen beginning at 4 days for PE16 and at 8 days for PE8 and PE12 composite scaffolds. PE16 composite scaffolds showed the highest calcium content compared to all other scaffold groups at 4 days (764 ± 150 µg), 8 days (799 ± 136 µg), and 16 days (1324 ± 253 µg).
Figure 5.
Calcium content of plain PCL scaffolds and PCL/ECM (PE) composite scaffolds seeded with MSCs and cultured in static conditions without dexamethasone for 16 days. PE4, PE8, PE12, and PE16 composite scaffolds contain mineralized matrix of various maturities generated in flow perfusion culture of increasing durations. Data are presented as mean ± standard deviation for n = 4. Within a specific scaffold group, significant difference (p < 0.05) between time points is noted with (#). At a specific time point, significant difference (p < 0.05) compared to plain PCL controls is noted with (*), with significant difference (p < 0.05) compared to all other scaffold groups noted with (†).
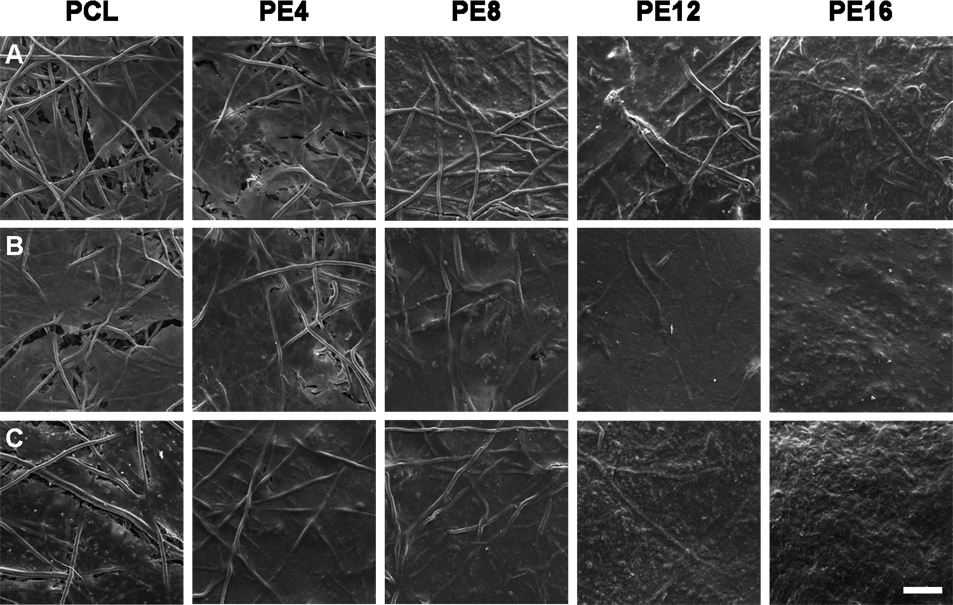
Scanning electron micrographs, as shown in Figure 6, were taken of the top surfaces of constructs to visualize the overall quality of the resulting extracellular matrix. Though PCL/ECM composite scaffolds started with an initial mineralized matrix while PCL scaffolds did not, the density and overall coverage of extracellular matrix on all scaffold groups increased over time, eventually to where scaffold fibers were no longer visible. The most striking differences in matrix quality were seen after 16 days of culture, where both PE12 and PE16 composite scaffolds developed a distinguishingly rough texture, whereas all other scaffold groups retained a smooth appearance. PE16 composite scaffolds seemed to take on this rough surface characteristic sooner than all other scaffold groups beginning after 8 days of culture.
Figure 6.
Representative scanning electron micrographs of the top surfaces of plain PCL scaffolds and PCL/ECM (PE) composite scaffolds seeded with MSCs and cultured in static conditions without dexamethasone for 16 days. PE4, PE8, PE12, and PE16 composite scaffolds contain mineralized matrix of various maturities generated in flow perfusion culture of increasing durations. For each scaffold group, three rows of images are shown for constructs after (A) 4 days of culture, (B) 8 days of culture, and (C) 16 days of culture. The scale bar shown represents 100 µm and applies to all images.
Discussion
The objective of this study was to evaluate the osteogenic capacity of PCL/ECM composite scaffolds in vitro. This study was designed to investigate the effects of mineralized extracellular matrix maturity on MSC differentiation down the osteogenic lineage in the absence of the osteogenic cell culture supplement dexamethasone. In order to determine whether exposing cells to a biomimetic microenvironment containing various compositions of matrix signals could influence their osteogenic differentiation response, PCL/ECM composite scaffolds were generated by coating electrospun PCL microfiber scaffolds with natural mineralized extracellular matrix of various maturities, then seeded with MSCs and cultured in medium without the addition of dexamethasone.
Exposing MSCs to both dexamethasone and fluid shear stresses in flow perfusion culture has been shown to synergistically enhance osteogenic differentiation and the distribution of mineralized extracellular matrix [4, 5]. Thus in this study, flow perfusion culture with dexamethasone was employed to generate PCL/ECM constructs. In generating these PCL/ECM constructs, we found that cells were able to penetrate throughout the interconnected porosity of electrospun PCL microfiber scaffolds and deposit increasing amounts of mineralized extracellular matrix with distinct compositions and morphologies over time. Since PCL/ECM constructs were generated with MSCs induced down the osteogenic pathway through exposure to osteogenic culture conditions, the resulting constructs at the end of each culture period contains extracellular matrix secreted by cells at various stages of osteogenesis after 4, 8, 12, and 16 days. As evident in our characterization of calcium, collagen, and glycosaminoglycan contents, we were able to generate PE4, PE8, PE12, and PE16 constructs containing various quantities of minerals and proteins by exposing MSCs to dexamethasone and fluid shear stresses for various culture durations. In addition to this quantitative difference in matrix composition, PCL/ECM constructs also differed in appearance, as seen through x-ray images and scanning electron micrographs demonstrating mineralized matrix. Overall, the trends observed here using electrospun PCL microfiber scaffolds are consistent with previous studies in our group using titanium fiber mesh scaffolds, nonwoven poly(L-lactic acid) scaffolds, and fiber bonded starch-poly(ε-caprolactone) scaffolds [4, 21, 22]. Although the flow rate in this study was chosen to match the fluid shear stress applied in our previous study using titanium fiber mesh scaffolds, electrospun PCL microfiber scaffolds have smaller pore sizes as compared to our previous scaffolding materials. Therefore, as extracellular matrix accumulates in the pore space over time, higher fluid shear forces are generated throughout the culture period. As a result, we were able to achieve much higher calcium deposition as compared to our previous scaffolding materials, since higher fluid shear stresses stimulate cells to deposit increasing amounts of matrix which mineralizes over time [23, 24].
PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds containing mineralized matrix of various maturities. In order to evaluate osteogenic properties and the influence of mineralized matrix maturity on the osteogenic differentiation of MSCs in vitro, we seeded PCL/ECM composite scaffolds along with plain PCL control scaffolds with MSCs and cultured them under static conditions without the addition of dexamethasone. Since dexamethasone is a potent synthetic glucocorticoid that is often necessary to drive osteogenic differentiation in vitro, we sought to isolate the effects of mineralized matrix on osteogenic differentiation by omitting this osteogenic supplement from the culture medium.
Our results showed that the presence of mineralized matrix in PCL/ECM composite scaffolds was able to induce the differentiation of MSCs down the osteogenic lineage as compared to plain PCL scaffolds. In general, we observed that mineralized matrix reduced cellular proliferation while stimulating alkaline phosphatase activity with increasing amounts of calcium deposition over time, thus indicating the progression of osteogenesis in vitro. Cells cultured on plain PCL scaffolds, on the other hand, exhibited minimal alkaline phosphatase activity and calcium deposition as expected, since they were not presented with any osteoinductive stimuli, specifically dexamethasone or extracellular matrix signals.
Mineralized matrix maturity did not seem to differentially influence cellular proliferation since all PCL/ECM composite scaffolds maintained similar cellularity over 16 days of culture. In fact, cells did not appear to proliferate on PCL/ECM composite scaffolds as compared to the proliferation seen on plain PCL scaffolds. This could be due to a difference in the physical morphology of the scaffolds, where PCL/ECM composite scaffolds are coated with mineralized matrix that may promote cell spreading to a confluent layer, while plain PCL scaffolds present a fiber morphology with a more open pore structure to support cellular proliferation. It is likely that cells seeded on the surface of PCL/ECM composite scaffolds grew to confluence since the layer of mineralized matrix may have been too dense for cells to remodel and penetrate under static culture conditions, as confirmed through histology (data not shown). Though in this study, in order to isolate the effects of mineralized matrix, it was necessary to evaluate osteogenic differentiation under static conditions and minimize confounding factors, namely fluid shear stresses introduced though flow perfusion culture, which would have facilitated cell penetration but also affected cellular response.
Alkaline phosphatase is an enzyme responsible for the dephosphorylation of phosphates, and is used as an early stage marker for osteogenic differentiation. ALP levels peak as cells progress from a proliferative stage to depositing a mature extracellular matrix containing calcium phosphate [25]. Since PE16 composite scaffolds induced significantly higher levels of ALP activity as compared to all other scaffold groups after just 8 days, with the highest levels achieved among scaffold groups at 8 and 16 days of culture, it appears that a more mature mineralized matrix containing greater quantities of calcium and collagen induces a more rapid and robust osteogenic differentiation response. As compared to our previous studies on titanium fiber mesh scaffolds, higher ALP levels were achieved in this study using extracellular matrix signals than with dexamethasone in static culture, while fluid shear stresses in flow perfusion culture without dexamethasone induced more ALP activity [5–7].
Although our results did not show a peak in ALP activity for PCL/ECM composite scaffolds, enzyme levels did not significantly increase from 8 to 16 days of culture, implying that cellular activity may just be starting to shift toward the synthesis of a more mature matrix. This shift in cellular activity is supported by the calcium deposition results, used here as a late stage marker of osteogenic differentiation. An increase in calcium content was only observed after 16 days of culture for PE8, PE12, and PE16 composite scaffolds. It is important to emphasize that PCL/ECM composite scaffold groups each began with different amounts of calcium deposited during the generation of mineralized matrix in flow perfusion culture. Although the initial quantity of minerals was characterized following each culture period, the actual mineral content of PCL/ECM composite scaffolds may be reduced following the freeze-thaw decellularization process. Nevertheless, even though minerals present in PCL/ECM composite scaffolds inherently contribute to the enhanced calcium contents seen for more mature scaffold groups, the increase in ALP activity is indicative of osteogenic differentiation, which is especially apparent in MSCs cultured for only 8 days on PE16 composite scaffolds. Due to the initial variation in calcium content among PCL/ECM composite scaffolds, it is difficult to compare the resulting calcium content between scaffold groups, but rather more informative to note the change in calcium content over time for each scaffold group. In doing so, we see that cells cultured on plain PCL scaffolds and PE4 composite scaffolds do not produce much matrix even after 16 days of culture. In contrast, cells cultured on PE8, PE12, and PE16 composite scaffolds require exposure to matrix signals for at least 16 days in order to differentiate and lay down a mineralized extracellular matrix of their own. The progression in matrix maturation is further observed through scanning electron micrographs showing the initial deposition of a smooth collagen matrix which eventually develops into a rough mineralized matrix over time.
We presume that the osteoinductive effects of mineralized extracellular matrix observed in this study involve specific cell-matrix interactions, since foreseeable confounding effects due to dexamethasone supplementation and fluid shear stresses were excluded from this study. In addition to the characterized presence of calcium, collagen, and glycosaminoglycan, mineralized matrix generated in the same flow perfusion bioreactor system has been shown to contain active bone-related growth factors, particularly BMP-2, FGF-2, VEGF, and TGF-β1, found to be localized and most prevalent at the surfaces of constructs as detected through immunohistochemical analysis [26]. Therefore, in generating PCL/ECM composite scaffolds of various maturities that contain increasing quantities of minerals and proteins with increasing culture duration, we expect the presence of these signaling molecules to increase as well. Cells seeded onto PCL/ECM composite scaffolds would directly interact with not only physical matrix components but also localized growth factors that together, regulate osteogenic differentiation. In addition to cell-matrix interactions with native bone tissue components, surface roughness due to calcium phosphate incorporation into the mineralized matrix on PCL/ECM composite scaffolds may have also influenced cellular response. The difference in surface morphology down to nanoscale features could affect cell attachment and migration, with possible effects on osteogenic differentiation [27–29]. Accordingly, we found that a more mature mineralized matrix containing more minerals, collagen, and glycosaminoglycan possesses greater osteogenic ability than less developed matrices, possibly due to the presence of more bone signaling molecules and increased surface roughness.
Conclusion
In this work, we demonstrate that the presence of mineralized extracellular matrix on electrospun PCL microfiber scaffolds imparts osteogenic properties to an otherwise inert biomaterial, as evident in its ability to stimulate osteogenic differentiation of MSCs in vitro in the absence of the osteogenic supplement dexamethasone. Furthermore, we show that the maturity of this mineralized matrix modulates osteogenic differentiation, providing insight towards the development of osteoinductive scaffolding materials with controllable characteristics for bone regeneration.
Acknowledgements
This work has been supported by the National Institutes of Health R01-AR57083.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19(4):513–521. doi: 10.1016/j.coms.2007.06.002. iv. [DOI] [PubMed] [Google Scholar]
- 2.Holtorf HL, Jansen JA, Mikos AG. Modulation of cell differentiation in bone tissue engineering constructs cultured in a bioreactor. Adv Exp Med Biol. 2006;585:225–241. doi: 10.1007/978-0-387-34133-0_16. [DOI] [PubMed] [Google Scholar]
- 3.Li WJ, Tuli R, Huang X, Laquerrierex P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26(25):5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A. 2003;64(2):235–241. doi: 10.1002/jbm.a.10365. [DOI] [PubMed] [Google Scholar]
- 5.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 6.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26(9):971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG. Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J Biomed Mater Res A. 2003;67(3):944–951. doi: 10.1002/jbm.a.10126. [DOI] [PubMed] [Google Scholar]
- 9.Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI. Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A. 2007;82(1):129–138. doi: 10.1002/jbm.a.31082. [DOI] [PubMed] [Google Scholar]
- 10.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 11.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9(3):549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 12.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254(2):317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 13.Medalie DA, Eming SA, Collins ME, Tompkins RG, Yarmush ML, Morgan JR. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997;64(3):454–465. doi: 10.1097/00007890-199708150-00015. [DOI] [PubMed] [Google Scholar]
- 14.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 15.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 16.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, Mikos AG. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15(9):2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249(2):228–238. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 20.Bretaudiere JP, Spillman T. Bergmeyer HU, Bergmeyer J, Grassl M. Methods of enzymatic analysis. Verlag Chemie; 1984. Alkaline phosphatases, routine method; pp. 75–82. [Google Scholar]
- 21.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33(1):63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 22.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J Biomed Mater Res A. 2003;67(1):87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 23.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtorf HL, Datta N, Jansen JA, Mikos AG. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res A. 2005;74(2):171–180. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 25.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 26.Gomes ME, Bossano CM, Johnston CM, Reis RL, Mikos AG. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12(1):177–188. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- 27.Keller JC, Collins JG, Niederauer GG, McGee TD. In vitro attachment of osteoblast-like cells to osteoceramic materials. Dent Mater. 1997;13(1):62–68. doi: 10.1016/s0109-5641(97)80010-3. [DOI] [PubMed] [Google Scholar]
- 28.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 29.Dalby MJ, Andar A, Nag A, Affrossman S, Tare R, McFarlane S, Oreffo RO. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J R Soc Interface. 2008;5(26):1055–1065. doi: 10.1098/rsif.2008.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]