Recent advances with functionalized nanoporous supports provide an innovative approach for entrapping proteins and for their subsequent controlled release and delivery.1-7 Functionalized mesoporous silica (FMS) can provide a confined and interactive nanoenvironment that increases protein activity and allow large amounts of protein loading compared to unfunctionalized mesoporous silica (UMS) or normal porous silica.5-7 First, the proteins can be spontaneously entrapped in FMS with rigid, uniform, open nanopore geometry of tens of nanometers via non-covalent interaction. Then, one can control the release of the entrapped proteins from FMS based on the function groups and pore sizes when the FMS-protein composites are dispersed in a fresh buffer solution in which a new thermodynamic balance can be reached. In this work, we found that antibodies can be spontaneously loaded in FMS with super-high density (0.4-0.8 mg of antibody/mg of FMS) due to their comprehensive non-covalent interaction. We hypothesize that therapeutic antibodies entrapped in FMS can be gradually released locally in vivo under physiological conditions and that this will help develop innovative therapies for many diseases. We performed pilot tests to investigate the anti-tumor activity of a monoclonal antibody (mAb) to CTLA4,8 an immunoregulatory molecule released from FMS at the tumor site. This strategy resulted in much greater and extended inhibition of tumor growth than the antibody given systematically.
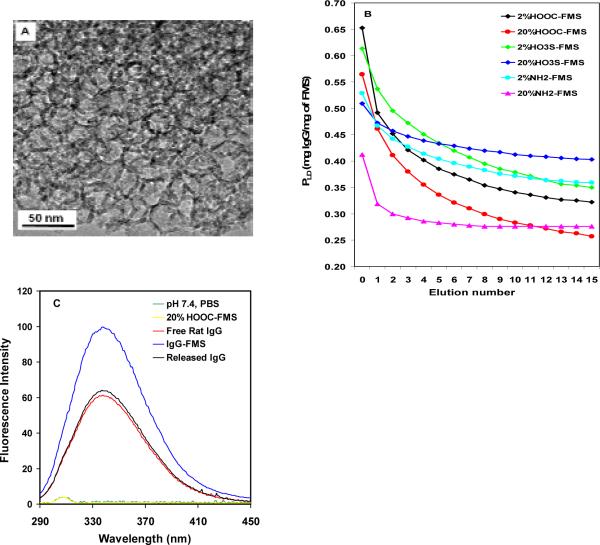
To ensure large loading of mAb molecules (M.W. 150kDa) in FMS, we prepared UMS with a pore size (diameter) as large as 30 nm, a surface area as great as 533 m2/g and an average bead size of 12-15 μm (Supporting information).9,10 A controlled hydration and condensation reaction was used to introduce functional groups into UMS.9,10 Coverage of 2% (or 20%) HOOC-FMS, HO3S-FMS or NH2-FMS means 2% (or 20%) of the total available silanol groups (5 × 1018 silanol groups per square meter9,10) of UMS would be silanized with trimethoxysilane with the functional group HOOC, HO3S or NH2.1-7 Figs. 1A shows the TEM image of 30 nm 20% HOOC-FMS. There is no significant difference between the TEM images of UMS and their corresponding FMS.6 Unlike 3-nm and 10-nm mesoporous silica, the 30-nm mesoporous silica has a large degree of disordering,11 but it still reveals more or less uniform cage-like porous structure.12
Fig. 1.
(A) TEM image of 30 nm 20% HOOC-FMS; (B) Rat IgG loading density in FMS and gradual release of the IgG from FMS in the simulated body fluid; (C) Fluorescence spectra of the free rat IgG, the FMS-IgG, and the released IgG from FMS. [IgG]: 0.03 mg/mL in pH 7.4, PBS. The excitation was at 278 nm.
FMS was incubated in the antibody solution, where the antibody would be spontaneously entrapped in FMS. We defined the protein amount (mg) of an antibody entrapped with 1 mg of FMS as the protein-loading density (PLD). We first exploited the large loading density of FMS for entrapping rat and mouse IgGs and studying their releasing ability in a physiological buffer (Fig. 1B and Supporting information, Fig. S1). IgGs were loaded in various FMSs. The resulting FMS-IgG composites were then transferred to fresh buffers and eluted multiple times to determine the release kinetics of antibody from the particles. The protein contents of the supernatants in between each cycle of shaking-elution-centrifugation were measured. Although different, PLD of IgGs in various FMSs were all super-high at the “0 elution” data point (0.4-0.8 mg of IgG/mg of FMS), which is much higher than previously reported for other proteins.1-7 The subsequent controllable release of the IgG from FMS was carried out in pH 7.4, 10 mM sodium phosphate, 0.14 M NaCl (PBS) or a simulated body fluid that has ion concentrations nearly equal to those of human blood plasma (buffered at pH 7.4 with 50 mM Tris-HCl) (Fig. 1B and Supporting information, Fig. S1). A decreasing PLD was observed along the series of elutions. For both rat and mouse IgGs, the 20% HOOC-FMS and 2% HO3S-FMS displayed faster releasing rates than other FMSs under the identical elution solutions. These results reflected the difference of the comprehensive non-covalent interaction of IgG with various FMSs; that is the electrostatic, H-bond, hydrophilic and hydrophobic interaction of the functional groups and spacers of FMS with the amino acid residues of protein molecules.5
Fig. 1C shows fluorescence emission spectra of the free rat IgG, the entrapped IgG in FMS, and the released IgG from FMS. Fluorescence emission was monitored at the excitation wavelength of 278 nm, allowing excitation of both tyrosinyl and tryptophanyl residues. Comparing the free IgG to FMS-IgG (Fig. 1C), there was no dramatic emission peak shift but increased emission intensity because of the interaction of IgG with FMS, which might result in less exposure of tyrosinyl and tryptophanyl residues to the aqueous environment. It is noteworthy that the released IgG displayed similar fluorescence spectra to that of the free IgG prior to the entrapment, indicating that the interaction of FMS with IgG did not induce dramatic change on the IgG protein structure. Our preliminary result also shows that in vitro released antibody from FMS still maintained its binding activity (Supporting information, Table S1).
Monoclonal antibodies have been used to treat many medical conditions, including cancer.13-15 For example, a systemic administration of an mAb to the immunoregulatory molecule CTLA4 has displayed anti-tumor activity by modifying the host response to tumors, both in mouse models and in human cancer patients.16 It is important that a sufficient amount of the mAb gets delivered to the tumor, as the tumor micro-environment is highly immunosuppressive because of its high concentration of tumor antigen, regulatory T lymphocytes, etc.17 However, to deliver sufficient amounts of the anti-CTLA4 mAb to a tumor to be therapeutically effective, there is a risk of side effects from inducing autoimmunity to normal tissue antigens. For example, a profound anti-tumor activity was marred by toxicity in several renal carcinoma patients who had been injected systemically with anti-CTLA4 mAb.18
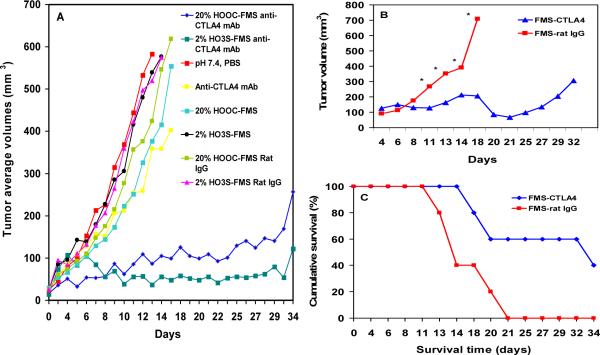
To test our strategy, we selected a rat IgG mAb to CTLA4 for entrapment into FMS particles.8 The FMS-entrapped antibody was injected directly into established mouse melanomas derived from s.c. injection of cells from the SW1 clone. We compared the results to several controls, including intraperitoneally injected anti-CTLA4 mAb; and intratumorally injected FMS particles, and FMS particles containing rat IgG and PBS buffer. Mice were injected with 106 SW1 cells s.c. on the back. When the mice had tumors of ~3 mm mean diameter, we randomized them according to tumor size into different groups, each comprised of three mice. Fig. 2A shows representative results from each treatment group. The results demonstrate that FMS-anti-CTLA4 inhibited tumor growth. We saw no evidence of toxicity from injecting FMS particles into tumors. In particular, the anti-tumor activity of FMS-Anti-CTLA4 (>50% tumor regression) was much more potent than that of anti-CTL4 alone (without FMS). We have repeated the experiment and got the similar results (Figs. 2B & 2C). To confirm the local release, we measured in vivo release of fluorescent dye-labeled IgG from FMS at the tumor site. The results demonstrate that FMS entrapping with IgG prolonged the antibody stay at the tumor site and thus facilitates sustained antibody release in tumors, offering an advantage over simply injecting antibodies into tumors (Supporting information, Fig. S2). Further optimization of functionalization and pore sizes of FMS,4,19 more extensive therapeutic and pathological experiments are ongoing, and the results will be reported elsewhere.
Fig. 2.
(A) Anti-tumor activity of FMS-anti-CTLA4 injected subcutaneously (s.c.) into small established, growing mouse melanomas (3 mice/group). 0.5 mg Anti-CTLA4 was used. Controls were the PBS buffer, anti-CTLA4, FMS (20% HOOC- and 2% HO3S-), and FMS-Rat IgG; (B) Summary results of anti-tumor activity of 20% HOOC-FMS-anti-CTLA4 from a repeat experiment with 5 mice/group which had small SW1 tumors on both sides of the back, providing 10 tumor sites/group. 2 tumors were completely regressed. *p <0.05; (C) Survival of mice in the repeat experiment.
We conclude that immunoglobulins can be loaded in FMS particles with super-high density to provide long-lasting local release, and our preliminary data indicate that FMS-entrapped anti-CTLA4 IgG mAb induces a much greater and extended therapeutic response than the same amount given systemically. Our results have also demonstrated that the rate and durability of the mAb release from FMS particles can be fine-tuned by changing the functional group types and coverages of FMS (Fig. 1B and Supporting information, Fig. S1). We expect that a similar approach of local release can be applied to other mAbs as well as other immunologically active proteins, delivered alone or in combination, and that a long-lasting local release will cause more effective tumor destruction with less dose amount, longer dose intervals, and thus fewer side effects than systemic administration.
Supplementary Material
ACKNOWLEDGMENT
This work is supported by the pilot funding programs of Pacific Northwest National Laboratory (PNNL), Washington Research foundation and UW Institute of Translational Health Sciences, the NIH grants R01GM080987 and RO1CA134487, and the US Dept. of Energy BES Award KC020105-FWP12152. We thank Drs. Mary Disis, Cheryl Baird and Karin Rodland for helpful discussions, and Dr Nancy Kiviat, Ms. Yean Yee Yip and Ms. Kristin D. Victry for facility and experimental support. PNNL is operated for the U.S. Dept. of Energy by Battelle under Contract DE-AC06-RLO1830.
Footnotes
Supporting Information Available: Experimental section and additional experimental data are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Takahashi H, Li B, Sasaki T, Miyazaki C, Kajino T, Inagaki S. Chem. Mater. 2000;12:3301. [Google Scholar]
- 2.Yiu HHP, Wright PA, Botting NP. Microporous and Mesoporous Materials. 2001;44-45:763. [Google Scholar]
- 3.Deere J, Magner E, Wall JG, Hodnett BK. J. Phy. Chem. B. 2002;106:7340. [Google Scholar]
- 4.Han YJ, Stucky GD, Butler A. J. Am. Chem. Soc. 1999;121:9897. [Google Scholar]
- 5.Lei C, Shin Y, Liu J, Ackerman EJ. J. Am. Chem. Soc. 2002;124:11242. doi: 10.1021/ja026855o. [DOI] [PubMed] [Google Scholar]
- 6.Lei C, Shin Y, Magnuson JK, Fryxell G, Lasure LL, Elliott DC, Liu J, Ackerman EJ. Nanotechnology. 2006;17:5531. doi: 10.1088/0957-4484/17/22/001. [DOI] [PubMed] [Google Scholar]
- 7.Chen BW, Lei CH, Shin YS, Liu J. Biochemical and Biophysical Research Communications. 2009;390:1177. doi: 10.1016/j.bbrc.2009.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leach DR, Krummel MF, Allison JP. Science. 1996;271:1734. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Shin Y, Nie ZM, Chang JH, Wang L-Q, Fryxell GE, Samuels WD, Exarhos GJ. J. Phys. Chem. A. 2000;104:8328. [PubMed] [Google Scholar]
- 10.Feng X, Fryxell GE, Wang L-Q, Kim AY, Liu J, Kemner KM. Science. 1997;276:923. [Google Scholar]
- 11.Zhao DY, Feng JL, Huo QS, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Science. 1998;279:548. doi: 10.1126/science.279.5350.548. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Shin Y, Nie ZM, Chang JH, Wang L-Q, Fryxell GE, Samuels WD, Exarhos GJ. J. Phys. Chem. A. 2000;104:8328. [PubMed] [Google Scholar]
- 13.Hellstrom KE, Hellstrom I. Journal of Cellular Biochemistry. 2007;102:291. doi: 10.1002/jcb.21468. [DOI] [PubMed] [Google Scholar]
- 14.Ye ZM, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Nature Medicine. 2002;8:343. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 15.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen LP. Nature Medicine. 1997;3:682. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 16.Egen JG, Kuhns MS, Allison JP. Nature Immunology. 2002;3:611. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 17.Hellstrom KE, Hellstrom I. Journal of Cellular Biochemistry. 2007;102:291. doi: 10.1002/jcb.21468. [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Hughes H, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Journal of Immunotherapy. 2007;30:825. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horcajada P, Ramila A, Perez-Pariente J, Vallet-Regi M. Microporous and Mesoporous Materials. 2004;68:105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.