Abstract
Background
Statins have been suggested to reduce expansion of abdominal aortic aneurysms (AAA) independent of lipid lowering effects.
Methods
We assessed the association of statin treatment and serum low density lipoprotein (LDL) concentrations with small AAA expansion. 652 patients undergoing surveillance of small AAAs were entered into the study from five vascular centers. In a subset fasting lipids (n=451) and other biomarkers (n=216) were measured. AAA diameter was followed by ultrasound surveillance for a median of 5 years.
Results
349 (54%) of the patients were prescribed statins. Adjusting for other risk factors statin prescription was not associated with AAA growth (odds ratio, OR, 1.23, 95% confidence interval, CI, 0.86–1.76). Above median AAA growth was positively associated with initial diameter (OR 1.78 per 4.35mm larger initial aortic diameter, 95% CI 1.49–2.14) and negatively associated with diabetes (OR 0.37, 95% CI 0.22–0.62). Above median serum LDL concentration was not associated with AAA growth. Patients receiving statins had lower serum C-reactive protein concentrations but similar matrix metalloproteinase-9 and interleukin-6 concentrations to those not prescribed these medications.
Conclusions
We found no association between statin prescription or LDL concentration with AAA expansion. The results do not support the findings of smaller studies and suggest that statins may have no benefit in reducing AAA progression.
Keywords: Abdominal aortic aneurysm, Statin, Low density lipoprotein
Introduction
Abdominal aortic aneurysm (AAA) is recognized as an important cause of death in older men [1, 2]. A number of trials have demonstrated the advantage of ultrasound screening for AAAs in older men and screening has been introduced in a number of countries [3]. Most AAAs detected by screening programs are small measuring <55mm in diameter [4]. Patients with AAAs of this size do not benefit from open surgery and the role of endovascular repair is controversial [5–7]. Thus most AAAs identified by surveillance are treated conservatively and monitored by repeated imaging unless the AAA expands above the local intervention threshold [8]. Currently only a few small randomized controlled trials have assessed the efficacy of medication in slowing AAA progression without clear evidence of an effective drug to date [9–12].
Data from some but not all observational studies suggest that concurrent prescription of statins is associated with reduced AAA expansion rates [13–17]. These studies have however been small and not all analyses have adjusted for risk factors associated with statin prescription or AAA progression [13, 15, 17].
The aim of this study was to assess the association of statin treatment with AAA expansion in a large cohort of patients with small AAA. In a sub-group of patients we also assessed the association of serum low density lipoprotein (LDL) with AAA progression.
Methods
Study design and patients
In order to assess the association of statin prescription with AAA progression we aimed to recruit at least 600 patients in order to have a power of >80% to detect a 25% difference in annual AAA growth (estimated as 1.2±1.2 mm/year, alpha 0.05) [18]. Patients were recruited from five vascular centers from Australia and New Zealand, including Fremantle Hospital, Western Australia; The Townsville Hospital, Queensland; Gosford Hospital, New South Wales; Box Hill Hospital, Victoria; and Christchurch Hospital, New Zealand.
The study was approved by the relevant ethics committees at each site and informed consent was obtained from participating patients. We included consenting patients with small AAA (infrarenal aortic diameter 30–50 mm) for whom the recruiting clinician had no current plans for AAA repair. Patients were assessed by clinical interview and physical examination in order to collect risk factor and medication history. Items recorded included age, gender, diabetes mellitus (DM), hypertension, coronary heart disease (CHD), stroke, peripheral artery disease (PAD), dyslipidaemia, smoking history (categorized as current or past smoker, or never smoked), family history of AAA or sudden death. Height, weight, waist and hip circumference, systolic and diastolic blood pressure were measured by a research nurse. Height, weight, waist and hip circumference were measured in order to calculate body mass index (BMI) and waist to hip ratio (WHR). Risk factors were defined by prior history or treatment, as previously described [19]. We routinely recorded details of medications on only one occasion. Medication prescription during surveillance was based on one interview with the patient and chart review according to drug classes including statins, angiotensin converting enzyme (ACE) inhibitors, angiotensin-2 receptor blockers, aspirin, beta-blockers, calcium-channel blockers, diabetic medications and non-steroidal anti-inflammatory drugs (NSAID). Duration of therapy and statin dose was not routinely recorded.
Ultrasound surveillance
Maximum antero-posterior and transverse infra-renal aortic diameter were measured by experienced sonographers using a 3.75 MHz transducer and ultrasound machines present in vascular laboratories of each centre (Toshiba Capasee; Philips HDI 5000; GE Logic 9; Siemens Acuson Antares; Philips IU22). The reproducibility of aortic diameter measurements were regularly assessed at each vascular laboratory, as previously described [20, 21]. An example of the results from one group of patients assessed in Queensland is given. Ten patients were assessed on two separate occasions and results analyzed with Bland-Altman plots. The mean of measurement differences (±2SD) was 0.4mm (−2.62, 3.42). The interval for repeated imaging was at the discretion of the local clinician but in general repeated scans were carried out yearly for 30–44 mm AAAs and 6 monthly for 45–50 mm AAAs.
Measurement of fasting lipids and serum inflammatory markers
Cholesterol, triglyceride, high and low density lipoprotein were measured in a subset (n=451) by automated enzymatic methods [22]. Serum C-reactive protein (CRP), matrix metalloproteinase-9 (MMP-9) and interleukin-6 (IL-6) concentrations were measured in a further subset (n=216). CRP concentration was measured by immunophelometry using the BNII analyzer as reported previously (Dade Behring, Milton Keynes, UK) [20]. MMP-9 and IL-6 concentrations were measured in serum using previously validated enzyme-linked immunosorbent assays (Duoset®; R&D Systems, Minneapolis, Minnesota, USA) [23].
Statistical analyses
Demographic and clinical patient characteristics of statin users compared to non-users were compared using χ2 and the Mann-Whitney U tests.
The annual growth rates of AAAs were assessed by taking into account all diameters measured during follow-up and calculating time-weighted average growth rates for each patient. Median yearly AAA growth rates for the whole cohort were calculated and expansion ≥ or < median noted. The relationship between statin treatment and AAA expansion ≥ or < median was examined using logistic regression analysis adjusting for variables known to influence AAA growth rate and risk factors disparate between patients receiving statins and those not prescribed this medication. Continuous variables were scaled by the standard deviation to calculate more easily comparable odds ratios. In a further analysis we also assessed the relationship between statin prescription and AAA growth above median during the year following collection of medication history using similar methods. In the sub-group for which fasting lipids were measured we also assessed the association of LDL with AAA growth using Spearman’s correlation and logistic regression (adjusting for initial aortic diameter, CHD and diabetes). The latter analysis was performed separately for patients who were receiving statins and those not prescribed this medication. For the latter purpose LDL concentrations were converted to ≥ or < median. A p-value of less than 0.05 was considered statistically significant.
Funding
Grants from the National Institute of Health, USA [RO1 HL080010]; The Townsville Hospital Private Practice Fund; The National Heart Foundation and National Health and Medical Research Council [project grants 964145 and 540404] supported this work. JG and PEN hold Practitioner Fellowships from the National Health and Medical Research Council, Australia [431503, 458505].
Results
Characteristics of patients in relation to statin prescription
652 patients were included in this study. Statins were prescribed to 349 (54%) patients, which included simvastatin (47%), atorvastatin (35%), pravastatin (17%) or fluvastatin (1%). AAA diameter was followed by ultrasound surveillance for a median of 5 years (inter-quartile range, IQR 3-6). Patients underwent a median of 6 (IQR 5-7) scans. Median AAA growth for all patients was 1.1mm/year (IQR 0.5-2.0). Table I shows the characteristics of the patients in relation to statin prescription. Patients prescribed statins were significantly younger, more likely to be female (although this was still rare), and to have a range of other risk factors, including hypertension, CHD, PAD and history of smoking. The initial AAA diameter was slightly larger in statin users. Patients receiving statins had lower serum total cholesterol and LDL (where these were measured). Statin users were also more likely to be prescribed aspirin, beta-blockers and ACE inhibitors (Table I). The subset of male patients in which serum inflammatory markers were measured was younger and less likely to have hypertension, CHD, PAD or previous stroke than the total patient group. The initial AAA diameter was also lower in these patients (Table I). Serum CRP was lower in statin users (2.39 mg/L, IQR 1.13–5.00, n=118) than non-users (3.84 mg/L, IQR 1.86–7.52, n=98, p<0.001). Serum MMP-9 and IL-6 concentrations were similar in both groups (Table I).
Table I.
Characteristics of patients in relation to statin prescription and biomarker measurement
| Characteristic | Prescribed statin n=349 | Not prescribed statin n=303 | P value | Total cohort n=652 | Patients with lipid measurements n=451 | Patients with inflammatory markers n=216 |
|---|---|---|---|---|---|---|
| Age (years) | 72 [69–76] | 74 [71–78] | <0.001* | 73 [70–77] | 73 [69–77]‡ | 72 [69–75]§ |
| Males (%) | 319 (91) | 291 (96) | 0.025† | 610 (94) | 410 (91)§ | 216 (100)§ |
| Females (%) | 30 (9) | 12 (4) | 42 (6) | 41 (9)§ | 0 (0) | |
| DM (%) | 50 (14) | 36 (12) | 0.420† | 86 (13) | 63 (14) | 29 (13) |
| Hypertension (%) | 226 (65) | 163 (54) | 0.006† | 389 (60) | 270 (60) | 107 (50)§ |
| CHD (%) | 210 (60) | 93 (31) | <0.001† | 303 (46) | 213 (47) | 79 (37)§ |
| PAD (%) | 91 (26) | 41 (14) | <0.001† | 132 (20) | 109 (24)§ | 21 (10)§ |
| Family history of AAA (%) | 36 (10) | 25 (8) | 0.486† | 61 (9) | 53 (12)‡ | 19 (9) |
| Previous stroke (%) | 50 (14) | 39 (13) | 0.683† | 89 (14) | 59 (13) | 16 (7)‡ |
| Smoking history (%) | 310 (89) | 250 (83) | 0.028† | 560 (86) | 387 (86) | 185 (86) |
| Initial aortic diameter (mm) | 33.9 [31.3–37.6] | 33.0 [31.0–36.5] | 0.037* | 33.2 [31.0–37.0] | 33.4 [31.0–37.3] | 32.6 [31.0–35.1]§ |
| BMI (kg/m2) | 27.2 [25.0–29.9] | 26.6 [24.7–29.1] | 0.058* | 26.9 [24.7–29.4] | 27.1 [24.6–29.5] | 27.2 [25.1–29.4] |
| WHR | 0.97 [0.92–1.01] | 0.96 [0.93–1.00] | 0.336* | 0.96 [0.93–1.00] | 0.96 [0.92–1.00] | 0.96 [0.93–1.01] |
| Cardiovascular medications | ||||||
| ACE inhibitors | 150 (43) | 92 (30) | 0.001† | 242 (37) | 170 (38) | 77 (36) |
| Angiotensin-2 receptor blockers | 51 (15) | 39 (13) | 0.597† | 90 (14) | 77 (17)§ | 37 (17) |
| Aspirin | 226 (65) | 137 (45) | <0.001† | 363 (56) | 267 (59)‡ | 123 (57) |
| Beta-blockers | 123 (35) | 59 (19) | <0.001† | 182 (28) | 136 (30) | 52 (24) |
| Calcium channel blockers | 92 (26) | 63 (21) | 0.116† | 155 (24) | 102 (23) | 51 (24) |
| Statins | 349 (54) | 277 (61)§ | 118 (55) | |||
| Fasting lipids (mmol/L) | n=277 | n=174 | ||||
| Total cholesterol | 4.2 [3.7–4.8] | 5.2 [4.5–5.8] | <0.001* | 4.5 [3.9–5.3] | 4.5 [3.9–5.2] | 4.7 [4.0–5.3] |
| Triglycerides | 1.4 [1.0–2.1] | 1.3 [0.9–1.9] | 0.046* | 1.4 [0.9–2.0] | 1.4 [0.9–2.0] | 1.3 [0.9–1.8] |
| High density lipoprotein | 1.2 [1.1–1.5] | 1.2 [1.0–1.5] | 0.639* | 1.2 [1.0–1.5] | 1.2 [1.1–1.5] | 1.2 [1.0–1.5] |
| Low density lipoprotein | 2.2 [1.8–2.7] | 3.2 [2.7–3.7] | <0.001* | 2.5 [2.0–3.2] | 2.5 [2.0–3.2] | 2.7 [2.1–3.4] |
| Serum inflammatory markers | n=118 | n=98 | ||||
| CRP (mg/L) | 2.39 [1.13–5.00] | 3.84 [1.86–7.52] | <0.001* | 2.92 [1.44–5.53] | 2.90 [1.42–5.53] | 2.42 [1.30–4.98] |
| MMP-9 (pg/μL) | 729 [494–1015] | 724 [526–1052] | 0.853* | 725 [503–1049] | 727 [503–1050] | 725 [501–1051] |
| IL-6 (pg/mL) | 4.31 [<1.17–10.98] | 5.70 [<1.17–24.08] | 0.303* | 4.89 [<1.17–16.05] | 4.89 [<1.17–16.05] | 4.82 [<1.17–15.89] |
Continuous values are medians [interquartile range];
Mann Whitney-U test;
Chi-square test;
p value <0.05 and
p value <0.001 compared to total cohort.
Relationship between statin prescription and AAA growth
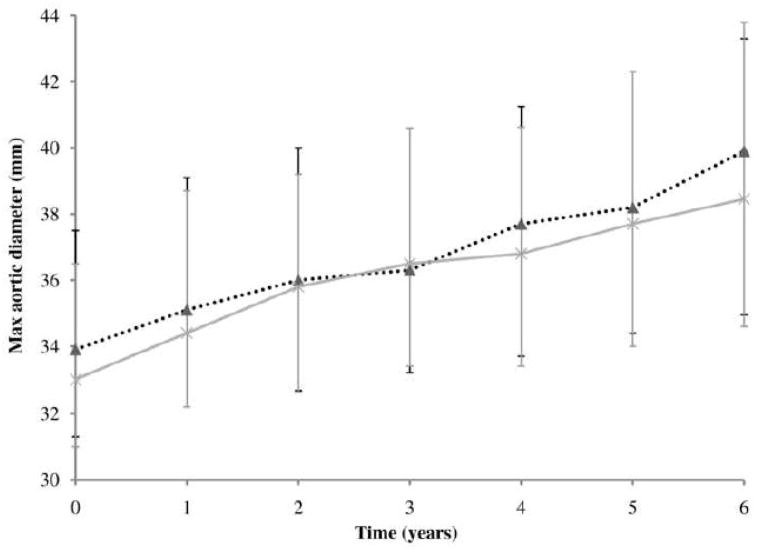
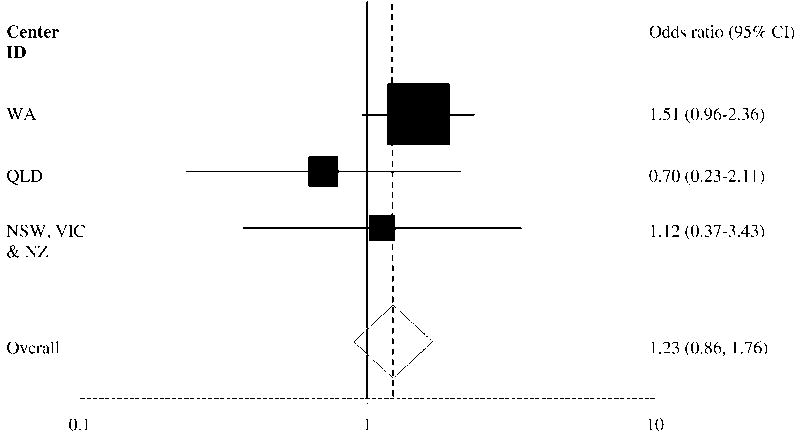
AAA growth rates were similar for patients who were and were not prescribed statins (Figure 1). Adjusting for other risk factors statin prescription was not associated with AAA growth (odds ratio, OR, 1.23, 95% confidence interval, CI, 0.86–1.76, Table II). There was no association with growth for any type of statin prescribed (simvastatin OR 1.22, 95% CI 0.72–2.05; atorvastatin OR 0.95, 95% CI 0.54–1.66; pravastatin OR 1.78, 95% CI 0.84–3.75) or when AAA growth was only considered within the year in which the statin prescription was recorded (statin use OR 1.04, 95% CI 0.73–1.49). Above median AAA annual growth was strongly associated with initial diameter (OR 1.78 per 4.35mm larger initial aortic diameter, 95% CI 1.49–2.14). Diabetes was negatively associated with above median growth (OR 0.37, 95% CI 0.22–0.62). The association of statin use with AAA growth was similar for all five centers (Figure 2).
Figure 1.
Median maximum infrarenal abdominal aortic diameter (mm) of patients treated with and without statins during six years of ultrasound surveillance. Vertical error bars represent interquartile range.
Table II.
Independent correlates of AAA growth
| 95% CI | ||||
|---|---|---|---|---|
| Variable | OR | Lower | Upper | P value |
| Age (per 5.55 years) | 1.10 | 0.93 | 1.30 | 0.278 |
| Female gender | 1.30 | 0.64 | 2.66 | 0.472 |
| DM | 0.37 | 0.22 | 0.62 | 0.000 |
| Hypertension | 0.92 | 0.64 | 1.31 | 0.638 |
| CHD | 0.67 | 0.46 | 0.97 | 0.033 |
| PAD | 0.96 | 0.62 | 1.48 | 0.849 |
| Smoking history | 0.75 | 0.47 | 1.20 | 0.230 |
| Initial aortic diameter (per 4.35mm) | 1.78 | 1.49 | 2.14 | 0.000 |
| ACE inhibitors | 0.91 | 0.64 | 1.31 | 0.621 |
| Aspirin | 1.10 | 0.78 | 1.56 | 0.575 |
| Beta-blockers | 1.13 | 0.76 | 1.67 | 0.554 |
| Statins | 1.23 | 0.86 | 1.76 | 0.263 |
Figure 2.
Association of statin prescription and AAA growth. Shown are adjusted odds ratios and 95% confidence intervals (CI) for different centers. AAA growth was defined as < or >= median. The New South Wales (NSW, n=30), Victoria (VIC, n=21) and New Zealand (NZ, n=34) cohorts were grouped since the cohorts were small. Western Australia (WA, n=458) and Queensland (QLD, n=109) cohorts were larger.
Relationship between serum lipids and AAA growth
451 patients (69%) had fasting serum lipids measured. Table I shows the characteristics of patients with fasting serum lipid measurements compared to the total cohort. Patients with lipid measurements were younger, more likely to be female, and to have PAD and a family history of AAA. This subset of patients was also more commonly prescribed a variety of medications (Table I). After adjustment for initial aortic diameter, CHD and diabetes, above median serum LDL was not significantly associated with AAA growth in patients prescribed statins (OR 1.64 95% CI, 0.99–2.70) or those not prescribed statins (OR 1.11 95% CI, 0.59–2.09). Serum LDL was not correlated with AAA growth rate in patients prescribed statins (r=0.072, p=0.229) or patients not prescribed statins (r =−0.012, p=0.873). There was also no correlation between total cholesterol and AAA growth rate in either group (patients prescribed statins, r =0.035, p=0.556; patients not prescribed statins r =−0.026, p=0.732).
Discussion
In this large observational study we found no relationship between statin prescription and AAA growth. We also found no association between serum LDL or total cholesterol and AAA growth. Our findings do not support those of some smaller studies which reported reduced AAA growth in statin users (Table III) including two studies that adjusted for other determinants of AAA growth [14, 16]. The current study includes more than 3 times the number of patients entered in previous investigations of the association between statin prescription and AAA growth (Table III). Our findings support those from a recent study of patients carried out in Tromso [24]. Forsdahl and colleagues carried out ultrasound imaging at recruitment and 7 years later in 4000 subjects and reported no reduction in the incidence of AAA development in individuals prescribed statins. The report suggests that statins do not limit the development of AAA. Statins were developed to lower serum LDL and have been demonstrated to effectively perform this in many studies [25]. Patients receiving this medication in the current study had lower serum LDL concentrations. We did not find any association between serum LDL and AAA growth, irrespective of whether patients were receiving statins. Thus our findings support other evidence suggesting that the mechanisms involved in AAA progression are distinct from those of atherosclerosis. We and others have for example previously demonstrated that diabetes is negatively associated with AAA progression [18, 26, 27], an association that was confirmed in the current study. Overall our findings suggest that targeting LDL is unlikely to be a successful strategy to reduce AAA progression although it will remain indicated in patients with current occlusive atherothrombosis, such as CHD.
Table III.
Cohort studies examining the association of statin treatment and AAA growth
| Growth rate (mm/year) | |||||
|---|---|---|---|---|---|
| Ref | n | Initial AAA diameter | Statin users | Non statin users | Risk factors adjusted for in analyses |
| [13] | 130 | 45 | −0.5 | 4 | No adjustment |
| [14] | 150 | 38 | AED −1.16 (95% CI, −1.99, −0.33) | Age, gender, initial AAA diameter, intermittent claudication, DM, COPD, NSAID prescription | |
| [15] | 121 | 39 | 1.9±1.8* | 2.6±2.4* | No adjustment |
| [16] | 147 | 41 (±8.0) | AED −1.2 (95% CI, −2.3, −.06) | Age, gender, initial AAA diameter, height, weight, DM, cerebral vascular disease, hypertension, creatinine clearance, hyperlipidaemia | |
| [17] | 213 | 40 (37–44) | 1.6 | 2.5 | No adjustment |
difference in AAA growth rate not statistically significant (p>0.05); AED, adjusted estimated difference for statin use; COPD, chronic obstructive pulmonary disease.
Evidence from some rodent models and biopsies of human AAA suggest that statins may decrease the expression of proteolytic enzymes within the aortic wall [28–32]. In the current study we found no evidence that circulating concentrations of MMP-9 were reduced in patients receiving statins. This finding is similar to that reported by other investigators [17, 33]. There are a number of possible explanations for these findings. It is possible that statins initially reduce the concentrations of proteolytic enzymes within the AAA however the on-going inflammatory response ultimately causes resistance to the effect. Alternatively the circulating concentrations of MMP-9 may not reflect those present within the aortic tissue [34]. The lack of effect of statins on circulating MMP-9 concentration does fit with our overall finding of no association between statin prescription and AAA expansion. We similarly found no significant reduction in circulating IL-6 concentrations in patients receiving statins, although this finding may have been different if larger numbers had been examined. This cytokine is likely to be released into the circulation from AAAs [35]. CRP concentrations were reduced in patients prescribed statins, in line with findings of other studies [36–39]. However, we have previously shown that CRP concentrations are not associated with AAA progression [20].
The current study has a number of limitations. Firstly we have used logistic regression to assess differences in AAA growth. It has been suggested that much more complex modeling of small AAA growth is required in order to predict accurately the growth of small AAAs [26]. We have previously assessed the growth pattern of a subset of our patients with small AAA and found that growth conformed to requirements of simpler statistical modeling [40]. The difference between our findings and that of Brady and colleagues likely reflects the small initial diameters of AAAs in our cohort. We currently do not have expertise required to carry out the complex modeling suggested by Brady and colleagues. Secondly the current study is not a randomized trial and thus we cannot make definitive conclusions on the effects of statins on AAA progression. Such a trial would now appear impossible to organize due to the increasing number of patients receiving this medication as part of other treatment targets. A recent observational study of patients with small AAAs reported a 40% incidence of statin users between 2002 and 2005 [14]. In the current cohort 68% of patients recruited over the last 2 years reported being statin users. These numbers are likely to increase with reports of reduced perioperative mortalities in patients prescribed statins. In the likely absence of a large statin trial we therefore believe our current study is an important association study and have adjusted our analyses for likely confounding factors. Thirdly we have not assessed patient compliance with prescribed medications in detail. The much lower serum LDL and cholesterol levels in the patients recorded as statin users are consistent with patients taking their medication as reported. Fourthly we only collected statin prescription on one occasion during ultrasound surveillance, thus it is possible that changes in statin prescription during follow-up confounded our analysis. In a sub-analysis in which we assessed AAA growth within the year in which the statin prescription was recorded we similarly found no relationship between statins and AAA progression.
Conclusion
Our findings suggest that treatment with statins and serum LDL levels are not associated with small AAA growth. We have confirmed the negative association of diabetes and AAA progression. Our findings support the need for on-going pre-clinical studies and randomized trials designed to develop medication targeted specifically at reducing AAA growth.
Acknowledgments
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Footnotes
Conflict of interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Begg S, Vos T, Barker B, et al. The burden of disease and injury in Australia 2003. Canberra: Australian Institute of Health and Welfare; 2007. [Google Scholar]
- 2.Minino AM, Heron MP, Murphy SL, et al. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55(19):1–119. [PubMed] [Google Scholar]
- 3.Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(3):203–11. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160(10):1425–30. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 6.United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–52. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 7.Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364(9437):843–8. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 8.Brewster DC, Cronenwett JL, Hallett JW, Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106–17. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 9.Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2002;35(1):72–9. doi: 10.1067/mva.2002.121308. [DOI] [PubMed] [Google Scholar]
- 10.Mosorin M, Juvonen J, Biancari F, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34(4):606–10. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 11.Vammen S, Lindholt JS, Ostergaard L, et al. Randomized double-blind controlled trial of roxi-thromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg. 2001;88(8):1066–72. doi: 10.1046/j.0007-1323.2001.01845.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindholt JS, Henneberg EW, Juul S, et al. Impaired results of a randomised double blinded clinical trial of propranolol versus placebo on the expansion rate of small abdominal aortic aneurysms. Int Angiol. 1999;18(1):52–7. [PubMed] [Google Scholar]
- 13.Sukhija R, Aronow WS, Sandhu R, et al. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am J Cardiol. 2006;97(2):279–80. doi: 10.1016/j.amjcard.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Schouten O, van Laanen JH, Boersma E, et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg. 2006;32(1):21–6. doi: 10.1016/j.ejvs.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Mosorin M, Niemela E, Heikkinen J, et al. The use of statins and fate of small abdominal aortic aneurysms. Interact Cardiovasc Thorac Surg. 2008 doi: 10.1510/icvts.2008.178103. [DOI] [PubMed] [Google Scholar]
- 16.Schlosser FJ, Tangelder MJ, Verhagen HJ, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47(6):1127–33. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson L, Bergqvist D, Lindback J, et al. Expansion of small-diameter abdominal aortic aneurysms is not reflected by the release of inflammatory mediators IL-6, MMP-9 and CRP in plasma. Eur J Vasc Endovasc Surg. 2009;37(4):420–4. doi: 10.1016/j.ejvs.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Golledge J, Karan M, Moran CS, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J. 2008;29(5):665–72. doi: 10.1093/eurheartj/ehm557. [DOI] [PubMed] [Google Scholar]
- 19.Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116(20):2275–9. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 20.Norman P, Spencer CA, Lawrence-Brown MM, et al. C-reactive protein levels and the expansion of screen-detected abdominal aortic aneurysms in men. Circulation. 2004;110(7):862–6. doi: 10.1161/01.CIR.0000138746.14425.00. [DOI] [PubMed] [Google Scholar]
- 21.Golledge J, Muller J, Shephard N, et al. Association between osteopontin and human abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2007;27(3):655–60. doi: 10.1161/01.ATV.0000255560.49503.4e. [DOI] [PubMed] [Google Scholar]
- 22.Golledge J, Jones L, Oliver L, et al. Folic acid, vitamin B12, MTHFR genotypes, and plasma homocysteine. Clin Chem. 2006;52(6):1205–6. doi: 10.1373/clinchem.2006.069849. [DOI] [PubMed] [Google Scholar]
- 23.Smallwood L, Allcock R, van Bockxmeer F, et al. Polymorphisms of the matrix metalloproteinase 9 gene and abdominal aortic aneurysm. Br J Surg. 2008;95(10):1239–44. doi: 10.1002/bjs.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsdahl SH, Singh K, Solberg S, et al. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119(16):2202–8. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 25.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326(7404):1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110(1):16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 27.Santilli SM, Littooy FN, Cambria RA, et al. Expansion rates and outcomes for the 3.0-cm to the 3.9-cm infrarenal abdominal aortic aneurysm. J Vasc Surg. 2002;35(4):666–71. doi: 10.1067/mva.2002.121572. [DOI] [PubMed] [Google Scholar]
- 28.Shiraya S, Miyake T, Aoki M, et al. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis. 2009;202(1):34–40. doi: 10.1016/j.atherosclerosis.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Kalyanasundaram A, Elmore JR, Manazer JR, et al. Simvastatin suppresses experimental aortic aneurysm expansion. J Vasc Surg. 2006;43(1):117–24. doi: 10.1016/j.jvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Abisi S, Burnand KG, Humphries J, et al. Effect of statins on proteolytic activity in the wall of abdominal aortic aneurysms. Br J Surg. 2008;95(3):333–7. doi: 10.1002/bjs.5989. [DOI] [PubMed] [Google Scholar]
- 31.Evans J, Powell JT, Schwalbe E, et al. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg. 2007;34(3):302–3. doi: 10.1016/j.ejvs.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Wilson WR, Evans J, Bell PR, et al. HMG-CoA reductase inhibitors (statins) decrease MMP-3 and MMP-9 concentrations in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2005;30(3):259–62. doi: 10.1016/j.ejvs.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Gottsater A, Flondell-Site D, Kolbel T, et al. Associations between statin treatment and markers of inflammation, vasoconstriction, and coagulation in patients with abdominal aortic aneurysm. Vasc Endovascular Surg. 2008;42(6):567–73. doi: 10.1177/1538574408320027. [DOI] [PubMed] [Google Scholar]
- 34.Wilson WR, Choke EC, Dawson J, et al. Plasma matrix metalloproteinase levels do not predict tissue levels in abdominal aortic aneurysms suitable for elective repair. Vascular. 2008;16(5):248–52. doi: 10.2310/6670.2008.00043. [DOI] [PubMed] [Google Scholar]
- 35.Dawson J, Cockerill G, Choke E, et al. Aortic aneurysms as a source of circulating interleukin-6. Ann N Y Acad Sci. 2006;1085:320–3. doi: 10.1196/annals.1383.009. [DOI] [PubMed] [Google Scholar]
- 36.Musial J, Undas A, Gajewski P, et al. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001;77(2–3):247–53. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 37.Riesen WF, Engler H, Risch M, et al. Short-term effects of atorvastatin on C-reactive protein. Eur Heart J. 2002;23(10):794–9. doi: 10.1053/euhj.2001.2967. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 40.Moran CS, Clancy P, Biros E, et al. Association of PPARgamma Allelic Variation, osteoprotegerin and abdominal aortic aneurysm. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03615.x. Epub 2009 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]