Abstract
Double balloon enteroscopy (DBE) is a new technique, first published and introduced into clinical practice in 2001 by Yamamoto, the inventor of this outstanding method. DBE allows complete visualization, biopsy and treatment of the small bowel. Nowadays, we have some experience of this method for evaluation of the complication rate. Severe complications are described in 1%-1.7% of patients. Acute pancreatitis is a rare complication of the investigation. The incidence of acute pancreatitis after diagnostic DBE is 0.3% in most studies. More than 50 cases of acute pancreatitis have been described in the literature so far. On the contrary, hyperamylasemia after DBE seems to be a rather common condition. Association with acute pancreatitis is supposed to be possible, but not obligatory. The causal mechanism of post-DBE acute pancreatitis is uncertain, and there are several theories in the literature. The most probable cause seems to be a mechanical straining of the endoscope with over-tube on the pancreas or in the papillary area.
Keywords: Double balloon endoscopy, Gastrointestinal endoscopy, Small intestine, Hyperamylasemia, Acute pancreatitis
INTRODUCTION
Double balloon endoscopy (DBE) is a method of enteroscopy that was introduced 8 years ago. Despite 8 years of experience, the complication rate is still under evaluation. Acute pancreatitis is the most feared complication in oral DBE. The cause of acute pancreatitis is uncertain. The aim of this paper is to provide an in-depth overview of possible risk factors for acute pancreatitis in DBE.
HISTORY
The small intestine was inaccessible to endoscopic methods for a long time. Too far from the mouth and the anus, it seemed to be unreachable for the endoscopist. The history of endoscopy investigation of the small bowel is quite short but accompanied by long-lasting skepticism.
In 1999, Mosse and Swain still stated in their work: “Enteroscopy remains the procedure in the gastrointestinal tract that is most inaccessible to endoscopy, and technical limitations severely impair the ability to advance and examine the small bowel reliably or completely”[1].
In 2000, Oates and Morris[2] published in their article: “It is now more than 25 years since small bowel enteroscopy was first described. For several reasons, this technique developed more slowly than other more usual forms of endoscopy. The small bowel disease is relatively rare in comparison with other gastrointestinal diseases. Also, there was lack of initial design agreement in different types of enteroscopes. Finally, commercial interests of the manufacturers of endoscopes were mainly focused on the more conventional, large volume markets. Problem areas remain, but with advancing technology and more professional interest in this area, these will be addressed during the next few years”[2].
Attempts to observe the entire gastrointestinal tract began even with early fibroscopes, but only two applicable methods were developed in addition to intra-operative enteroscopy: the ropeway method described by Deyhl et al[3] and Classen et al[4] in 1972 and the sonde endoscope described by Tada et al[5] in 1977. The first successful total enteroscopy was performed in March 1971 by Hiratsuka, using the ropeway method[6]. Both methods were soon abandoned due to the complexity of the technique, patient discomfort, and the long time needed to complete the procedure (and the high rate of complications of ropeway enteroscopy).
Push enteroscopy using a long endoscope was regarded as the gold standard then, but most of the small intestine remained beyond its reach. The first procedure was performed by Parker using a colonoscope in 1983[7]. Push enteroscopy can definitely not evaluate the non-resected small bowel in its entire length. Nowadays, push enteroscopy is reserved exclusively for investigation of the duodenum and oral end of the jejunum[8].
Recent innovations and introduction of two new methods (wireless capsule endoscopy and DBE) have made observation of the entire small intestine possible[6]. Both of these techniques are now available in clinical practice and are complementary: capsule endoscopy for screening and DBE for further diagnostics and/or therapy.
Capsule enteroscopy was invented by Swain and initial experiences in 4000 patients were published by Fritscher-Ravens and Swain in 2002[9]. The introduction of DBE for investigation of the small bowel in 2001 was a milestone in gastrointestinal endoscopy because it allows us to carry out therapeutic interventions as well as diagnostic procedures in the small bowel[10,11].
Double balloon (push-and-pull) enteroscopy (Fujinon, Inc., Saitama, Japan) represents an endoscopic method that enables us to investigate a substantial part or even the entire small bowel. The device was developed by Yamamoto and colleagues, introduced by him into clinical practice, and their first experiences were published in 2001[12]. Several subsequent studies[13-18] have suggested that this method is feasible for diagnostic and therapeutic purposes. Nevertheless, DBE is still under evaluation and its yield and safety aspects must be further determined[19-22].
Single balloon enteroscopy (SBE) is a modification of DBE, and is another system for small bowel enteroscopy (Olympus, Tokyo, Japan). The endoscope (XSIF-Q160Y) consists of a high-resolution enteroscope with a working length of 200 cm. The device is equipped with a transparent silicone overtube with a silicone balloon attached to its distal part. In contrast to the DBE device, there is no balloon attached to the enteroscope, and therefore stable position of the device must be maintained by hooking the distal tip of the enteroscope on the small-bowel wall[23].
Balloon-guided enteroscopy (BGE) is another modification of the DBE method. The advantage of this novel push-pull technique is that it is cheaper than other balloon-assisted methods (DBE and SBE). The device can be used with standard endoscopic equipment. The BGE device comprises a double balloon added onto a disposable element and an air supply unit (NavAid ASU; Smart Medical Systems, Ra’anana, Israel) to control the inflation and deflation of the balloons. The disposable BGE element is slipped over the tip of a standard endoscope and then fixed to the endoscope. At the tip of the endoscope is an inflatable stabilizing balloon. During diagnostic or therapeutic interventions through the endoscope’s instrument channel, the advancing catheter with its front balloon deflated can be pulled back and “parked” in its dedicated channel in the BGE device[24].
Spiral enteroscopy is a new technique for deep small-bowel intubation that uses a special overtube (Endo-Ease Discovery SB; Spirus Medical, Stoughton, MA, USA) to pleat the small bowel. Any type of enteroscope can be passed through the overtube, which has helical spirals at its distal end and rotates independently from the endoscope. The enteroscope can be locked in the overtube, which allows the option of spiral enteroscopy, or unlocked and advanced through the overtube[25,26]. Now, it is necessary to gain more data on its usefulness and safety and to compare this method with balloon-assisted enteroscopy[27].
Intra-operative enteroscopy is still a useful method for a specific group of patients (in case of failure of DBE, adhesions, multiple small transmural lesions unresolvable by endoscopic methods, such as carcinoids, and blue rubber bleb nevus syndrome), and therefore, it is necessary to be able to use this method in carefully considered cases[28-31].
PARTICULARITY OF DBE
As DBE is a lengthy procedure, a large volume of air is usually insufflated, which leads to significant distension of the small bowel (with the formation of distended bowel loops and acute angulations with increasing amounts of gases intraluminally). CO2, unlike air, is rapidly absorbed from the bowel. Preliminary data indicate that bowel insufflation with CO2, instead of air, enhances patient comfort and decreases the need for sedation[23,31]. We have used CO2 insufflation in DBE procedures regularly from 2007. We had no complications with hyperinflation, and the comfort of the patient rapidly increased. This type of insufflation is helpful for easier and deeper insertion of the endoscope, because the absorption of CO2 is 150 times faster than absorption of air in the bowel. Indeed, a recent randomized double-blind trial showed that insufflation with CO2 is safe, reduces patient discomfort, and significantly improves intubation depth[32].
Combination of water with simethicone is used routinely to do away with bubbles in the intestine. During withdrawal of the endoscope and during therapeutic interventions, spasmolytics might improve visualization of the small-bowel mucosa by reducing motility of the small bowel[23]. We administer intravenous crystalloids, mostly saline solution, to all patients during DBE lasting over 30 min. Conscious sedation is thought to be sufficient for DBE[23]. It seems to be much better in DBE in comparison with general anesthesia, according to our experience. Abdominal pain is an important warning signal, and it is necessary to terminate the procedure immediately in such cases. Intense pain may be a sign of inadequate pressure on the pancreas and poses a high risk of post-DBE pancreatitis[21,22]. We use small intravenous repetitive doses of midazolam and pentazocine for conscious sedation (batch-wise). The duration of the procedure and discomfort for the patient caused by oral passage of the overtube requires deep analgosedation. The procedure requires an experienced endoscopist and the possibility of fluoroscopy if needed, especially during the learning period[33].
COMPLICATIONS
DBE has been reported as a safe endoscopic technique. In initial series on DBE, no complications during or after the procedure were reported[34-37]. Recently, the overall complication rate is stated as being about 1.7%[38,39].
A complication of endoscopy is defined as any event that negatively changes the health status of the patient, and that occurs during the 30-d period after the investigation. Complications are usually categorized as minor when requiring up to 3 d of hospitalization, moderate when requiring 3-10 d and major or severe when requiring > 10 d, and/or an endoscopic, radiological or surgical intervention, and/or contribute to the death of the patient[38,40]. Procedure-related mortality is defined as mortality within 30 d of DBE[38]. Complications are divided into two main categories, those directly attributed to the procedure and those secondary to anesthesia or conscious sedation[23]. The most common complications secondary to anesthesia or conscious sedation are respiratory depression, aspiration, and pneumonia, with a frequency of < 1%[23].
Until now, no standards or definitions for complications during or after DBE have been established. Potential complications during or after DBE might be: perforation, bleeding, balloon dislocation, sedation-related, segmental enteritis after argon plasma coagulation[41], intestinal necrosis after epinephrine injection[42], paralytic intestinal ileus[43], and acute parotitis[44].
Recently, post-DBE pancreatitis has been recognized as a complication[39,45]. In diagnostic procedures via the anterograde approach, pancreatitis is the most common and most severe complication[23]. The very first post-DBE acute pancreatitis was reported by Honda et al[46] in 2006.
An international symposium held in Atlanta, GA, USA in 1992 has established a clinically based classification system for acute pancreatitis[47,48]. The goal was to establish international standards for definition of acute pancreatitis and its complications, to facilitate valid comparisons of severity of illness and results of therapy, and also to establish criteria for patient selection in randomized prospective trials. According to the Atlanta symposium, acute pancreatitis is defined as an acute inflammatory process of the pancreas that may also involve peripancreatic tissues and/or remote organ systems. Mild acute pancreatitis is defined as pancreatitis associated with minimal organ dysfunction and uneventful recovery. Severe pancreatitis is defined as pancreatitis associated with organ failure and/or local complications (necrosis, abscess, or pseudocyst). Criteria for severity included organ failure (particularly shock, pulmonary insufficiency, and renal failure) and/or local complications (especially pancreatic necrosis but also including abscess and pseudocyst). Early predictors of severity within 48 h of initial hospitalization included Ranson signs and APACHE II (Acute Physiology and Chronic Health Evaluation II) points[47-49].
In the Atlanta symposium, a uniform threshold was not established for serum amylase and/or lipase for the diagnosis of acute pancreatitis. In recently published articles, the threshold varies from ≥ 2 to ≥ 4 times the upper limit of normal. Criteria for severe pancreatitis include organ failure and/or local complications. This broad definition describes a heterogeneous group of patients with varying levels of severity. For example, the prognosis of pancreatic necrosis is more serious than a pseudocyst or pancreatic abscess. Also, almost all patients with necrotizing pancreatitis without organ failure survive, whereas those with multisystem organ failure do not[49].
Bollen et al[50] have revised the Atlanta symposium in their review. The authors propose the following recommendations for revision of the classification of acute pancreatitis. (1) The diagnosis should incorporate two of the following three items: upper abdominal pain, amylase and/or lipase levels ≥ 3 times the upper limit of normal (as this cut-off is used most frequently in the literature), and computed tomography (CT) or magnetic resonance imaging findings compatible with acute pancreatitis; (2) Persistent organ failure (for at least 48 h) should have an important role in defining the severity of acute pancreatitis; and (3) Decisions should be made as to which predictive scoring system, including cut-off value, should be used to define predicted severe acute pancreatitis, based on a systematic review of the available data.
Progress in the field of acute pancreatitis is hampered greatly when various author groups use their own idiosyncratic definitions[50].
According to the literature on post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis [American Society for Gastrointestinal Endoscopy (ASGE) guidelines], post-DBE pancreatitis is defined as newly developed or worsened abdominal pain after the procedure, with a serum amylase ≥ 3 times the upper limit of normal as the upper limit 24 h after the procedure and requiring at least 2 d of unplanned hospitalization after the procedure[51]. According to these guidelines, the severity of the disease has been classified as follows: mild, requiring 2-3 d hospitalization; moderate, 4-10 d hospitalization; and severe, > 10 d hospitalization, and/or the occurrence of pseudocyst and/or the need for surgery[52]. The duration of pain after the procedure is crucial for defining post-endoscopy pancreatitis[53].
We found the definition from UpToDate 2009 to be fundamental. Acute pancreatitis is an acute inflammatory process of the pancreas. It is usually associated with severe acute upper abdominal pain and elevated blood levels of pancreatic enzymes. Acute pancreatitis can be suspected clinically, but requires biochemical, radiologic, and sometimes histological evidence to confirm the diagnosis. Clinical, biochemical, and radiologic features need to be considered together since none of them alone is diagnostic of acute pancreatitis. Acute pancreatitis is an important cause of acute upper abdominal pain. Because its clinical features are similar to a number of other acute illnesses, it is difficult to base a diagnosis only on symptoms and signs. The disease varies in severity and the diagnosis is often missed at the extreme ends of the spectrum[54].
It is not usually difficult to recognize severe pancreatitis. The mild form may pose a problem. Abdominal pain and hyperamylasemia after DBE need not mean pancreatitis. On the other hand, a lot of mild pancreatitis could be missed in patients with DBE performed on an outpatient basis.
The complication rate of diagnostic procedures is low (0.4%-0.8%) according to the literature[10,11,38,45]. The overall complication rate of therapeutic DBE is 3%-4%. However, difficult therapeutic endoscopic procedures (e.g. resection of large polyps) may increase the risk to 10%[10,11,23,38]. The perforation rate is significantly elevated in patients with postsurgical anatomical changes undergoing diagnostic retrograde DBE examinations[55].
The overall complication rate was reported to be about 1.7% in a recent international multicenter survey of 2362 DBE procedures. The complications were rated minor in 0.9%, moderate in 0.3% and severe in 0.6% of procedures. The complication rate is significantly higher in therapeutic procedures in comparison with diagnostic ones (4.3% vs 0.8%). An exception to the rule is acute pancreatitis, the most common complication in diagnostic DBE procedures. Acute pancreatitis was reported in 0.3% of DBEs[38,39].
A recent report from the National German DBE Register showed an overall complication rate of 1.2% in a large series of 3894 DBE procedures. The incidence of acute pancreatitis was also 0.3% in that study[11].
A study by May et al[41] evaluated the acute complication rate of DBE in 353 patients. Only therapeutic procedures were evaluated, with a complication rate of 3.4%. No acute pancreatitis was reported[41].
In general, DBE is associated with a higher complication rate compared with standard endoscopic procedures.
OUR OWN EXPERIENCE
We began with DBE in our department in March 2006. All of our investigations were performed using EN-450T5 and EN-450P5 endoscopes (Fujinon, Inc., Saitama, Japan). Being aware of the possibility of acute pancreatitis as a complication of DBE, we started with our prospective study on hyperamylasemia after DBE right from the start of DBE in 2006. This prospective study is still continuing. All patients were admitted to our department and followed prospectively for at least 2 d after oral DBE.
Urine and serum amylase, serum pancreatic lipase and C-reactive protein (CRP) were investigated before and 4 h and 24 h after DBE. Abdominal pain was evaluated using a three-step scale. Hyperamylasemia (exceeding the upper normal limit) and marked hyperamylasemia (reaching ≥ 3 times the upper limit of normal) were distinguished.
Normal ranges of the following values in our laboratory were: serum amylase 0.44-1.67 μkat/L (i.e. 28-100 U/L); urine amylase 0-7.67 μkat/L (i.e. 0-460 U/L); serum lipase 0.22-1 μkat/L (i.e. 13-60 U/L); and serum CRP 0-5 mg/L.
Acute pancreatitis was diagnosed in accordance with clinical signs (abdominal pain, fluid sequestration and lack of peristalsis), CRP level and CT scan in addition to the above.
Risk factors for acute pancreatitis and the importance of hyperamylasemia associated with DBE have not been satisfactorily resolved yet. The aim of our prospective study was to clarify the relationship between oral DBE and amylasemia and lipasemia, and to address the possible role of the learning curve for DBE for risk of acute pancreatitis.
A total of 138 DBEs were carried out from March 2006 to November 2009 in 60 men and 56 women under deep conscious sedation (midazolam and pentazocine). The mean time of DBE was 110 min (range 12-270 min), and the mean number of push-and-pull cycles was 15 (range 1-47).
Amylase was set after 128 DBEs; elevation was found in 61 (48%), and marked hyperamylasemia in 27/128 (21%). Abdominal pain was recorded in 19/96 (20%). Elevated lipase levels were found in 55/94 (59%); including 38/94 (40%) with ≥ 3 times the upper limit of normal. We observed elevation of CRP after DBE in only 18/100 (18%). Peak values of serum amylase and lipase levels were found 4 h after DBE, and peak values of CRP at 24 h after the procedure.
Total panenteroscopy (i.e. investigation of the entire small intestine by one oral approach in one session) was accomplished in 12 DBEs (9%). In this subgroup, we found only four patients with hyperamylasemia and two with marked hyperamylasemia. We had no complications in this subgroup. Compared to the total number of patients, in this subgroup, there was a younger mean age and longer duration of DBE (mean 148 min vs 106 min; P = 0.010), but there was no acute pancreatitis and no significant difference in amylase and lipase and/or abdominal pain.
In all DBEs, we did not identify any risk factor for abdominal pain and/or elevated pancreatic amylases (sex, age, previous abdominal surgery, panenteroscopy, indication or endoscopic finding, type of endoscope, number of push-and-pull procedures, diagnostic or therapeutic procedure).
Three patients (2.1%) developed acute pancreatitis after DBE, one necrotizing and two edematous. Two had inflammatory affection of the pancreatic tail, the third of the head region. All of them had abdominal tenderness during the procedure.
Subsequently, we divided our patients into two groups (the first 69 and second 69 procedures) to assess possible influence of the learning curve. Diagnostic and therapeutic DBEs (using Fujinon EN 450P5 or 450T5) were proportionally included in both groups. Neither group differed in age, sex, diagnoses or previous surgery. Differences in amylase and lipase (4 or 24 h after minus basal values before DBE) were used as a major indicator.
The difference in abdominal pain, DBE duration, amylase or lipase was not significant in either of the two groups. Marked hyperamylasemia (≥ 3 times above the upper limit; in 21% after DBE) was not associated with marked pain. There was a weak but significant correlation between amylase difference and abdominal pain (P = 0.003), the number of push-and-pull cycles (P = 0.018), and negative correlation with age (P = 0.029). Lipase difference 4 h after DBE weakly correlated with abdominal pain (P = 0.034). Our three cases of acute pancreatitis were numbers 24, 50 and 57 of 138 DBE procedures, without any evidence of further consequences. DBE lasted 12-270 min (mean: 110 min). Shorter (≤ 120 min, 64%) and longer (> 120 min; 50/138, 36%) DBEs did not differ in terms of post-DBE abdominal pain (P = 0.784). There was a borderline significant difference between longer and shorter DBEs for amylase (P = 0.047) but not lipase (P = 0.225) differences 4 h after DBE.
Elevation of amylase and lipase is often associated with DBE, but acute pancreatitis is however a rare complication. Duration of DBE is not a risk factor for post-DBE acute pancreatitis. Abdominal pain during DBE should be considered as a possible risk factor for acute pancreatitis. That is why we prefer conscious sedation to general anesthesia in oral DBE. The initial learning curve for DBE is not associated with higher amylase or lipase in our setting, and it does not signify a risk factor for post-DBE acute pancreatitis[21,22,56]. The most important point of our study was that it was prospective, solely on an inpatient basis, and all consecutive patients who underwent oral DBE were included.
CLINICAL OUTCOMES
Our center has long-term experience (since 1994) with push-enteroscopy[57] and intraoperative enteroscopy[28-31]. We have never registered acute pancreatitis as a complication of either push-enteroscopy or intraoperative enteroscopy in our setting. However, acute pancreatitis as a complication of push-enteroscopy, caused by an overtube, has been described previously by other authors[58]. Acute pancreatitis has even been described after uneventful upper and lower gastrointestinal endoscopy[59-61]. Blackwood et al[62] have detected asymptomatic hyperamylasuria in 6.6% of patients undergoing gastrointestinal endoscopy[62].
Pelletier et al[63] have studied the prevalence of hyperamylasemia 2 and 24 h after upper gastrointestinal endoscopy in 50 consecutive patients. In the 2-h sample, hyperamylasemia was observed in nine patients (18%), and in the 24-h sample, in five patients. Pelletier et al[63] have concluded that the cause of hyperamylasemia may be due to hypersalivation during the procedure. In our opinion, hypersalivation cannot affect the serum amylase level in such a way (most of the saliva runs out of the mouth during endoscopy and is not swallowed). Furthermore, it cannot affect abdominal pain or pancreatic lipase elevation[21,22,56].
DBE was initially described as a safe procedure[6], with the rate of severe complications ranging from 0 to 1.4%[14,18,64]. However, abdominal pain lasting 1-2 d occurred in 9% of patients in one study[13], or even in 20% according to another[14]. Abdominal discomfort reducing within 72 h was reported in three out of six patients after a DBE procedure[65].
There have been 51 published cases of post-DBE acute pancreatitis on PubMed to date.
Eisen and Schreiner[45] have presented a study of 275 consecutive patients who underwent DBE at two tertiary referral hospitals. The most common complication of DBE was abdominal pain which was seen in 20% of cases. This was typically self-limiting, yet a systematic analysis was not performed. Three cases of pancreatitis occurred (1%), two of which were mild and one of which was of intermediate severity[45]. It is hard to say how many patients with self-limited abdominal post-DBE pain had mild acute pancreatitis, because of inadequate follow-up.
Honda et al[46,66] described one and Groenen et al[67] two (one necrotizing) cases of acute pancreatitis after DBE. Heine et al[14] and Jarbandhan et al[52] from the same Dutch group have studied 603 DBE procedures (441 oral DBEs) on an outpatient basis, with six cases of post-DBE acute pancreatitis (1% of all DBEs, i.e. 1.4% of oral DBEs); all cases of pancreatitis were diagnosed after oral procedures. None of the cases of pancreatitis were in the head of the pancreas[52]. In a retrospective analysis of 378 DBEs by Zhong et al[68], two patients (0.7%) suffered from abdominal pain with an unspecified elevation of serum amylase. Unfortunately, it was not stated if they required hospitalization[68]. Möschler et al[10,11], in a German multicenter retrospective study, have reported 3894 DBEs with an overall complication rate of 1.2%. They quoted nine cases of acute pancreatitis with one lethal disease course after oral DBE, with a complication rate of 0.34%[10,11]. A retrospective study by Gerson et al[55] in nine United States centers collected data from 2478 DBEs, with a total of 0.9% major complications (22 DBEs), among which there were six cases of acute pancreatitis (0.2%). Surprisingly, one case of acute pancreatitis was reported after an anal DBE procedure[55]. Another multicenter study by Mensink et al[38] investigated 2362 DBEs. The majority of these (87%) were performed on an inpatient basis. The overall complication rate was 1.7%; six patients suffered from acute pancreatitis (0.3%). The location of the pancreatitis was the body and/or the tail of the pancreas in four patients, the entire pancreas in two, and the head of the pancreas in one[38]. One case of post-DBE acute pancreatitis that affected the pancreatic tail has been reported at the Mayo Clinic (Rochester, MN, USA)[69], along with another case from Osaka, Japan[70], and Rotterdam (one case of pancreatitis of unknown location from 135 patients, 0.7%)[71]. In a recent study by Sunada and Yamamoto, four cases of acute pancreatitis in 1092 DBEs (0.37%) were diagnosed, although the severity and location were not mentioned[72].
In agreement with the Dutch study, we believe that post-DBE pancreatitis is underestimated in retrospective studies on an outpatient basis. In particular, retrospective questionnaire-based surveys might be at risk from an inaccurate report or inclusion bias[52]. As the distinction between clinically mild pancreatitis and hyperamylasemia with transient abdominal discomfort is somewhat arbitrary, it seems likely that an under-diagnosis of post-DBE pancreatitis might have occurred, especially in outpatients.
There was no association with sex, duration of the procedure, or type of endoscope in most of the studies mentioned above.
Surprising results have been reported by Pata et al[73] in their study of 48 oral DBE procedures. Pancreatitis was observed in six patients (12.5%). Acute pancreatitis was diagnosed when amylase and/or lipase reached ≥ 3 the upper limit of normal in the presence of pancreatic-type abdominal pain[73]. The question is whether the diagnostic criteria of acute pancreatitis are sufficient in the case of post-DBE pancreatitis. Asymptomatic hyperamylasemia may occur in nearly half of DBE procedures[21,22,27,56,66,74]. Honda et al[66] have investigated 13 patients who underwent DBE. Hyperamylasemia occurred in six of them after the procedure, and one of these developed acute pancreatitis. In agreement with our results, this study demonstrates that latent hyperamylasemia without the development of pancreatitis occurs after peroral DBE more frequently than was previously thought[66]. It may be that some other criteria for the diagnosis of post-DBE acute pancreatitis should be added: ultrasound and/or CT scanning, peristalsis weakening, or compartment sequestration syndrome. Time will tell if the significance of post-DBE and post-ERCP enzyme elevation is absolutely comparable. Pata et al[73] have verified only two reported cases of pancreatitis using CT.
The causal mechanism of post-DBE acute pancreatitis is uncertain, and there are several hypotheses. Nevins assumes local trauma to the pancreas during the procedure or release of as-yet-undefined inflammatory mediators[59]. May et al[75] assume that the length of the examination time is an important factor, because they did not observe pancreatitis in more than 500 DBEs and had a strict maximum examination time limit of 2 h[75]. However, data from other authors (including our own data) do not support this assumption. In our study, in agreement with other studies[66,70], we found hyperamylasemia and pancreatitis in patients with shorter procedures, and we had fewer patients with hyperamylasemia and no case of pancreatitis in the subgroup of 12 patients with panenteroscopy via an oral approach (mean time: 148 min)[21,22,56]. We therefore believe, like Honda et al[76], that it is the technique of DBE itself, with the shortening of the small bowel, that may be a factor in pancreatitis after peroral DBE, rather than the length of the examination time (Figure 1)[21,22,56].
Figure 1.
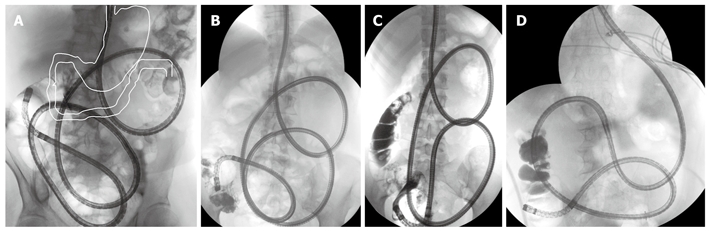
DBE, panenteroscopy via oral approach (plain radiograph) in supine position. In comparison with the normal situation (A, white outline), the duodenum is shifted markedly to the left, straightened and shortened. A-C: Normal anatomy; D: After BII resection.
Honda et al[66] think that, in peroral DBE, the duodenum and proximal jejunum are markedly shortened, and the duodenum is sometimes found to be nearly straight from the pyloric ring to the ligament of Treitz on fluoroscopy. In these conditions, the pancreas body and/or tail may be subject to severe strain, with traumatic injury and/or ischemia. Intraluminal pressure in the duodenum may increase in such a way as to disturb the secretion of pancreatic juice. This mechanism might be associated with the occurrence of hyperamylasemia and pancreatitis after peroral DBE[66]. This hypothesis is supported by the finding that, in most cases in which early CT was performed, pancreatitis was located in the tail or body-tail region of the pancreas[52].
Heine et al[14] state that pancreatitis probably results from prolonged mechanical stress on the organ due to repeated stretching by the endoscope[14]. Sunada and Yamamoto suggest that the possible mechanism is mechanical torsion of the pancreatic body during insertion of the endoscope. Therefore, for oral insertion, extreme shortening should not be performed, and counter-clockwise rotation is preferred[72].
Another possibility is ischemia of the pancreas, which could induce acute pancreatitis. This has been confirmed in an animal model[77].
Groenen et al[67] hypothesize that acute pancreatitis is caused by an increase in intraluminal duodenal pressure during the endoscopic procedure caused by inflation of the two balloons, which leads to reflux of duodenal fluids into the pancreatic duct[67]. A closed duodenal loop has indeed become an established animal model for acute pancreatitis[78,79]. However, the question is whether the duodenal intraluminal pressure during DBE really can reach such a high level. On the other hand, the pancreatitis caused by reflux should affect the whole pancreas, with diffuse swelling, not only in the body or pancreatic tail.
Some endoscopists prefer to inflate the balloons after passing the ligament of Treitz[52,71], or after two insertions (being at least 50 cm distal from the papilla of Vater)[39]. Unfortunately, precise description of this technique and its control (by fluoroscopy?) was not incorporated in their work. Frankly, it is difficult to believe that by pushing the endoscope with the overtube, without pulling back and straightening out the looping, they were able to pass the ligament with a 129-cm long device (the balloon on the tip is 7 cm long and the rest of working part of the overtube is 129 cm). On the other hand, the papilla is about 80-90 cm from the incisors when inserting the endoscope, therefore, both balloons are probably beyond the papilla of Vater. However, even with this safe technique of endoscope insertion, the Dutch group had a post-DBE acute pancreatitis rate of 1.4%, using the oral approach[52].
Other possible causes are direct trauma to the ampullary area or a direct obstruction of the pancreatic duct by the insufflated balloon[67]. In our opinion, this hypothesis seems also to be unlikely.
Another consideration is the question of whether the elevation of amylase is always a result of pancreatitis or whether it could be of intestinal origin and related to manipulation of the gut[80,81]. However, this mechanism could hardly explain the elevation of pancreatic lipase.
The method of insertion and withdrawal of the endoscope might be a factor in the origin of post-DBE pancreatitis. However, in accordance with the literature[52], we did not find any differences in technique between our center and others that are performing DBE in Europe, and we did not see any differences even observing the masterful work of Professor Yamamoto during his live workshops at our endoscopy unit.
Differences in definitions of post-procedural complications offer a likely explanation for the difference in reported post-DBE pancreatitis[52].
In our opinion, mechanical stress on the pancreas seems possible, and the increased level of pancreatic lipase could be a correlate of this. We considered a possible influence of our learning curve on the incidence of hyperamylasemia in our patients, but we did not confirm this by subsequent analysis. The second point of our reflection was the type of endoscope. We used the thicker and stiffer EN-450T5 endoscope, so we were afraid of more forceful pressing of this device onto the pancreas. However, three cases of acute pancreatitis after peroral DBE using the EN-450P5 have been reported in the Netherlands[14] and one after DBE using the EN-450T5[66], and there were no differences in the type of endoscope in large studies[38].
Another question is prevention of post-DBE pancreatitis. We use parenteral hydration during after the oral procedure. The usual dose is 1 L of saline solution during a 2-h procedure. We believe that hydration could improve blood supply to the splanchnic region, pancreatic microcirculation, and post-procedure recovery. The use of proteinase inhibitors such as gabexate mesylate for the prevention of post-endoscopic pancreatitis has been disappointing[53,82]. There have been some studies of intravenous nitroglycerin[83], ulinastatin[84], somatostatin[53,82,85,86], rectal diclofenac[84], and other drugs for the prevention of post-procedure pancreatitis, but the results have not been significant.
CONCLUSION
Acute pancreatitis is a feared complication of oral DBE (51 cases of acute pancreatitis have been described in the literature to date, one of them fatal). Acute pancreatitis is the most common complication seen after diagnostic oral DBE (complications of therapy itself prevail in therapeutic procedures). Hyperamylasemia and elevation of pancreatic lipase after DBE seems to be a common occurrence. Association with acute pancreatitis is possible, but not obligatory. The diagnosis of acute pancreatitis is complex. It can be suspected clinically, but requires biochemical, radiological, and sometimes histological evidence to confirm the diagnosis. The complication rate of acute pancreatitis is reported at about 0.3% of DBEs according to large studies, almost solely after oral DBE. Drawbacks and possible bias of those studies are that they were mostly retrospective, a substantial number of DBEs were performed on an outpatient basis, and the follow-up was inadequate. Nowadays, it is clear that the oral DBE procedure is of higher risk in comparison with the anal one. It would be more precise to count the pancreatitis risk from oral procedures separately. By including anal procedures in determination of post-DBE pancreatitis rate, we obtain much lower and biased numbers. The presumable number of cases of acute pancreatitis after oral DBE is 1.5%-2%. In all patients with abdominal pain during the procedure and/or after oral DBE, diagnosis of acute pancreatitis should be considered and treatment should be provided in good time, the same as in post-ERCP pancreatitis. From the results of our study, we established the following rules in our clinical practice. Conscious sedation seems to be more favorable than general anesthesia due to monitoring of the patient’s pain during the procedure. Intense pain during the procedure may be a sign of inadequate pressure on the pancreas and pose a high risk of post-DBE pancreatitis. CO2 insufflation during DBE is highly recommended as it prevents over-inflation of the small bowel, however, a possible preventive relationship to post-DBE pancreatitis has not been determined yet.
Footnotes
Supported by The Research Project: MZO 00179906 from the Ministry of Health, Czech Republic
Peer reviewer: Michel M Murr, MD, Professor of Surgery, USF Health, Director of Bariatric Surgery, Tampa General Hospital, 1 Tampa General Circle, Tampa, FL 33647, United States
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
References
- 1.Mosse CA, Swain CP. Technical advances and experimental devices for enteroscopy. Gastrointest Endosc Clin N Am. 1999;9:145–161. [PubMed] [Google Scholar]
- 2.Oates BC, Morris AI. Enteroscopy. Curr Opin Gastroenterol. 2000;16:121–125. doi: 10.1097/00001574-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Deyhl P, Jenny S, Fumagalli J. Endoscopy of the whole small intestine. Endoscopy. 1972;4:155–157. [Google Scholar]
- 4.Classen M, Fruhmorgan P, Kock H. Peroral enteroscopy of the small and large intestine. Endoscopy. 1972;4:157–162. [Google Scholar]
- 5.Tada M, Akasaka Y, Misaki F, Kwaie K. Clinical evaluation of a sonde-type small intestinal fiberscope. Endoscopy. 1977;9:33–38. doi: 10.1055/s-0028-1098483. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Kita H. Enteroscopy. J Gastroenterol. 2005;40:555–562. doi: 10.1007/s00535-005-1645-5. [DOI] [PubMed] [Google Scholar]
- 7.Parker HW, Agayoff JD. Enteroscopy and small bowel biopsy utilizing a peroral colonoscope. Gastrointest Endosc. 1983;29:139–140. doi: 10.1016/s0016-5107(83)72558-7. [DOI] [PubMed] [Google Scholar]
- 8.Pohl J, Delvaux M, Ell C, Gay G, May A, Mulder CJ, Pennazio M, Perez-Cuadrado E, Vilmann P. European Society of Gastrointestinal Endoscopy (ESGE) Guidelines: flexible enteroscopy for diagnosis and treatment of small-bowel diseases. Endoscopy. 2008;40:609–618. doi: 10.1055/s-2008-1077371. [DOI] [PubMed] [Google Scholar]
- 9.Fritscher-Ravens A, Swain CP. The wireless capsule: new light in the darkness. Dig Dis. 2002;20:127–133. doi: 10.1159/000067484. [DOI] [PubMed] [Google Scholar]
- 10.Möschler O, May AD, Müller MK, Ell C. [Complications in double-balloon-enteroscopy: results of the German DBE register] Z Gastroenterol. 2008;46:266–270. doi: 10.1055/s-2007-963719. [DOI] [PubMed] [Google Scholar]
- 11.Möschler O, May AD, Müller MK, Ell C, DBE-Studiengruppe Deutschland. Complicatons and more: Results of the German prospectiove DBE-database by the German DBE Study Group. Gastrointest Endosc. 2008;67:AB262. [Google Scholar]
- 12.Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 13.Ell C, May A, Nachbar L, Cellier C, Landi B, di Caro S, Gasbarrini A. Push-and-pull enteroscopy in the small bowel using the double-balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613–616. doi: 10.1055/s-2005-870126. [DOI] [PubMed] [Google Scholar]
- 14.Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Sugano K. A new method of enteroscopy--the double-balloon method. Can J Gastroenterol. 2003;17:273–274. doi: 10.1155/2003/309532. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K. New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders. Gastroenterology. 2003;125:1556; author reply 1556–1557. doi: 10.1016/j.gastro.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Yano T, Yamamoto H. Current state of double balloon endoscopy: the latest approach to small intestinal diseases. J Gastroenterol Hepatol. 2009;24:185–192. doi: 10.1111/j.1440-1746.2008.05773.x. [DOI] [PubMed] [Google Scholar]
- 18.Mönkemüller K, Weigt J, Treiber G, Kolfenbach S, Kahl S, Röcken C, Ebert M, Fry LC, Malfertheiner P. Diagnostic and therapeutic impact of double-balloon enteroscopy. Endoscopy. 2006;38:67–72. doi: 10.1055/s-2005-921190. [DOI] [PubMed] [Google Scholar]
- 19.Cave D. Wireless video capsule endoscopy. UpToDate on line, 18.1. Wellesley. 2010. Available from: http://www.uptodate.com. [Google Scholar]
- 20.Travis AC, Saltzman JR. Evaluation of occult gastrointestinal bleeding. UpToDate on line, 18.1. Wellesley. 2010. Available from: http://www.uptodate.com. [Google Scholar]
- 21.Kopácová M, Rejchrt S, Tachecí I, Bures J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133–1138. doi: 10.1016/j.gie.2007.03.1085. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita M, Shimatani M, Uchida K, Okazaki K. Association of hyperamylasemia and longer duration of peroral double-balloon enteroscopy: present and future. Gastrointest Endosc. 2008;68:811; author reply 811–811; author reply 812. doi: 10.1016/j.gie.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 23.Pohl J, Blancas JM, Cave D, Choi KY, Delvaux M, Ell C, Gay G, Jacobs MA, Marcon N, Matsui T, et al. Consensus report of the 2nd International Conference on double balloon endoscopy. Endoscopy. 2008;40:156–160. doi: 10.1055/s-2007-966994. [DOI] [PubMed] [Google Scholar]
- 24.Adler SN, Bjarnason I, Metzger YC. New balloon-guided technique for deep small-intestine endoscopy using standard endoscopes. Endoscopy. 2008;40:502–505. doi: 10.1055/s-2007-995677. [DOI] [PubMed] [Google Scholar]
- 25.Akerman PA, Agrawal D, Cantero D, Pangtay J. Spiral enteroscopy with the new DSB overtube: a novel technique for deep peroral small-bowel intubation. Endoscopy. 2008;40:974–978. doi: 10.1055/s-0028-1103402. [DOI] [PubMed] [Google Scholar]
- 26.Akerman PA, Agrawal D, Chen W, Cantero D, Avila J, Pangtay J. Spiral enteroscopy: a novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest Endosc. 2009;69:327–332. doi: 10.1016/j.gie.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Mönkemüller K, Olano C, Fry LC, Ulbricht LJ. Small-bowel endoscopy. Endoscopy. 2009;41:872–877. doi: 10.1055/s-0029-1215139. [DOI] [PubMed] [Google Scholar]
- 28.Kopácová M, Bures J, Rejchrt S, Siroký M, Bedrna J, Ferko A, Hajzman Z, Hladík P, Holecek T, Hroch T, et al. [Intraoperative enteroscopy--personal experience from 1995 to 2002] Cas Lek Cesk. 2003;142:303–306. [PubMed] [Google Scholar]
- 29.Kopácová M, Bures J, Vykouril L, Hladík P, Simkovic D, Jon B, Ferko A, Tachecí I, Rejchrt S. Intraoperative enteroscopy: ten years’ experience at a single tertiary center. Surg Endosc. 2007;21:1111–1116. doi: 10.1007/s00464-006-9052-4. [DOI] [PubMed] [Google Scholar]
- 30.Kopácová M, Tachecí I, Koudelka J, Králová M, Rejchrt S, Bures J. A new approach to blue rubber bleb nevus syndrome: the role of capsule endoscopy and intra-operative enteroscopy. Pediatr Surg Int. 2007;23:693–697. doi: 10.1007/s00383-006-1843-0. [DOI] [PubMed] [Google Scholar]
- 31.Kopacova M, Tacheci I, Rejchrt S, Bures J. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397–5408. doi: 10.3748/wjg.15.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domagk D, Bretthauer M, Lenz P, Aabakken L, Ullerich H, Maaser C, Domschke W, Kucharzik T. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064–1067. doi: 10.1055/s-2007-966990. [DOI] [PubMed] [Google Scholar]
- 33.Gay G, Delvaux M. Small-bowel endoscopy. Endoscopy. 2006;38:22–26. doi: 10.1055/s-2005-921120. [DOI] [PubMed] [Google Scholar]
- 34.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 35.Su MY, Liu NJ, Hsu CM, Chiu CT, Chen PC, Lin CJ. Double balloon enteroscopy-the last blind-point of the gastrointestinal tract. Dig Dis Sci. 2005;50:1041–1045. doi: 10.1007/s10620-005-2701-y. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T, Esaki M, Moriyama T, Nakamura S, Iida M. Comparison of capsule endoscopy and enteroscopy with the double-balloon method in patients with obscure bleeding and polyposis. Endoscopy. 2005;37:827–832. doi: 10.1055/s-2005-870207. [DOI] [PubMed] [Google Scholar]
- 37.Wu CR, Huang LY, Song B, Yi LZ, Cui J. Application of double-balloon enteroscopy in the diagnosis and therapy of small intestinal diseases. Chin Med J (Engl) 2007;120:2075–2080. [PubMed] [Google Scholar]
- 38.Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613–615. doi: 10.1055/s-2007-966444. [DOI] [PubMed] [Google Scholar]
- 39.Mensink PB. Complications of double balloon enteroscopy. Tech Gastrointest Endosc. 2008;10:66–69. [Google Scholar]
- 40.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 41.May A, Nachbar L, Pohl J, Ell C. Endoscopic interventions in the small bowel using double balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007;102:527–535. doi: 10.1111/j.1572-0241.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 42.Yen HH, Chen YY, Su WW, Soon MS, Lin YM. Intestinal necrosis as a complication of epinephrine injection therapy during double-balloon enteroscopy. Endoscopy. 2006;38:542. doi: 10.1055/s-2006-925184. [DOI] [PubMed] [Google Scholar]
- 43.Attar A, Maissiat E, Sebbagh V, Cellier C, Wind P, Bénamouzig R. First case of paralytic intestinal ileus after double balloon enteroscopy. Gut. 2005;54:1823–1824. doi: 10.1136/gut.2005.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen HH, Su WW, Chiu YH, Chen YY, Soon MS. Acute parotitis after double-balloon endoscopy. Gastrointest Endosc. 2008;68:1017–1019. doi: 10.1016/j.gie.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 45.Eisen GM, Schreiner M. Small-bowel endoscopy. Endoscopy. 2007;39:113–117. doi: 10.1055/s-2007-966141. [DOI] [PubMed] [Google Scholar]
- 46.Honda K, Mizutani T, Nakamura K, Higuchi N, Kanayama K, Sumida Y, Yoshinaga S, Itaba S, Akiho H, Kawabe K, et al. Acute pancreatitis associated with peroral double-balloon enteroscopy: a case report. World J Gastroenterol. 2006;12:1802–1804. doi: 10.3748/wjg.v12.i11.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 48.Bradley EL. The necessity for a clinical classification of acute pancreatitis: The Atlanta system. In: Bradley EL, editor. Acute pancreatitis: Diagnosis and therapy. New York: Raven Press; 1994. pp. 27–32. [Google Scholar]
- 49.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 50.Bollen TL, van Santvoort HC, Besselink MG, van Leeuwen MS, Horvath KD, Freeny PC, Gooszen HG. The Atlanta Classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 51.Mallery JS, Baron TH, Dominitz JA, Goldstein JL, Hirota WK, Jacobson BC, Leighton JA, Raddawi HM, Varg JJ 2nd, Waring JP, et al. Complications of ERCP. Gastrointest Endosc. 2003;57:633–638. doi: 10.1053/ge.2003.v57.amge030576633. [DOI] [PubMed] [Google Scholar]
- 52.Jarbandhan SV, van Weyenberg SJ, van der Veer WM, Heine DG, Mulder CJ, Jacobs MA. Double balloon endoscopy associated pancreatitis: a description of six cases. World J Gastroenterol. 2008;14:720–724. doi: 10.3748/wjg.14.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andriulli A, Caruso N, Quitadamo M, Forlano R, Leandro G, Spirito F, De Maio G. Antisecretory vs antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. JOP. 2003;4:41–48. [PubMed] [Google Scholar]
- 54.Vege SS, Chari ST. Clinical manifestations and diagnosis of acute pancreatitis. UpToDate on line, 18.1. Wellesley. New York: Raven Press; 2010. Available from: http://www.uptodate.com. [Google Scholar]
- 55.Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177–1182, 1182.e1-3. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Kopacova M, Rejchrt S, Tacheci I, Bures J. Association of hyperamylasemia and acute pancreatitis with oral double balloon enteroscopy: 100 consecutive oral procedures. Endoscopy. 2009;41(Suppl 1):A234. [Google Scholar]
- 57.Bures J, Rejchrt S. Enteroscopy. In: Bures J, Rejchrt S, editors. Small bowel investigation & atlas of enteroscopy. Praha: Grada Publishing; 2001. p. 480. [Google Scholar]
- 58.Gay G, Pennazio M, Delmotte JS, Rossini EP. Push enteroscopy. In: Rossini FP, Gay G, editors. Atlas of Enteroscopy. Milan: Springer Verlag; 1998. pp. 43–50. [Google Scholar]
- 59.Nevins AB, Keeffe EB. Acute pancreatitis after gastrointestinal endoscopy. J Clin Gastroenterol. 2002;34:94–95. doi: 10.1097/00004836-200201000-00019. [DOI] [PubMed] [Google Scholar]
- 60.Deschamps JP, Allemand H, Janin Magnificat R, Camelot G, Gillet M, Carayon P. Acute pancreatitis following gastrointestinal endoscopy without ampullary cannulation. Endoscopy. 1982;14:105–106. doi: 10.1055/s-2007-1021593. [DOI] [PubMed] [Google Scholar]
- 61.Thomas AW, Mitre RJ. Acute pancreatitis as a complication of colonoscopy. J Clin Gastroenterol. 1994;19:177–178. doi: 10.1097/00004836-199409000-00024. [DOI] [PubMed] [Google Scholar]
- 62.Blackwood WD, Vennes JA, Silvis SE. Post-endoscopy pancreatitis and hyperamylasuria. Gastrointest Endosc. 1973;20:56–58. doi: 10.1016/s0016-5107(73)73873-6. [DOI] [PubMed] [Google Scholar]
- 63.Pelletier G, Nee N, Brivet M, Etienne JP, Lemonnier A. Upper gastrointestinal endoscopy. An unrecognized cause of hyperamylasemia. Dig Dis Sci. 1987;32:254–256. doi: 10.1007/BF01297050. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010–1016. doi: 10.1016/s1542-3565(04)00453-7. [DOI] [PubMed] [Google Scholar]
- 65.Jones BH, Harrison ME, Fleischer DE, Maltby NL, Leighton JA. Double balloon enteroscopy: New information and limitations defined. Gastrointest Endosc. 2005;61:AB229. [Google Scholar]
- 66.Honda K, Itaba S, Mizutani T, Sumida Y, Kanayama K, Higuchi N, Yoshinaga S, Akiho H, Kawabe K, Arita Y, et al. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endoscopy. 2006;38:1040–1043. doi: 10.1055/s-2006-944831. [DOI] [PubMed] [Google Scholar]
- 67.Groenen MJ, Moreels TG, Orlent H, Haringsma J, Kuipers EJ. Acute pancreatitis after double-balloon enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endoscopy. 2006;38:82–85. doi: 10.1055/s-2005-921179. [DOI] [PubMed] [Google Scholar]
- 68.Zhong J, Ma T, Zhang C, Sun B, Chen S, Cao Y, Wu Y. A retrospective study of the application on double-balloon enteroscopy in 378 patients with suspected small-bowel diseases. Endoscopy. 2007;39:208–215. doi: 10.1055/s-2007-966190. [DOI] [PubMed] [Google Scholar]
- 69.Decker GA, Leighton JA, Harrison ME, Nguyen CC, Das A, Pasha SF, Moss AA, Miller LJ. New technology, new complications: pancreatitis complicating double-balloon enteroscopy. Gastroenterol Hepatol. 2007;3:920–924. [PMC free article] [PubMed] [Google Scholar]
- 70.Matsushita M, Shimatani M, Uchida K, Okazaki K. Mechanism of acute pancreatitis after peroral double-balloon enteroscopy. Endoscopy. 2007;39:480; author reply 481. doi: 10.1055/s-2007-966258. [DOI] [PubMed] [Google Scholar]
- 71.Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41:670–673. doi: 10.1055/s-0029-1214976. [DOI] [PubMed] [Google Scholar]
- 72.Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1–12. doi: 10.1007/s00535-008-2292-4. [DOI] [PubMed] [Google Scholar]
- 73.Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post-procedure Elevated Amylase and Lipase Levels After Double-Balloon Enteroscopy: Relations with the Double-Balloon Technique. Dig Dis Sci. 2009:Epub ahead of print. doi: 10.1007/s10620-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 74.Pennazio M. Small-bowel endoscopy. Endoscopy. 2008;40:835–842. doi: 10.1055/s-2008-1077594. [DOI] [PubMed] [Google Scholar]
- 75.May A, Ell C. Push-and-pull enteroscopy using the double-balloon technique/double-balloon enteroscopy. Dig Liver Dis. 2006;38:932–938. doi: 10.1016/j.dld.2006.07.101. [DOI] [PubMed] [Google Scholar]
- 76.Honda K, Nakamura K, Itaba S, Akiho H, Arita Y, Takayanagi R. Reply to M. Matsushita et al. Endoscopy. 2007;39:481. [Google Scholar]
- 77.Weinbroum AA. Mannitol prevents acute lung injury after pancreas ischemia-reperfusion: a dose-response, ex vivo study. Lung. 2009;187:215–224. doi: 10.1007/s00408-009-9154-6. [DOI] [PubMed] [Google Scholar]
- 78.Chetty U, Gilmour HM, Taylor TV. Experimental acute pancreatitis in the rat--a new model. Gut. 1980;21:115–117. doi: 10.1136/gut.21.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrie MM, O’Hare R, Joffe SN. Acute and chronic pancreatitis in the rat caused by a closed duodenal loop. Digestion. 1978;18:280–285. doi: 10.1159/000198211. [DOI] [PubMed] [Google Scholar]
- 80.Mönkemüller K, Fry LC, Malfertheiner P. Double-balloon enteroscopy: beyond feasibility, what do we do now? Endoscopy. 2007;39:229–231. doi: 10.1055/s-2006-945193. [DOI] [PubMed] [Google Scholar]
- 81.Lo SK, Simpson PW. Pancreatitis associated with double-balloon enteroscopy: how common is it? Gastrointest Endosc. 2007;66:1139–1141. doi: 10.1016/j.gie.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 82.Andriulli A, Clemente R, Solmi L, Terruzzi V, Suriani R, Sigillito A, Leandro G, Leo P, De Maio G, Perri F. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002;56:488–495. doi: 10.1067/mge.2002.128130. [DOI] [PubMed] [Google Scholar]
- 83.Beauchant M, Ingrand P, Favriel JM, Dupuychaffray JP, Capony P, Moindrot H, Barthet M, Escourrou J, Plane C, Barrioz T, et al. Intravenous nitroglycerin for prevention of pancreatitis after therapeutic endoscopic retrograde cholangiography: a randomized, double-blind, placebo-controlled multicenter trial. Endoscopy. 2008;40:631–636. doi: 10.1055/s-2008-1077362. [DOI] [PubMed] [Google Scholar]
- 84.Hoogerwerf WA. Pharmacological management of pancreatitis. Curr Opin Pharmacol. 2005;5:578–582. doi: 10.1016/j.coph.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Vila JJ, Jiménez FJ, Prieto C, Borobio E, Juanmartiñena JF, Borda F. [Utility of bolus somatostatin administration in preventing pancreatitis after endoscopic retrograde cholangiopancreatography: a controlled, non-randomized study] Gastroenterol Hepatol. 2006;29:231–236. doi: 10.1157/13085969. [DOI] [PubMed] [Google Scholar]
- 86.Xia Q, Yuan L, Yang XN, Tang WF, Jiang JM. Comparison of integrated Chinese and Western medicine with and without somatostatin supplement in the treatment of severe acute pancreatitis. World J Gastroenterol. 2005;11:1073–1076. doi: 10.3748/wjg.v11.i7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]


