Abstract
Pressure-tip catheters (PTCs) are used to evaluate ventricular mechanics during surgical repair of congenital heart disease in children. Studies in infants require miniaturized sensors. We compared the safety and accuracy of a 2 French (Fr) ultraminiature PTC to a 5 Fr-PTC. In 10 piglets (19-22 kg), a 5 Fr-PTC was inserted through a 3 mm apical puncture with a # 11 blade. A 20-gauge angiocatheter was inserted using a separate site. A 2 Fr-PTC was threaded through the angiocatheter lumen. The angiocatheter was withdrawn, leaving the 2 Fr-PTC within the LV. LV pressure (LVP) changes were measured during three inferior vena caval occlusions. Reliability coefficients demonstrated correlation between the 2 Fr-PTC and 5 Fr-PTC for LV end-diastolic pressure (0.90-0.95), peak LVP (0.92-0.99), and the maximal (0.87-0.93) and minimal (0.89-0.94) first derivatives of LVP. Bland-Altman analysis demonstrated agreement for all variables. Blood loss was trivial with pressure manipulation and catheter placement and removal. Pressure measurements using the 2 Fr-PTC were accurate and comparable to those from the 5 Fr-PTC. Transventricular placement of a 2 Fr-PTC is feasible and should allow evaluation of ventricular mechanics during surgical repair of congenital heart disease.
Keywords: Ventricular Mechanics, Pressure-tip Micromanometer, Congenital Heart Disease
Introduction
Accurate intracardiac pressure measurement is fundamental to assessing ventricular mechanics intraoperatively. Fluid-filled catheter systems are relatively inexpensive and durable; however, as these pressure signals are transmitted through an interposed fluid column to an external transducer, they may be distorted with respect to time and amplitude. These transducer systems are limited by a 0-30 Hz frequency response,1-3 a resonant frequency of 5-75 Hz, and damping (damping coefficient of 0.16-0.50),2 making them inadequate for these purposes.4, 5 Trapped air bubbles or small blood clots also contribute to the system’s limitations. Measurement errors as much as 20 mmHg have been previously reported.2 Placing the sensor directly at the catheter tip made more precise pressure measurement possible.6 High-fidelity pressure-tip catheters (PTCs) with a 0-10 kHz frequency response have been validated and are now used to record left ventricular pressure (LVP) and its derivatives.5, 7-10
Increasingly, advanced surgical techniques are being applied to palliate complex forms of congenital heart disease, especially in infants and children born with single ventricle physiology. These patients initially have a parallel circulation, in which their single ventricle pumps to both systemic and pulmonary circulations. The first goal of palliative surgery in this setting is to stabilize systemic and pulmonary blood flow.11 Subsequently, the bidirectional Glenn (BDG) and Fontan procedures are performed, which separate the systemic and pulmonary circulations, allowing the single ventricle to pump to the systemic circulation while blood flows passively through the pulmonary circulation.
Ventricular function and cardiac work are crucial determinants of the outcome of patients with palliated single ventricle anatomy. Optimizing the ventricular mass-volume ratio and controlling ventricular afterload are critical to the long-term success of these procedures. The intraoperative assessment of ventricular mechanics during these palliative surgeries could lead to insight and innovation, with respect to surgical technique, myocardial preservation, or immediate pre- and post-operative management of this vulnerable population.
New technologies have allowed materials to be miniaturized, and, presently, a 2 French (Fr) ultraminiature PTC is available for clinical use. This catheter has been widely utilized in small animal preparations to assess ventricular function.12, 13
Our study compares dynamic measurements of LVP and its first derivative (dP/dt), over a broad physiological range, simultaneously obtained with a 2 Fr-PTC and a larger 5 Fr-PTC. The 5 Fr-PTC has been widely used in patients, is currently used in our laboratory, and is being utilized as the “gold standard” in this experiment. Additionally, we evaluated a novel transventricular catheter insertion technique. Demonstration of reliability, safety, and feasibility of use will allow methods of assessing intraoperative ventricular mechanics, currently used in older patients with congenital heart disease, to be applied to infants weighing <4 kg, undergoing staged single ventricle palliation.
Methods
All animals received humane care, in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 85-23, revised 1985). The Columbia University Institutional Animal Care and Use Committee approved the experimental protocol.
Animal Preparation
Data was collected in 10 Yorkshire piglets (19-22 kg). These pigs were part of a broader study requiring serial pressure measurement recordings, during an evaluation of the benefits of biventricular pacing optimization. They were chosen because their LV volume and pressure are similar to those of a neonate with single ventricle physiology. Pigs were anesthetized intramuscularly with atropine sulfate (0.02 mg/kg), ketamine hydrochloride (20 mg/kg), and xylazine (0.5 mg/kg), followed by oral endotracheal intubation. They were mechanically ventilated using 100% oxygen and titrated isoflurane (1.75-2.5%). Normal saline was administered intravenously at 10 mL/kg/h for the first hour and 5 mL/kg/h thereafter. Heart rate and body temperature were monitored.
Instrumentation
A median sternotomy was performed, the pericardium was opened longitudinally, and the inferior vena cava (IVC) was snared. Heparin was administered intravenously (100 U/kg). The first pressure catheter, a 5 Fr-PTC (Millar Instruments, Houston, TX), was placed via a 3 mm apical puncture with a # 11 blade through a 4-0 prolene purse-string suture at the apex. Without using a purse-string suture, the second PTC was placed at a separate LV site. A 20-gauge angiocatheter with an introducer was inserted through the LV wall near the apex. The introducer was withdrawn, and a 2 Fr-PTC (Millar Instruments, Houston, TX) was threaded through the lumen. The angiocatheter was then withdrawn, leaving the 2 Fr-PTC within the LV cavity.
The LV apex in piglets is very thin and was chosen as the catheter placement site to approximate the size of an infant’s LV anterior wall. Throughout the experiment, the myocardium was inspected for bleeding from catheter insertion sites. Both catheters were calibrated at 0 mmHg and 20 mmHg, using room temperature normal saline in a graduated cylinder, immediately before insertion and after each experiment.
Pressure Measurements
LVP was acutely reduced by transient IVC occlusion. During the dynamic pressure manipulation period, beat-to-beat pressure changes were simultaneously recorded by both catheters. These pressure changes were analogous to those seen intraoperatively upon initiation of cardiopulmonary bypass (CPB). Three consecutive IVC occlusions, each lasting 10-15 seconds, were performed per experiment. The lungs were held at end-expiration during the entire IVC occlusion. Pressure and electrocardiographic data were digitized at 200 Hz, using a 16-channel analog-to-digital converter (AD Instruments, Milford, CA), and recorded on a portable computer (Apple Computer, Cuperino, CA).
Pressure Sensor
The 2 Fr-PTC (SPC-320 – Millar Instruments, Inc. Houston, TX) consists of an electronic interface and a polyurethane catheter without a lumen. The catheter contains a piezo-electric strain gauge (full Wheatstone bridge), side-mounted at the tip. The pressure sensitivity is 5 mV/V/mmHg, with a range of −50-300 mmHg and a temperature band of +/− 3 mmHg, from −20-38 degrees Celsius, with <6 mmHg drift over a 12-hour span.
The 5 Fr-PTC (MPC-500 – Millar Instruments, Inc. Houston, TX) is also a non-lumen polyurethane catheter, with an electronic interface and pressure sensor side-mounted at its tip. It also has a pressure sensitivity of 5 mV/V/mmHg, with a range of −50-300 mmHg and a temperature band of +/− 1 mmHg, from −20-38 degrees Celsius, with <6 mmHg drift over a 12-hour span.
Data Analysis
Pressure analysis was performed offline using custom routines implemented in Matlab (The MathWorks, Inc., Natick, MA). Thirty episodes of dynamic LV pressure change were recorded. LV pressure was digitally differentiated. LV end-diastolic pressure (LVEDP) was identified as the point before the LVP rapid upstroke, before dP/dt exceeded 10% of its maximal value (dP/dtmax). For each serial beat acquired during IVC occlusion, LVEDP, peak systolic pressure (peak LVP), and the dP/dtmax and minimal (dP/dtmin) first derivative were analyzed for each PTC. The beat preceding the first LVEDP decrease defined the first beat, and the lowest LVEDP defined the last beat analyzed during IVC occlusion.
Statistical Analysis
Reliability coefficients (intra-class correlations) were generated for each LVP variable (LVEDP, peak LVP, dP/dtmax, dP/dtmin), for all 30 IVC occlusions, using mixed model technology (SAS, PROC MIXED). A random intercept model was fit, which generated the intra-class correlation: d/ (d + s2), where d = intersubject variability and s2 = intrasubject variability.
Bland-Altman14 analysis was also performed for each pressure variable (LVEDP, peak LVP, dP/dtmax, dP/dtmin), for all 30 IVC occlusions. Bias and 95% confidence intervals were calculated using the following formulas: Bias = d (where d = the mean difference between the two measurements), and 95% confidence intervals = d +/− 2s (where s = the standard deviation of the differences). The multiple measurements taken for each IVC occlusion were summarized as means and the difference in means. Bland-Altman analysis was then performed for each pressure variable.
Results
Recorded pressure data, obtained during dynamic preload reduction, allowed for simultaneous comparison of the two catheters.
Pressures
During each transient IVC occlusion, beat-to-beat pressure variations were elicited and simultaneously recorded. This procedure was repeated three times per experiment, with peak LVP and LVEDP measured and compared for each heartbeat during dynamic pressure reduction. Figure 1 shows representative pressure data obtained by IVC occlusion. Peak LVP ranged from 111.6-34.0 mmHg for the 2 Fr-PTC and 108.2-34.0 mmHg for the 5 Fr-PTC. LVEDP ranged from 20.5-1.1 mmHg for the 2 Fr-PTC and 19.5-1.1 mmHg for the 5 Fr-PTC.
Figure 1.
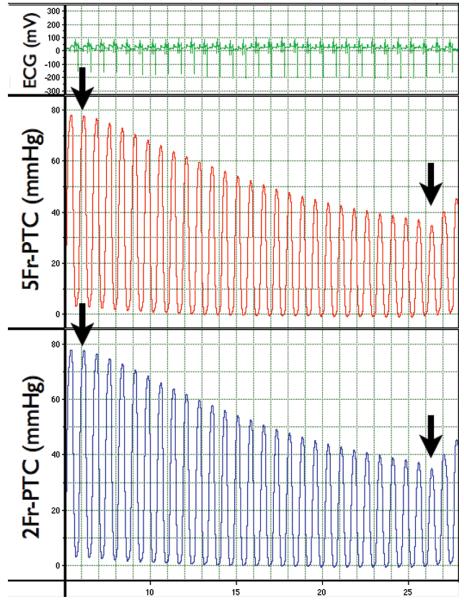
Representative simultaneous LVP recordings with 2 Fr- and 5 Fr-PTC, including electrocardiographic monitoring during acute preload reduction by IVC occlusion. The black arrows indicate the initiation and termination of IVC occlusion.
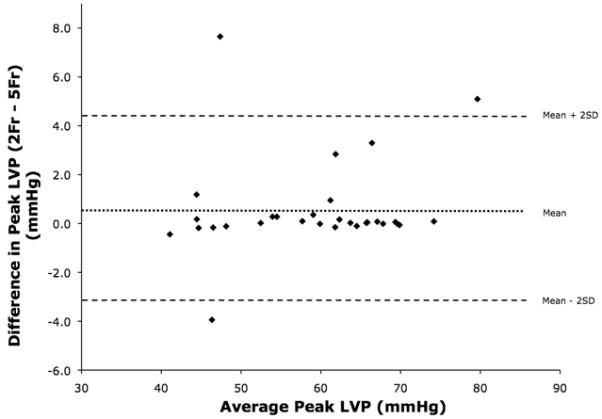
The results from reliability coefficients generated during the 10 experiments are shown in Table 1 and demonstrate correlation between the catheters for peak LVP and LVEDP, with reliability coefficients of ≥0.92 and ≥0.90, respectively. Figure 2 is a Bland-Altman plot of peak LVP, comparing measurements obtained by the 2 Fr-PTC and 5 Fr-PTC. Over 30 episodes of pressure manipulation, peak LVP had a 0.6 mmHg bias, with a 95% confidence interval of −3.4-4.5 mmHg. Figure 3 is a Bland-Altman plot comparing LVEDP measurements. Analysis demonstrated a 0.4 mmHg bias, with a 95% confidence interval of −1.9-2.6 mmHg.
TABLE I. Reliability Coefficients.
| Variable | IVC 1 | IVC 2 | IVC 3 |
|---|---|---|---|
| Peak LVP | 0.92 | 0.96 | 0.99 |
| LVEDP | 0.91 | 0.90 | 0.95 |
| dP/dtmax | 0.93 | 0.90 | 0.87 |
| dP/dtmin | 0.89 | 0.92 | 0.94 |
IVC, Inferior vena caval occlusion; Peak LVP, peak left ventricular pressure; LVEDP, left ventricular end-diastolic pressure; dP/dtmax, maximum rate of increase of ventricular pressure; dP/dtmin, maximum rate of decrease of ventricular pressure.
Figure 2.

Bland-Altman plot of peak LVP.
Figure 3.
Bland-Altman plot of LVEDP.
Derivatives
The dP/dt measurements, obtained during each of the 30 episodes, were digitally calculated. dP/dtmax ranged from 4668-280 mmHg/sec for the 2 Fr-PTC and 5120-288 mmHg/sec for the 5 Fr-PTC. dP/dtmin ranged from −211 to −1964 mmHg/sec for the 2 Fr-PTC and −210 to −1948 mmHg/sec for the 5 Fr-PTC.
Reliability coefficients generated to compare dP/dt measurements from the two PTCs are shown in Table 1. Correlation between the catheters, with respect to dP/dtmax and dP/dtmin, is demonstrated with reliability coefficients of ≥0.87 and ≥0.89, respectively.
Figure 4 is a Bland-Altman plot of dP/dtmax, performed to compare pressure measurements from the 2 Fr-PTC and 5 Fr-PTC. Bland-Altman analysis demonstrated a −24 mmHg/sec bias, with a 95% confidence interval of −176-128 mmHg/sec. Figure 5 is a Bland-Altman plot of dP/dtmin. Analysis demonstrated a 22.9 mmHg/sec bias, with a 95% confidence interval of −65-111 mmHg/sec.
Figure 4.

Bland-Altman plot of dP/dtmax.
Figure 5.

Bland-Altman plot of dP/dtmin.
Insertion Technique
The transventricular insertion technique, which did not require a purse-string suture, was successfully employed using the 2 Fr-PTC, in all 10 experiments. Only trivial blood loss occurred during pressure manipulation and catheter placement and removal, and any bleeding due to catheter withdrawal was limited and resolved spontaneously. The catheter position was stable during IVC occlusion, and it was not displaced during any of the experiments.
Discussion
Ventricular function and cardiac work are crucial determinants of outcomes for patients with palliated single ventricle anatomy. These patients often require multiple re-operations utilizing CPB, with the long-term effects of such repeated insults on myocardial function largely unknown.15 The intraoperative assessment of ventricular mechanics during these palliative surgeries could lead to insight and innovation, with respect to surgical technique, myocardial preservation, or immediate pre- and post-operative management.
Our group has previously studied patients undergoing Fontan completion, linking increased preoperative ventricular stiffness to increased postoperative morbidity.9 Tanoue et al. investigated older patients with single ventricle physiology before and after BDG and Fontan procedures.16 They found that end-diastolic ventricular volume decreased step-wise following both surgeries, allowing for increased ventricular efficiency, and concluded that the interposed BDG procedure is an important consideration factor in treating high-risk Fontan candidates.
Currently, there is controversy concerning the type of initial palliation for single ventricle patients, with respect to the pulmonary blood flow supply method, and it is the subject of an ongoing randomized controlled trial.17 Two options are available and differ from sites of anastomosis: the systemic artery to pulmonary artery conduit and right ventricular to pulmonary artery conduit. Bove and colleagues have developed multi-scale computer models to predict postoperative hemodynamic changes based on initial surgical palliation.18, 19 The use of miniaturized catheters should allow our methods to be applied to directly evaluate ventricular mechanics, in these vulnerable patients undergoing initial surgical palliation.
This work requires high-fidelity ventricular pressure measurements. Fluid-filled catheters with externally placed transducers have insufficient temporal resolution to accurately assess the ventricular pressure changes necessary for studying ventricular mechanics.4-8 Therefore, only PTCs should be used for high-fidelity measurements. We compared a smaller 2 Fr-PTC with a catheter currently used at Columbia University Medical Center. The 5 Fr-PTC has been utilized intraoperatively in older children undergoing Fontan completion9 and tetralogy of Fallot repair,10 and more than 175 such studies employing this catheter have been performed since 1997 at our medical center.
Before CPB, the catheter was inserted via a 3 mm apical puncture with a # 11 blade within a purse-string suture, placed in the ascending aorta, and advanced retrograde across the aortic valve into the ventricular cavity. Upon initiation and cessation of CPB, high-fidelity pressure measurements were then obtained and simultaneously recorded, along with transesophageal echocardiographic volume measurements, allowing dynamic pressure-volume analyses to be performed.20 The smaller heart size and mediastinal space constraints make this catheter insertion method technically challenging in younger children and infants. We hypothesized that the smaller 2 Fr-PTC would compare favorably to our 5 Fr-PTC “gold standard” and that a transventricular insertion technique would be safe and effective, thus permitting these methods to be applied to much smaller patients.
Both catheters were inserted through the LV apex. The 5 Fr-PTC was inserted via a 3 mm apical puncture with a # 11 blade within a purse-string suture; the 2 Fr-PTC was inserted using the transventricular needle insertion technique described above. Pressure changes induced by transient IVC occlusion were then recorded simultaneously.
Pressures
The 2 Fr-PTC satisfies all requirements for a high-fidelity manometer, with pressure sensitivities comparable to the 5 Fr-PTC currently used. Reliability coefficient analysis of peak LVP and LVEDP recordings showed that the two catheters compared very well, with coefficients of ≥0.90.
Bland-Altman analyses were also performed to assess agreement between the two clinical measurement methods.14 Bland-Altman analysis of peak LVP demonstrated agreement between the two catheters with a <1 mmHg bias, with a 95% confidence interval of −3.4-4.5 mmHg, given that the peak LVP ranged from 112-34 mmHg. In fact, Figure 3 demonstrates most values clustered along the 0.6 mmHg bias line, with relatively few outliers. Analysis of LVEDP demonstrated somewhat less agreement. The 0.4 mmHg bias was low, with a modest 95% confidence interval range of −1.9-2.6 mmHg; however, the overall LVEDP pressure range was 20.5-1.1 mmHg, much lower than peak LVPs encountered.
Overall, the pressures encountered were within normal limits. The LVEDP range found in Fontan patients is 2-13 mmHg, with the mean LVEDP of 7.7±2.6 in right dominant ventricles and 11.2±1.9 in left dominant ventricles.9 When reviewing Figure 2, it appears that most LVEDP measurements clustered near the bias line at pressures of <7 mmHg, with more variation noted at pressures >7 mmHg. This could be secondary to catheter tip entrapment within a ventricular trabeculation during pressure manipulation, or thrombus formation at the catheter tip. Alternatively, if the micromanometers were positioned at different levels within the ventricular cavity, physiologic pressure gradients arising during diastole could account for the variations encountered.21,22
Derivatives
Reliability coefficient analysis demonstrated very comparable pressure recordings for dP/dtmax and dP/dtmin, with coefficients of ≥0.87. Bland-Altman analysis also demonstrated acceptable agreement between measurements from the two catheters. A −24 mmHg/sec dP/dtmax bias, with a 95% confidence interval of +/− 152 mmHg/sec, is acceptable, given the dP/dtmax range (5120-280 mmHg/sec). Similarly, a 23 mmHg/sec dP/dtmin bias, with a 95% confidence interval of +/− 88 mmHg/sec, is also acceptable,22 given the dP/dtmin range (−210 to −1948 mmHg/sec).
Insertion Technique
The transventricular insertion technique rationale was based upon the routine use at our institution of 18- to 20-gauge needles to de-air the heart (ventricles) during withdrawal from CPB. This insertion technique was easily performed and provided stable catheter position, without excessive bleeding or ectopy. Additionally, all bleeding stopped spontaneously immediately after catheter removal. This technique avoided excessive catheter manipulation retrograde across the aortic valve during placement, thereby minimizing the risks of aortic valve or coronary artery injury, while allowing the high-fidelity ventricular pressure measurements necessary for studying ventricular mechanics.
Limitations
An important limitation was the inability to verify baseline calibrations after insertion, an advantage that fluid-filled pressure systems have over PTCs. A PTC with a lumen capable of pressure passage to an external port has been reported to address this issue.23 Standardization of insertion depth or visualization of exact catheter tip position within the ventricle during catheter insertion may allow for tighter agreement in future experiments, especially regarding LVEDP measurements. Furthermore, while it was unlikely that the catheters were in mechanical contact, electrical field interference induced by currents in the sensors was possible. In the future, one ventricular catheter will be used at a time to overcome this possible limitation. Recent advancements in the development of ultraminiature PTCs have allowed for further downsizing of the catheters to 1.0 Fr. These catheters are currently widely used in studies of ventricular mechanics in mice24 and will ultimately be refined for clinical application.
Conclusion
In infants and young children undergoing congenital heart defect repair, thorough evaluation of intraoperative ventricular function has been limited by available technology. This study has shown that, in piglet hearts, pressure recordings obtained from the ultraminiature 2 Fr-PTC are comparable to those from the larger 5 Fr-PTC currently used. Additionally, our novel transventricular insertion technique remained stable throughout our experiments and was easily performed and well tolerated. We hope to eventually use these methods and technologies to study intraoperative ventricular mechanics in infants and small children undergoing open-heart surgical procedures.
ACKNOWLEDGEMENTS
The work was supported by a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01-HL48109, PI: Dr. Spotnitz) and by the Surgery and Pediatric Cardiology Departments at Columbia University. Dr. Spotnitz is the George H. Humphreys, II, Professor of Surgery. The authors would also like to thank Lauren Bedrosian for editorial assistance.
Footnotes
Disclosures: The work was supported by a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01-HL48109, PI: Dr Spotnitz) and by the Departments of Surgery and Pediatric Cardiology at Columbia University.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zimmer HG, Millar HD. Technology and application of ultraminiature catheter pressure transducers. Can J Cardiol. 1998;14:1259–1266. [PubMed] [Google Scholar]
- 2.Rothe CF, Kim KC. Measuring systolic arterial blood pressure. Possible errors from extension tubes or disposable transducer domes. Crit Care Med. 1980;8:683–689. doi: 10.1097/00003246-198011000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Gould KL, Trenholme S, Kennedy JW. In vivo comparison of catheter manometer systems with the catheter-tip micromanometer. J Appl Physiol. 1973;34:263–267. doi: 10.1152/jappl.1973.34.2.263. [DOI] [PubMed] [Google Scholar]
- 4.Colan SD. Combined fluid-filled and micromanometer-tip catheter system for high-fidelity pressure recordings in infants. Cathet Cardiovasc Diagn. 1984;10:619–623. doi: 10.1002/ccd.1810100615. [DOI] [PubMed] [Google Scholar]
- 5.Aubert AE, Vrolix M, De Geest H, Van de Werf F. In vivo comparison between two tip pressure transducer systems. Int J Clin Monit Comput. 1995;12:77–83. doi: 10.1007/BF01142487. [DOI] [PubMed] [Google Scholar]
- 6.Gauer OH, Gienapp E. A miniature pressure-recording device. Science. 1950;112:404–405. doi: 10.1126/science.112.2910.404. [DOI] [PubMed] [Google Scholar]
- 7.Millar HD, Baker LE. A stable ultraminiature catheter-tip pressure transducer. Med Biol Eng. 1973;11:86–89. doi: 10.1007/BF02477303. [DOI] [PubMed] [Google Scholar]
- 8.Kar S, Drury JK, Tokioka H, Meerbaum S, Corday E. Experimental evaluation of a new transducer tipped catheter. Indian Heart J. 1989;41:213–220. [PubMed] [Google Scholar]
- 9.Garofalo CA, Cabreriza SE, Quinn TA, et al. Ventricular diastolic stiffness predicts perioperative morbidity and duration of pleural effusions after the Fontan operation. Circulation. 2006;114:156–161. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 10.Richmond ME, Cabreriza SE, Van Batavia JP, et al. Direction of preoperative ventricular shunting affects ventricular mechanics after Tetralogy of Fallot repair. Circulation. 2008;118:2338–2344. doi: 10.1161/CIRCULATIONAHA.107.761080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernovsky G, Ghanayem N, Ohye RG, et al. Hypoplastic left heart syndrome: consensus and controversies in 2007. Cardiol Young. 2007;17:75–86. doi: 10.1017/S1047951107001187. [DOI] [PubMed] [Google Scholar]
- 12.Suarez J, Belke DD, Gloss B, et al. In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;286:H68–H75. doi: 10.1152/ajpheart.00245.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kirn B, Starc V. Contraction wave in axial direction in free wall of guinea pig left ventricle. Am J Physiol Heart Circ Physiol. 2004;287:H755–H759. doi: 10.1152/ajpheart.01053.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 15.Chaturvedi RR, Lincoln C, Gothard JW, et al. Left ventricular dysfunction after open repair of simple congenital heart defects in infants and children: quantitation with the use of a conductance catheter immediately after bypass. J Thorac Cardiovasc Surg. 1998;115:77–83. doi: 10.1016/s0022-5223(98)70446-5. [DOI] [PubMed] [Google Scholar]
- 16.Tanoue Y, Sese A, Imoto Y, Joh K. Ventricular mechanics in the bidirectional glenn procedure and total cavopulmonary connection. Ann Thorac Surg. 2003;76:562–566. doi: 10.1016/s0003-4975(03)00467-3. [DOI] [PubMed] [Google Scholar]
- 17.Ohye RG, Devaney EJ, Hirsch JC, Bove EL. The modified Blalock-Taussig shunt versus the right ventricle-to-pulmonary artery conduit for the Norwood procedure. Pediatr Cardiol. 2007;28:122–125. doi: 10.1007/s00246-006-1449-2. [DOI] [PubMed] [Google Scholar]
- 18.Migliavacca F, Balossino R, Pennati G, et al. Multiscale modelling in biofluidynamics: application to reconstructive paediatric cardiac surgery. J Biomech. 2006;39:1010–1020. doi: 10.1016/j.jbiomech.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Hsia TY, Migliavacca F, Pennati G, et al. Management of a stenotic right ventricle-pulmonary artery shunt early after the Norwood procedure. Ann Thorac Surg. 2009;88:830–838. doi: 10.1016/j.athoracsur.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 20.Hart JP, Cabreriza SE, Gallup CG, Hsu D, Spotnitz HM. Validation of left ventricular end-diastolic volume from stroke volume and ejection fraction. ASAIO J. 2002;48:654–657. doi: 10.1097/00002480-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Courtois M, Kovacs SJ, Jr, Ludbrook PA. Transmitral pressure-flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation. 1988;78:661–671. doi: 10.1161/01.cir.78.3.661. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YQ, Sun K, Zhu SL, et al. Doppler myocardial performance index in assessment of ventricular function in children with single ventricles. World J Pediatr. 2008;4:109–113. doi: 10.1007/s12519-008-0021-y. [DOI] [PubMed] [Google Scholar]
- 23.Kinefuchi Y, Fukuyama H, Suzuki T, Kanazawa M, Takiguchi M. Development of a new catheter-tip pressure transducer. Tokai J Exp Clin Med. 1999;24:85–92. [PubMed] [Google Scholar]
- 24.Hartley CJ, Reddy AK, Taffet GE. In-vitro evaluation of sensors and amplifiers to measure ventricular pressure in mice. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:965–968. doi: 10.1109/IEMBS.2008.4649315. [DOI] [PMC free article] [PubMed] [Google Scholar]


