Abstract
Objective
As associations between endogenous sex hormones and the vasculature are not well characterized, the objective was to examine the cross-sectional associations of menopausal status and endogenous sex hormones with vascular characteristics.
Design
Common carotid artery adventitial diameter and intima-media thickness were determined using B-mode ultrasound among 483 middle-aged women enrolled in the Pittsburgh and Chicago sites of the Study of Women’s Health Across the Nation.
Results
Sixty-two percent of women were pre- or early perimenopausal (<3 months amenorrhea), 12% were late perimenopausal (3-12 months amenhorrhea), and 27% were postmenopausal (≥12 months amenorrhea). After adjustment for age, compared to pre-/early perimenopause, late perimenopause was associated with a 0.28 mm larger adventitial diameter (p=0.001), while postmenopause was associated with a 0.15 mm larger adventitial diameter (p=0.040). Adjustment for traditional cardiovascular risk factors slightly attenuated these associations, but the association with late perimenopause remained statistically significant (p=0.001). Each standard deviation lower log estradiol value was associated with a 0.07 mm larger adventitial diameter after adjustment for traditional cardiovascular risk factors (p=0.023), while other endogenous hormones showed no associations. Intima-media thickness values were not significantly associated with menopausal status or endogenous sex hormones after adjustment for age.
Conclusions
The menopausal transition and declining estrogen levels are associated with alterations of the peripheral vasculature, which may help to explain the increased risk of cardiovascular disease with postmenopause.
Keywords: estradiol, endogenous sex hormones, menopause, arteriosclerosis
INTRODUCTION
The development of cardiovascular disease in women compared to men is delayed by approximately 10 years and cardiovascular disease (CVD) risk rises among women following the menopause.1 Declining endogenous estrogen levels have been assumed to be responsible for this observation.2 However, clinical trials have demonstrated an increased long-term risk of CVD events among postmenopausal women treated with estrogen therapy, rather than the expected protective effect.3,4 It has been suggested that these unexpected results of exogenous hormone therapy in postmenopausal women may be due to the relatively older age of women enrolled in hormone therapy clinical trials, who may already have a significant atherosclerotic burden; the “timing hypothesis” suggests that hormone therapy administered to younger menopausal women with a lesser atherosclerotic burden may be beneficial to the cardiovascular system.5,6 Age-stratified analyses of the Women’s Health Initiative (WHI) data suggest a cardioprotective effect of hormone therapy among younger women, and recent data from the WHI coronary-artery calcium ancillary study found a lower coronary calcium burden among women randomized to hormone therapy between 50-59 years of age.7,8
Studies of endogenous hormones and either clinical CVD or subclinical atherosclerosis among women are rare.9-12 Given that estrogen and androgens are known to have numerous molecular and cellular actions that affect all components of the vessel wall,13,14 examination of the relationships between endogenous sex hormones and vascular characteristics may inform our understanding of the risks associated with the menopausal transition. Therefore, the primary purpose of the current study is to evaluate the effect of endogenous sex hormones with common carotid intima-media thickness (IMT) and adventitial diameters in a population of women at different stages of the menopausal transition, reporting no use of hormone therapy. Understanding associations with endogenous hormones may provide insight not only into the rise in CVD among women post-menopause, but also into the unexpected risks found to be associated with exogenous hormones among postmenopausal women.
METHODS
Study Participants
The current study includes participants from an ancillary study to the Study of Women’s Health Across the Nation (SWAN). SWAN is a multicenter, multiethnic longitudinal study designed to characterize the biological and psychosocial changes that occur during the menopausal transition in a community-based sample. Details of the study design and recruitment have been previously published.15 Briefly, SWAN is being conducted at seven sites: Boston, Chicago, the Detroit area, Los Angeles, Newark, NJ, Pittsburgh, PA and Oakland CA. A total of 3,302 women aged 42-52 years were enrolled from 1996 to 1997.
The ancillary project is a study of the natural history of subclinical atherosclerosis during the menopausal transition and involves only the University of Pittsburgh and Chicago sites. The ancillary study began approximately 4 years into the SWAN study. Thus, all participants were between 45 and 58 years of age at the ancillary study visit. To be eligible, participants could not have evidence of clinical atherosclerosis (myocardial infarction, angina, intermittent claudication, cerebral ischemia, or revascularization). A total of 608 women (382 Caucasian, 226 African American) were enrolled in the ancillary study.
This study was approved by the institutional review boards of the participating institutions and all women signed informed consent prior to participation.
Ultrasound Measures
Ultrasound measures were assessed at the time of the ancillary study visit. Common carotid artery (CCA) IMT was assessed by duplex scanning using a Toshiba SSA-270A scanner. Images were taken from the near and far walls of the distal common carotid artery, one centimeter proximal to the carotid bulb, and the lumen-intima interface and the media-adventitia interface were electronically traced across a 1 cm segment. CCA adventitial diameters were measured directly as the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole. Replicate readings were performed on 20 scans from these women to evaluate reproducibility of the measures. The intraclass correlation was 0.98 for IMT and 0.99 for adventitial diameter.
Definitions of Menopausal Status and Hormone Therapy Use
Menopausal status was based on menstrual bleeding patterns in the previous 12 months and was categorized as: 1) premenopausal = menstrual period in the past three months with no change in regularity in the past 12 months; 2) early perimenopausal = menstrual period in the past three months with some change in regularity over the previous 12 months; 3) late perimenopausal = no menstrual period within the past 3 months, but some menstrual bleeding within the past 12 months; 4) post menopausal = no menstrual period within the past 12 months. To maintain sufficient numbers of women in each category, menopausal status was collapsed for statistical analyses as premenopausal/early perimenopausal (Pre/Early Peri), late perimenopausal (Late Peri), and postmenopausal (Post). Hormone therapy use was ascertained by self-reported use of birth control pills, estrogen pills, estrogen injection or patch, combination estrogen/progestin, or progestin pills in the previous year. Women reporting hormone therapy use in the 12 months preceding their ancillary study visit were excluded from the current analyses.
Blood Assays
A fasting blood draw was targeted to the early follicular phase of the menstrual cycle (days two to five). All samples were maintained at 4° C until separated and then were frozen at −80 ° C and shipped on dry ice to a central laboratory. Hormone assays were conducted at the University of Michigan SWAN Endocrine Laboratory using the ACS-180 automated analyzer (Bayer Diagnostics Corp, Norwood, MA) on blood samples drawn concurrently with ultrasound measurements, as previously described.16 The free androgen index (FAI) was used to estimate the amount of testosterone unbound by SHBG and thus immediately biologically active. FAI was calculated as 100*T/(28.84*SHBG). This results in the amount of free testosterone in nmol/L. Cycle day of blood draw was recorded for menstruating women as within days 2-5 or outside of days 2-5. For non-menstruating women, cycle day of blood draw was coded as outside of days 2-5. Results were similar when non-menstruating women were coded as their own cycle day category. Standard cardiovascular risk factors were assayed at the Medical Research Laboratories, (Lexington, Kentucky, USA) which is certified by the National Heart Lung and Blood Institute, Centers for Disease Control Part III program, as previously described.16 LDL was calculated using the Friedewald equation,17 excluding women with values of triglycerides >400 mg/dL. The HOMA insulin resistance index was calculated from fasting insulin and glucose as (fasting insulin x fasting glucose)/22.5.18
Physical Measures
Blood pressure was measured in the right arm with the participant seated following at least 5 minutes of rest. Two sequential blood pressure values were completed and averaged. Height and weight were measured without shoes with participants wearing light clothing. Portable scales were calibrated weekly and stationary clinic devices were calibrated monthly. Body mass index was calculated as weight in Kg divided by height in M2. Waist circumference was measured with the respondent in non-restrictive undergarments, at the level of the natural waist, defined as the narrowest part of the torso as seen from the anterior aspect. In cases where a waist narrowing was difficult to identify, the measure was taken at the smallest horizontal circumference in the area between the ribs and the iliac crest.
Statistical Methods
Of the 608 women enrolled in the ancillary study, 52 had unusable IMT or diameter values, 13 were excluded for having had a hysterectomy between the time of SWAN enrollment and their ancillary study visit, and 57 were excluded for reporting hormone therapy use in the 12 months preceding their ancillary study visit. Additionally, 3 had incomplete menopausal status data, leaving data from 483 women for menopausal status analyses, and 32 women had missing hormone values, leaving data from 451 women for endogenous hormone analyses. Women included in these analyses were more likely to be African American (39.3% vs. 28.8%), were slightly younger (50.0 vs. 51.2 years; p<0.001), had lower DBP values (74.2 vs. 76.2 mmHg; p=0.041), and had higher total cholesterol values (207.7 vs. 199.8 mg/dL; p=0.037) compared to women not included in these analyses (excluded or with missing data).
Adventitial diameter values were normally distributed, while CCA IMT values were slightly skewed. All hormone variables were highly skewed and were log-transformed for analyses. Characteristics of the study population were calculated by menopausal status, and compared via chi-square for categorical variables and ANOVA for continuous, normally distributed characteristics. The Wilcoxon rank sum test was used for continuous characteristics that were not normally distributed. Relationships between cardiovascular risk factors and CCA adventitial diameter and IMT were examined via Spearman correlation coefficients to accommodate the non-normal CCA IMT distribution. Linear regression analyses were performed to examine relationships between menopausal status and endogenous sex hormones with CCA adventitial diameters and IMT, after initial adjustment for age, and after further adjustment for race/ethnicity, study site, education, current smoking, systolic blood pressure, height, body mass index, HDL cholesterol, LDL cholesterol, log triglycerides, log glucose, and log HOMA insulin resistance index. Hormone analyses were also adjusted for cycle day of blood draw. The covariates adjusted for in multivariable regression modeling were included based on those with significant p-values from either Table 1 or Table 2 (associations with either menopause status or adventitial diameter or IMT). For scenarios where collinearity was identified as an issue via examination of variance inflation factors, such as the inclusion of both systolic and diastolic blood pressure, the variable with the strongest association with diameter or IMT measurements was chosen. Regression model fit was assessed by normality of model residuals. Endogenous sex hormones were converted to units of standard deviation for linear regression analyses by taking each woman’s log hormone value, subtracting the sample mean log hormone value, and dividing by the sample standard deviation of the log hormone. To determine whether menopause status or hormone results differed between African Americans and Caucasians, multiplicative status-by-race/ethnicity or hormone-by-race/ethnicity interaction terms were tested using general linear regression modeling, after adjustment for confounders. As results were similar whether log-transformed CCA IMT values were utilized or not, results are given using raw CCA IMT values for ease of presentation. Least square mean CCA diameter and IMT values were estimated by quintile of estradiol after multivariate adjustment using general linear modeling. Statistical significance was considered at p<0.05.
Table 1. Characteristics* of the study population by menopausal status.
| Variable | Pre/Early Peri (n=297) |
Late Peri (n=58) |
Post (n=128) |
P-value |
|---|---|---|---|---|
| Age, years | 48.8 (2.2) | 50.8 (2.3) | 52.6 (2.6) | <0.001 |
| % Black | 107 (36.0) | 28 (48.3) | 55 (43.0) | 0.134 |
| Education | 0.091 | |||
| % ≤ High School | 35 (12.3) | 11 (19.3) | 22 (17.7) | |
| % Post High School | 78 (27.4) | 18 (31.6) | 44 (35.5) | |
| % ≥College | 172 (60.4) | 28 (49.1) | 58 (46.8) | |
| % Current Smokers | 45 (16.9) | 7 (12.7) | 16 (14.4) | 0.670 |
| Systolic Blood Pressure, mmHg | 118.1 (16.2) | 121.8 (15.0) | 123.0 (17.8) | 0.015 |
| Diastolic Blood Pressure, mmHg | 75.3 (10.4) | 78.0 (8.3) | 77.7 (9.7) | 0.029 |
| Cholesterol, mg/dL | 191.6 (33.7) | 209.4 (44.4) | 214.7 (33.6) | <0.001 |
| LDL, mg/dL | 113.7 (28.7) | 127.7 (40.8) | 128.4 (35.4) | <0.001 |
| HDL, mg/dL | 56.4 (13.8) | 54.3 (12.8) | 61.1 (17.2) | 0.004 |
| Triglycerides, mg/dL† | 91.0 (1132.0) | 107.5 (396.0) | 111.0 (509.0) | <0.001 |
| Glucose, mg/dL† | 87.0 (167.0) | 88.0 (76.0) | 92. (242.0) | 0.002 |
| Insulin, uIu/mL† | 8.4 (120.2) | 12.3 (70.5) | 10.6 (49.3) | <0.001 |
| HOMA Insulin Resistance Index† | 1.7 (27.5) | 2.7 (16.7) | 2.3 (40.0) | <0.001 |
| Waist Circumference, cm | 88.2 (14.6) | 90.1 (12.7) | 90.8 (14.8) | 0.163 |
| BMI, kg/m2 | 29.0 (6.5) | 30.4 (5.7) | 29.7 (6.8) | 0.259 |
| Estradiol, pg/mL† | 45.7 (597.4) | 22.7 (317.8) | 15.9 (431.1) | <0.001 |
| Testosterone, ng/dL† | 34.5 (89.5) | 42.6 (72.6) | 36.7 (95.3) | <0.001 |
| FSH, mIU/mL† | 18.2 (159.5) | 70.7 (148.9) | 94.3 (266.9) | <0.001 |
| SHBG, nM† | 44.5 (208.4) | 39.5 (116.0) | 38.2 (133.7) | 0.108 |
| FAI† | 2.8 (169.6) | 3.9 (18.5) | 3.3 (30.2) | 0.001 |
| CCA Adventitial Diameter (mm) | 6.65 (0.54) | 6.96 (0.75) | 6.87 (0.57) | <0.001 |
| CCA IMT (mm)† | 0.65 (0.70) | 0.69 (0.40) | 0.67 (0.58) | 0.011 |
values are mean (SE) or n (%), unless otherwise indicated
values are median (interquartile range)
BMI: Body Mass Index; CCA: Common Carotid Artery; FAI: Free Androgen Index; FSH: Follicle stimulating hormone; HDL: High Density Lipoprotein Cholesterol; LDL: IMT: Intima-media Thickness; Low Density Lipoprotein Cholesterol; SHBG: Sex Hormone Binding Globulin
Table 2.
Spearman correlations between traditional cardiovascular risk factors and CCA diameter and IMT levels
| Risk Factor | CCA Adventitial Diameter |
CCA IMT |
||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Age | 0.16 | <0.001 | 0.22 | <0.001 |
| Systolic Blood Pressure | 0.38 | <0.001 | 0.35 | <0.001 |
| Diastolic Blood Pressure | 0.30 | <0.001 | 0.19 | <0.001 |
| Total Cholesterol | 0.05 | 0.279 | 0.05 | 0.331 |
| LDL | 0.05 | 0.254 | 0.09 | 0.077 |
| HDL | −0.14 | 0.003 | −0.08 | 0.109 |
| Triglycerides | 0.10 | 0.036 | 0.04 | 0.394 |
| Glucose | 0.21 | <0.001 | 0.26 | <0.001 |
| Insulin | 0.32 | <0.001 | 0.25 | <0.001 |
| HOMA | 0.33 | <0.001 | 0.28 | <0.001 |
| Waist Circumference | 0.38 | <0.001 | 0.32 | <0.001 |
| BMI | 0.38 | <0.001 | 0.32 | <0.001 |
BMI: Body Mass Index; CCA: Common Carotid Artery; HDL: High Density Lipoprotein Cholesterol; IMT: Intima-media Thickness; LDL: Low Density Lipoprotein Cholesterol
RESULTS
Two hundred and ninety-seven (61.5%) women were classified as premenopausal or early perimenopausal, 58 (12.0%) as late perimenopausal, and 128 (26.5%) as postmenopausal. Cardiovascular disease risk factors differed among women at earlier versus later stages of the menopausal transition (Table 1). The menopause transition was associated with older age, and higher systolic blood pressure, total cholesterol, LDL, HDL, triglyceride, glucose, insulin, HOMA insulin resistance, FSH, and FAI levels, and lower estradiol levels. Diastolic blood pressure, testosterone, CCA adventitial diameter, and CCA IMT levels were higher among late perimenopausal women and appeared to remain at approximately the same level among postmenopausal women. With the exception of cholesterol levels, higher levels of traditional cardiovascular risk factors were associated with significantly larger CCA adventitial diameters and greater IMT (Table 2).
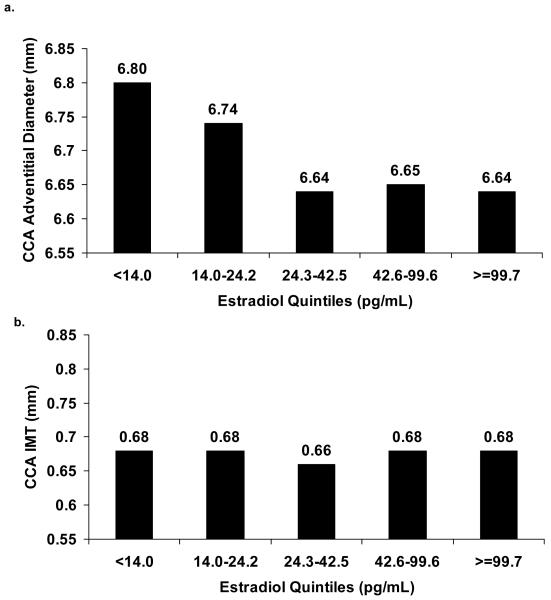
Late perimenopause and postmenopause were associated with significantly larger adventitial diameter values after adjustment for age (Table 3). Further multivariable adjustment attenuated the association with postmenopause but did not alter the association with late perimenopause. After multivariable adjustment, late perimenopause vs. pre/early perimenopause was associated with a 0.29 mm larger CCA adventitial diameter. CCA IMT did not differ between menopausal status groups after age adjustment. There were no significant interactions with race/ethnicity. Lower estradiol was associated with significantly larger adventitial diameters, and this persisted after adjustment for age, cycle day of blood draw, and other CVD risk factors (Table 4). Higher SHBG levels were also associated with smaller adventitial diameter and with smaller IMT. However, adjustment for CVD risk factors attenuated these associations. This attenuation was primarily due to adjustment for body mass index in the case of CCA adventitial diameter, and systolic blood pressure in the case of CCA IMT. No other hormones showed significant associations with either arterial diameter or IMT. There were no significant interactions between any endogenous hormone and race/ethnicity. Figure 1 presents the mean CCA adventitial diameter and IMT values by quintiles of estradiol, from a model with multivariate adjustments. A threshold effect appears to emerge for estradiol with CCA adventitial diameters, where-by the association between estradiol and CCA adventitial diameter levels off at the 3rd quintile, or at an estradiol value of approximately 24.2 pg/mL. When estradiol was dichotomized at this point, women with estradiol values below this point had significantly larger adventitial diameters than women with estradiol values at or above this point (6.77 vs. 6.64 mm, p=0.047), even after adjustment for age, race/ethnicity, site, education, current cigarette smoking, systolic blood pressure, height, BMI, HDL cholesterol, LDL cholesterol, triglycerides, log glucose, log HOMA insulin resistance, and cycle day of blood draw. This value is very close to the median estradiol value among late perimenopausal women (Table 1). Examination of quintiles of other hormones did not demonstrate possible non-linear or threshold effects.
Table 3.
Regression estimates of CCA diameter and IMT levels associated with late peri and postmenopause compared to pre/early perimenopause
| Menopause Status Category |
CCA Adventitial Diameter (mm) |
CCA IMT (mm) |
|---|---|---|
| Age-Adjusted | ||
| Late Perimenopausal | ||
| β (SE) | 0.28 (0.09) | 0.009 (0.01) |
| p-value | 0.001 | 0.505 |
| Postmenopausal | ||
| β (SE) | 0.15 (0.07) | −0.009 (0.01) |
| p-value | 0.040 | 0.470 |
| Multivariable-Adjusted * | ||
| Late Perimenopausal | ||
| β (SE) | 0.29 (0.09) | 0.009 (0.01) |
| p-value | 0.001 | 0.520 |
| Postmenopausal | ||
| β (SE) | 0.11 (0.08) | −0.007 (0.01) |
| p-value | 0.188 | 0.581 |
Multivariable adjustment for age, race/ethnicity, site, education, current smoking, systolic blood pressure, height, body mass index, HDL cholesterol, LDL Cholesterol, triglycerides, log glucose, and log HOMA insulin resistance index.
CCA: Common Carotid Artery; HDL: High Density Lipoprotein; IMT: Intima-media Thickness; LDL: Low Density Lipoprotein; SE: Standard Error
Table 4.
Regression estimates between endogenous sex hormones and CCA adventitial diameter and IMT
| CCA Adventitial Diameter (mm) | CCA IMT (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 1* | Model 2† | |||||
| Hormone | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value |
| Log Estradiol, per SD (1.09 pg/mL) | −0.09 (0.03) | 0.001 | −0.07 (0.03) | 0.023 | −0.007 (0.005) | 0.139 | −0.002 (0.005) | 0.663 |
| Log Testost., per SD (0.50 ng/dL) | −0.006 (0.03) | 0.841 | −0.04 (0.03) | 0.244 | −0.0001 (0.005) | 0.985 | −0.005 (0.005) | 0.259 |
| Log FSH, per SD (1.01 mIU/mL) | −0.004 (0.03) | 0.914 | 0.04 (0.04) | 0.313 | −0.001 (0.005) | 0.827 | 0.004 (0.006) | 0.525 |
| Log SHBG, per SD (0.65 nM) | −0.06 (0.03) | 0.036 | −0.01 (0.03) | 0.649 | −0.01 (0.004) | 0.035 | −0.002 (0.005) | 0.708 |
| Log FAI, per SD (0.83) | 0.04 (0.03) | 0.124 | −0.01 (0.03) | 0.712 | 0.008 (0.005) | 0.097 | −0.002 (0.005) | 0.681 |
Model 1 adjusted for: age and cycle day of blood draw.
Model 2 adjusted for: age, race/ethnicity, site, education, current cigarette smoking, systolic blood pressure, height, body mass index, HDL cholesterol, LDL cholesterol, triglycerides, log glucose, log HOMA insulin resistance index, and cycle day of blood draw.
CCA: Common Carotid Artery; FAI: Free Androgen Index; FSH: Follicle Stimulating Hormone; IMT: Intima-media Thickness; SD: LDL: Low Density Lipoprotein; Standard Deviation; SHBG: Sex Hormone Binding Globulin
Figure 1.
Adjusted* least square mean CCA adventitial diameter (a) and CCA IMT (b) levels by quintile of estradiol.
*Multivariable adjustment for age, race/ethnicity, education, site, current smoking, diastolic blood pressure, height, body mass index, HDL cholesterol, LDL Cholesterol, triglycerides, log glucose, and log HOMA insulin resistance.
To ensure that results were not affected by the possible vascular effects of anti-hypertensive, lipid-lowering, or oral hypoglycemic agents, all of the above analyses were repeated adjusting for the use of these medications as well as excluding women on these medications (n=71) and results were similar (data not shown).
DISCUSSION
These data demonstrate that lower estradiol levels accompanying ovarian aging are associated with larger arterial diameters among menopausal women, and highlight the late perimenopause, specifically, as a critical time period for these vascular effects. Larger carotid artery diameters at end diastole are correlated with adverse cardiovascular risk factor levels, as shown in the Atherosclerosis Risk in Communities Study population and others,19-21 and are also associated with an increased risk of coronary heart disease. In the Rotterdam study, each standard deviation higher baseline lumen diameter was associated with a 10% increase in risk of incident myocardial infarction.22 A dilated artery has less ability to enlarge further in response to a stimulus, which may make the artery more vulnerable to damage by heightened risk factor levels. In the current study, the late perimenopause was identified as the most relevant window for diameter enlargement, coinciding with larger increases in blood pressure and lipids over the transition from pre/early perimenopause to late perimenopause.
Whether such associations result from functional or structural changes is, as yet, unknown. Given the relatively short time course of the menopausal transition over which these effects are implied to occur, acute functional mechanisms related to vascular tone may be most likely. The vascular effects of estrogen administration on both coronary and brachial endothelial function occur within 15 minutes of estradiol infusion.23 Estrogen has been shown to enhance activation of nitric oxide24 and to decrease endothelin levels.25 Additionally, estrogen may affect both vasodilation and vasoconstriction by increasing adrenergic activity.26,27 The diameter enlargement observed in the current study may also signal a compensatory response to alterations in sheer stress levels resulting from adverse stimuli such as increased blood pressure or enhanced wall thickening.28,29 CCA adventitial diameters were significantly larger among late perimenopausal and postmenopausal women and with lower estradiol levels, while CCA IMT values showed no association with menopause status or sex hormones after adjustment for chronological age. This pattern is consistent with the early compensatory process, where changes in IMT may be masked by dilation; associations may be absent with CCA IMT because it is maintained at a constant level via dilation of the arteries. The SWAN study has just finished collecting prospective diameter and IMT change data which can be used to verify whether compensatory dilation is occurring in these menopausal women.
Although less likely, structural changes may also underlie the current results. Larger vessel diameters may be a secondary result of changes in the connective tissue content of the vessel wall. The connective tissue content of the body, including the cardiovascular system, has been shown to decrease with menopause and increase with hormone therapy use.13 Lower estrogen levels may also result in an enhanced inflammatory response which could elicit structural alterations to the vasculature.30 Existing literature concerning the relationships between androgens and the vasculature among women presents conflicting results. A case-control study among postmenopausal women in the Atherosclerosis Risk in Communities study found that women in the highest quartile of testosterone had the smallest odds of having subclinical atherosclerosis.11 A cross-sectional study of postmenopausal women found similar results, though did not make multivariable adjustments.12 However, prospective data from the Rancho Bernardo study detected no association between androgens and incident cardiovascular disease among postmenopausal women.9 Our data are in agreement with Rancho Bernardo, finding no association between either testosterone or the free androgen index and arterial diameter or IMT.
This study identified late perimenopause, specifically, as a critical time period for larger arterial diameters. We have previously shown that a dietary and exercise intervention slowed the progression of subclinical atherosclerosis only among women who had reached perimenopause or postmenopause, and did not affect progression among premenopausal women.31 Future research is needed to confirm these findings. However, should the perimenopause continue to be identified as the point at which the most dramatic vascular changes occur, this would have great relevance to the targeting of risk reduction strategies among women.
The results of this study need to be viewed within the context of its limitations. This study did not measure free estrogen and androgen values directly. Direct measurement would likely have strengthened the results demonstrated here. Additionally, the cross-sectional nature of this study precludes inferences about causality between endogenous sex hormones and vascular characteristics. The hypotheses generated from the current study need to be tested with longitudinal data among menopausal women, such as those which will soon be available from the two-year follow-up scans of the SWAN atherosclerosis ancillary study. This study had several strengths. Blood draws were standardized to cycle day for women who were menstruating. Although the days chosen (days 2-5 of menses) may not have been optimum for all hormones evaluated, standardization by cycle day likely enabled much clearer interpretations of hormone results than in previous studies in which blood draws were typically not timed to day of cycle. Additionally, to our knowledge these are the first data to assess the relationships between menopausal status or endogenous sex hormones in a population including perimenopausal women. Finally, these data are among the first to include a large percentage of African American women, enabling us to assess the consistency of associations across race/ethnicity. The negative effects of declining endogenous estrogens on arterial diameters seen in the current study, when viewed in light of the recent null or harmful results of the WHI and the HERS trials concerning exogenous estrogen administration, underscore the complexities of the effects of hormone levels on the cardiovascular system. It has been suggested that the unexpected results of the WHI and HERS trials may have been due, in part, to the older age of the study participants and the relatively lengthy number of years since the final menstrual period, hypothesizing that the effects of exogenous estrogen therapy on the vasculature may be beneficial if initiated early in the menopausal transition.5 Two recent WHI publications support this hypothesis. First, re-analyses of the WHI trial data found no increased CVD risk among younger women or women who initiated hormone therapy closer to their final menstrual period, and though not significant, suggested the possibility of a cardioprotective effect of HT among these women.7 Second, data from the WHI coronary-artery calcification ancillary study (WHI-CACS) showed that among women aged 50-59 years at the time of HT initiation in the WHI, HT was associated with a 22% lower odds of the presence of coronary-artery calcification.8 Data from the current study show that the menopausal transition and loss of endogenous estrogen in perimenopausal women is associated with diameter enlargement, and though speculative, suggest that estrogen administration in perimenopausal women may help to retain vessel health. Given the effects of the menopausal transition on CVD risk, this hypothesis warrants further examination. Additional research is needed into whether these effects are structural or functional, whether they are acute or permanent, and whether they alter the effects of exogenous hormones.
ACKNOWLEDGMENTS
SWAN Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell; (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair, Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Dr. Wildman received partial support for the analysis and interpretation of these data from National Institutes of Health grant K12 HD43451. The Study of Women’s Health Across the Nation (SWAN) is supported by the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health and the SWAN Heart Study is supported by the National Heart, Lung, and Blood Institute (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495; HL065581, HL065591). The Chicago site of the SWAN Heart Study also is supported by the Charles J. and Margaret Roberts Trust.
Contributor Information
Rachel P. Wildman, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY.
Alicia B. Colvin, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA.
Lynda H. Powell, Departments of Preventive Medicine and Behavioral Sciences, Rush University Medical Center, Chicago, IL.
Karen A. Matthews, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, and Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Susan A. Everson-Rose, Department of Preventive Medicine, and Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL.
Steven Hollenberg, Section of Cardiology, Cooper University Hospital, Camden, NJ.
Janet M. Johnston, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA.
Kim Sutton-Tyrrell, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA.
REFERENCES
- (1).Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- (2).Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- (3).Hulley S, Grady D, Bush T, et al. Heart and Estrogen/progestin Replacement Study (HERS) Research Group Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- (4).Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- (5).Harman SM, Brinton EA, Clarkson T, et al. Is the WHI relevant to HRT started in the perimenopause? Endocrine. 2004;24:195–202. doi: 10.1385/ENDO:24:3:195. [DOI] [PubMed] [Google Scholar]
- (6).Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- (7).Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- (8).Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- (9).Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ. 1995;311:1193–1196. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Price JF, Lee AJ, Fowkes FG. Steroid sex hormones and peripheral arterial disease in the Edinburgh Artery Study. Steroids. 1997;62:789–794. doi: 10.1016/s0039-128x(97)00103-7. [DOI] [PubMed] [Google Scholar]
- (11).Golden SH, Maguire A, Ding J, et al. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155:437–445. doi: 10.1093/aje/155.5.437. [DOI] [PubMed] [Google Scholar]
- (12).Bernini GP, Sgro’ M, Moretti A, et al. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab. 1999;84:2008–2012. doi: 10.1210/jcem.84.6.5824. [DOI] [PubMed] [Google Scholar]
- (13).Jayachandran M, Miller VM. Molecular and Cellular Mechanisms of Estrogen’s Actions. In: Douglas PS, editor. Cardiovascular Health and Disease in Women - Section III: Hormones and Heart Disease. Second Edition W.B. Saunders Company; Philadelphia, PA: 2004. pp. 207–30. [Google Scholar]
- (14).Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- (15).Sowers MF, Crawford S, Sternfeld B, et al. Design, survey sampling and recruitment methods of SWAN: a multi-cetner, multi-ethnic community-based cohort study of women and the menopausal transition. In: Lobos R, Marcus R, Kelsey JL, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego: 2000. pp. 175–88. [Google Scholar]
- (16).Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- (17).Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- (18).Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- (19).Crouse JR, Goldbourt U, Evans G, et al. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27:69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
- (20).Jensen-Urstad K, Jensen-Urstad M, Johansson J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke. 1999;30:1572–1576. doi: 10.1161/01.str.30.8.1572. [DOI] [PubMed] [Google Scholar]
- (21).Bonithon-Kopp C, Touboul PJ, Berr C, Magne C, Ducimetiere P. Factors of carotid arterial enlargement in a population aged 59 to 71 years: the EVA study. Stroke. 1996;27:654–660. doi: 10.1161/01.str.27.4.654. [DOI] [PubMed] [Google Scholar]
- (22).Bots ML, Grobbee DE, Hofman A, Witteman JC. Common carotid intima-media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke. 2005;36:762–767. doi: 10.1161/01.STR.0000158924.71069.94. [DOI] [PubMed] [Google Scholar]
- (23).Gilligan DM, Quyyumi AA, Cannon RO., III Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- (24).Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17_- Estradiol Regulation of Human Endothelial Cell Basal Nitric Oxide Release, Independent of Cytosolic Ca2+ Mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- (25).Ylikorkala O, Orpana A, Puolakka J, Pyorala T, Viinikka L. Postmenopausal hormonal replacement decreases plasma levels of endothelin-1. J Clin Endocrinol Metab. 1995;80:3384–3387. doi: 10.1210/jcem.80.11.7593457. [DOI] [PubMed] [Google Scholar]
- (26).Hamlet MA, Rorie DK, Tyce GM. Effects of estradiol on release and disposition of norepinephrine from nerve endings. Am J Physiol. 1980;239:H450–H456. doi: 10.1152/ajpheart.1980.239.4.H450. [DOI] [PubMed] [Google Scholar]
- (27).Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33:323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- (28).Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- (29).Polak JF, Kronmal RA, Tell GS, et al. Compensatory increase in common carotid artery diameter. Relation to blood pressure and artery intima-media thickness in older adults. Cardiovascular Health Study. Stroke. 1996;27:2012–2015. doi: 10.1161/01.str.27.11.2012. [DOI] [PubMed] [Google Scholar]
- (30).Harnish DC, Scicchitano MS, Adelman SJ, Lyttle CR, Karathanasis SK. The role of CBP in estrogen receptor cross-talk with nuclear factor-kappaB in HepG2 cells. Endocrinology. 2000;141:3403–3411. doi: 10.1210/endo.141.9.7646. [DOI] [PubMed] [Google Scholar]
- (31).Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579–585. doi: 10.1016/j.jacc.2004.03.078. [DOI] [PubMed] [Google Scholar]