17-1A antibody, a mouse monoclonal antibody, has proved to be efficacious in the adjuvant treatment of colorectal carcinoma of Dukes’s type C, reducing the death rate by 30% and the relapse rate by 27%.1,2 Cutaneous side effects have been reported. We report a case in which skin lesions persisted for months after treatment was discontinued.
A 73 year old woman was diagnosed as having adenocarcinoma of the colon in 1996 and was treated by subtotal colectomy. According to an established treatment protocol, she was given intravenous 17-1A antibody (Panorex) at a dose of 500 mg two weeks before tumour resection, followed by four infusions of 100 mg at intervals of four weeks thereafter. Four days after the second infusion she developed a burning rash characterised by red macules and weals, but she did not have any systemic side effects. Six weeks after the last drug infusion she had sharply demarcated erythematous macules, some of which were as large as the palm of a hand. The lesions were blanched at the centre with discrete brownish discolouration and looked like urticaria (figure). Histopathology of the lesions showed a superficial perivascular dermatitis. Direct immunofluorescence analysis showed a positive reaction at the vessels with C3 complement. Laboratory findings were normal. The lesions did not totally resolve between treatments, and readministration of the drug always slightly increased their severity. The skin lesions disappeared around four months after the last infusion.
The clinical and histological findings as well as the link between repeated infusions and development of the lesions indicated a drug rash, but a causal relation was not finally proved. However, infused antibody elicits both a humoral and a T cell response against idiotopes. Although the induction of an immune response like a cascade might be important for destroying tumour residues, in patients who are almost disease free the concentrations of antibodies might induce allergic reactions.3–5
Figure.
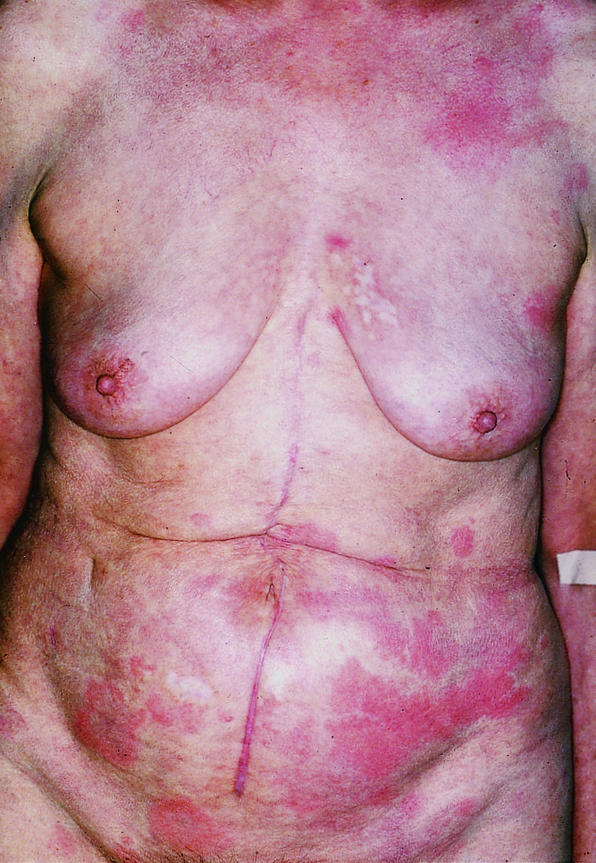
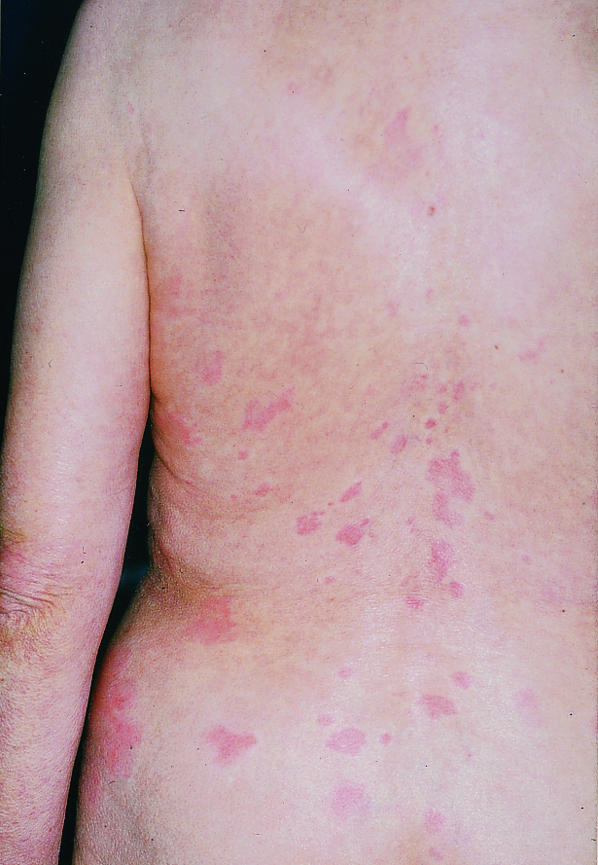
Urticarial rash with 17-1A antibody. Reproduced with patient’s permission
References
- 1.Haller DG. An overview of adjuvant therapy for colorectal cancer. Eur J Cancer. 1995;31A:1255–1263. doi: 10.1016/0959-8049(95)00258-k. [DOI] [PubMed] [Google Scholar]
- 2.Riethmüller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, et al. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes’ C colorectal carcinoma. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 3.LoBuglio AF, Wheeler RH, Trang K, Haynes A, Rogers K, Harvey EB, et al. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci. 1989;8G:4220–4224. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagerberg J, Ragnhammar P, Liljefors M, Hjelm A-L, Mellstedt H, Frodin J-L. Humoral antiidiotypic and anti-anti-idiotypic immune response in cancer patients treated with monoclonal antibody 17-1A. Cancer Immunol Immunother. 1985;42:81–87. doi: 10.1007/s002620050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagerberg J, Steinitz M, Wigzell H, Askelof P, Mellstedt H. Human anti-idiotypic antibodies induced a humoral and cellular immune response against a colorectal carcinoma associated antigen in patients. Proc Natl Acad Sci. 1995;92:4773–4777. doi: 10.1073/pnas.92.11.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]


