Abstract
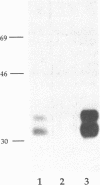
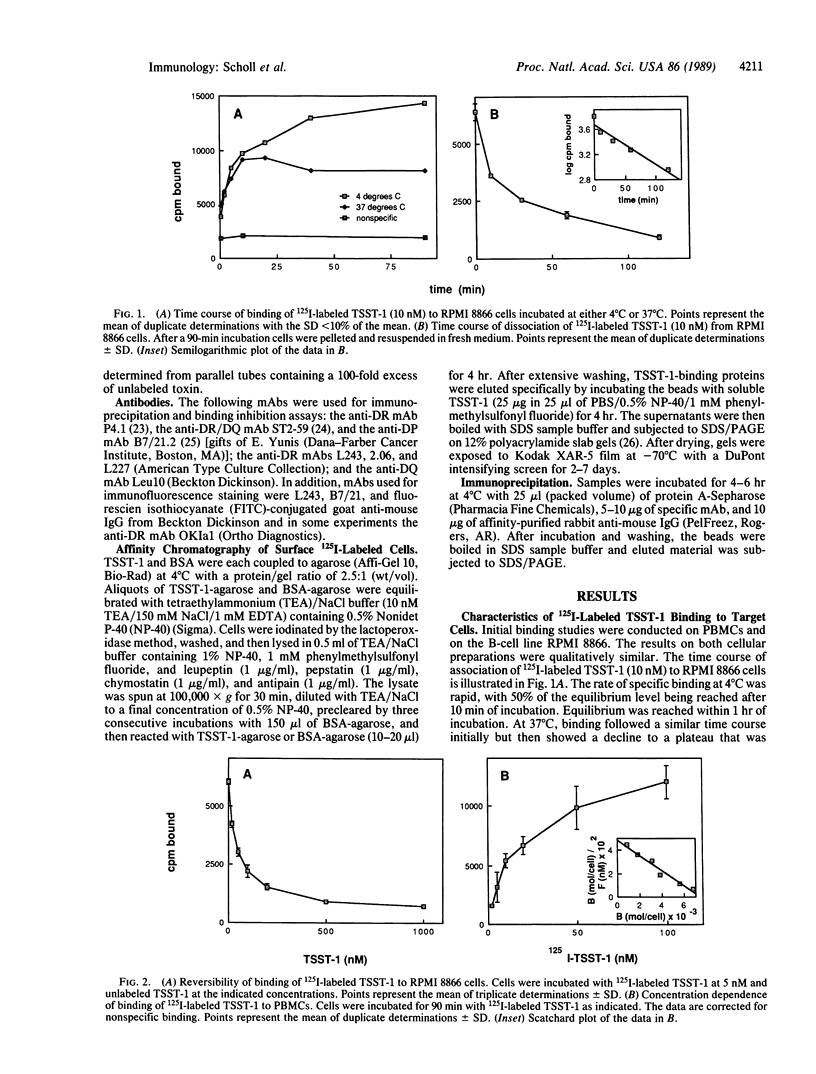
Toxic shock syndrome toxin 1 (TSST-1) is a 22-kDa exotoxin produced by strains of Staphylococcus aureus and implicated in the pathogenesis of toxic shock syndrome. In common with other staphylococcal exotoxins, TSST-1 has diverse immunological effects. These include the induction of interleukin 2 receptor expression, interleukin 2 synthesis, proliferation of human T lymphocytes, and stimulation of interleukin 1 synthesis by human monocytes. In the present study, we demonstrate that TSST-1 binds with saturation kinetics and with a dissociation constant of 17-43 nM to a single class of binding sites on human mononuclear cells. There was a strong correlation between the number of TSST-1 binding sites and the expression of major histocompatibility complex class II molecules, and interferon-gamma induced the expression of class II molecules as well as TSST-1 binding sites on human skin-derived fibroblasts. Monoclonal antibodies to HLA-DR, but not to HLA-DP or HLA-DQ, strongly inhibited TSST-1 binding. Affinity chromatography of 125I-labeled cell membranes over TSST-1-agarose resulted in the recovery of two bands of 35 kDa and 31 kDa that comigrated, respectively, with the alpha and beta chains of HLA-DR and that could be immunoprecipitated with anti-HLA-DR monoclonal antibodies. Binding of TSST-1 was demonstrated to HLA-DR and HLA-DQ L-cell transfectants. These results indicate that major histocompatibility complex class II molecules represent the major binding site for TSST-1 on human cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdoll M. S., Crass B. A., Reiser R. F., Robbins R. N., Davis J. P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981 May 9;1(8228):1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Novick R. P., Schlievert P. M. Localization of biologic functions of toxic shock syndrome toxin-1 by use of monoclonal antibodies and cyanogen bromide-generated toxin fragments. J Immunol. 1986 Dec 1;137(11):3572–3576. [PubMed] [Google Scholar]
- Bonventre P. F., Thompson M. R., Adinolfi L. E., Gillis Z. A., Parsonnet J. Neutralization of toxic shock syndrome toxin-1 by monoclonal antibodies in vitro and in vivo. Infect Immun. 1988 Jan;56(1):135–141. doi: 10.1128/iai.56.1.135-141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano S. E., Quimby F. W., Antonacci A. C., Reiser R. F., Bergdoll M. S., Dineen P. Analysis of the mitogenic effects of toxic shock toxin on human peripheral blood mononuclear cells in vitro. Clin Immunol Immunopathol. 1984 Oct;33(1):99–110. doi: 10.1016/0090-1229(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Chatila T., Wood N., Parsonnet J., Geha R. S. Toxic shock syndrome toxin-1 induces inositol phospholipid turnover, protein kinase C translocation, and calcium mobilization in human T cells. J Immunol. 1988 Feb 15;140(4):1250–1255. [PubMed] [Google Scholar]
- Dasgupta J. D., Relias V., Awdeh Z. L., Alper C. A., Yunis E. J. Two variants of DRw52, DR3, and DQw2 specificities distinguish two different DR3-bearing extended haplotypes. Hum Immunol. 1988 Feb;21(2):133–142. doi: 10.1016/0198-8859(88)90088-2. [DOI] [PubMed] [Google Scholar]
- Davis J. P., Chesney P. J., Wand P. J., LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980 Dec 18;303(25):1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Human lymphocyte mitogenic factor: synthesis by sensitized thymus-derived lymphocytes, dependence of expression on the presence of antigen. Cell Immunol. 1974 Jan;10(1):86–104. doi: 10.1016/0008-8749(74)90154-3. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klohe E. P., Watts R., Bahl M., Alber C., Yu W. Y., Anderson R., Silver J., Gregersen P. K., Karr R. W. Analysis of the molecular specificities of anti-class II monoclonal antibodies by using L cell transfectants expressing HLA class II molecules. J Immunol. 1988 Sep 15;141(6):2158–2164. [PubMed] [Google Scholar]
- Kushnaryov V. M., MacDonald H. S., Reiser R., Bergdoll M. S. Staphylococcal toxic shock toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect Immun. 1984 Sep;45(3):566–571. doi: 10.1128/iai.45.3.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Nepom B. S., Antonelli P., Mickelson E., Silver J., Goyert S. M., Hansen J. A. The HLA-DR4 family of haplotypes consists of series of distinct DR and DS molecules. J Exp Med. 1984 Feb 1;159(2):394–404. doi: 10.1084/jem.159.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Pier G. B. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis. 1986 Jul;154(1):55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Richter A. G., Pier G. B. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987 May;55(5):1070–1076. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Hickman R. K., Eardley D. D., Pier G. B. Induction of human interleukin-1 by toxic-shock-syndrome toxin-1. J Infect Dis. 1985 Mar;151(3):514–522. doi: 10.1093/infdis/151.3.514. [DOI] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Binding of toxic-shock-syndrome toxin-1 to human peripheral blood mononuclear cells. J Infect Dis. 1987 Jul;156(1):122–129. doi: 10.1093/infdis/156.1.122. [DOI] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Suppression of immunoglobulin-secreting cells from human peripheral blood by toxic-shock-syndrome toxin-1. J Infect Dis. 1986 Apr;153(4):772–779. doi: 10.1093/infdis/153.4.772. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Shands K. N., Schmid G. P., Dan B. B., Blum D., Guidotti R. J., Hargrett N. T., Anderson R. L., Hill D. L., Broome C. V., Band J. D. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980 Dec 18;303(25):1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978 Nov 25;2(8100):1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Katzen D., Chatila T., Miller R., Jabara H. H., Maher M., Oettgen H., Terhorst C., Geha R. S. Requirements for activation of human peripheral blood T cells by mouse monoclonal antibodies to CD3. Clin Immunol Immunopathol. 1987 Apr;43(1):48–64. doi: 10.1016/0090-1229(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Katzen D., Jabara H. H., Geha R. S. Antigen presentation by human dermal fibroblasts: activation of resting T lymphocytes. J Immunol. 1986 Jan;136(2):440–445. [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]