Abstract
Recent reports indicate that (−)-epicatechin can exert cardioprotective actions, which may involve eNOS-mediated nitric oxide production in endothelial cells. However, the mechanism by which (−)-epicatechin activates eNOS remains unclear. In this study, we proposed to identify the intracellular pathways involved in (−)-epicatechin-induced effects on eNOS, utilizing human coronary artery endothelial cells in culture. Treatment of cells with (−)-epicatechin leads to time- and dose-dependent effects, which peaked at 10 min at 1 μmol/L. (−)-Epicatechin treatment activates eNOS via serine-633 and serine-1177 phosphorylation and threonine-495 dephosphorylation. Using specific inhibitors, we have established the participation of the PI3K pathway in eNOS activation. (−)-Epicatechin induces eNOS uncoupling from caveolin-1 and its association with calmodulin-1, suggesting the involvement of intracellular calcium. These results allowed us to propose that (−) epicatechin effects may be dependent on actions exerted at the cell membrane level. To test this hypothesis, cells were treated with the phospholipase C inhibitor U73122, which blocked (−)-epicatechin-induced eNOS activation. We also demonstrated inositol phosphate accumulation in (−)-epicatechin-treated cells. The inhibitory effects of the pre-incubation of cells with the CaMKII inhibitor KN-93 indicate that (−)-epicatechin-induced eNOS activation is at least partially mediated via the Ca2+/CaMKII pathway. The (−)-epicatechin stereoisomer catechin was only able to partially stimulate nitric oxide production in cells. Altogether, these results strongly suggest the presence of a cell surface acceptor-effector for the cacao flavanol (−)-epicatechin, which may mediate its cardiovascular effects.
Keywords: flavonoids, ischemia, polyphenols, endothelial cells, endothelial nitric oxide synthase
INTRODUCTION
Cardiovascular diseases (CVD) are is a leading cause of morbidity and mortality, affecting Western industrialized countries as well as developing countries [1, 2]. CVD imposes major direct and indirect costs on health care systems, ranging from hospitalizations, drugs and rehabilitation services to losses of productivity due to premature mortality and short/long-term disability [1]. The incidence of CVD continues to increase; thus, it is important to identify potential therapeutic agents to prevent and treat CVD.
CVD is importantly linked to endothelial cell dysfunction. Endothelial cell dysfunction is associated with conditions, such as obesity, smoking, diabetes and hormonal changes. The vascular endothelium mediates many important physiological functions, such as responses to shear stress, angiogenesis, vascular remodeling, inflammation and coagulation. It also participates in metabolic and synthetic processes [3]. A key modulator of endothelial cell activity is nitric oxide (NO), which under physiological conditions is mainly produced by the endothelial nitric oxide synthase (eNOS) isoform. NO regulates vascular tone, proliferation of vascular smooth muscle cells and hemostasis amongst other important functions. Disruptions in the physiological production of NO triggers endothelial cell dysfunction, resulting in an increased susceptibility to CVD [4]. Therefore, strategies aimed at “physiologically” increasing NO bioavailability are promising for the prevention and therapy of CVD.
The induction of NO synthesis by flavonoid-containing compounds has received widespread attention, as their effects appear to positively impact CVD. Epidemiological studies indicate that the regular dietary intake of plant-derived foods and beverages high in flavonoids is inversely associated with the incidence of CVD [5]. Although these compounds are pleiotropic in nature, many of their effects may be explained by improving endothelial function. The regular consumption of cacao products high in flavonoid content has also been demonstrated to provide beneficial cardiovascular effects [5]. Natural cacao products are rich in monomeric and polymeric forms of the flavonoids EPI and catechin (60/40 ratio) and can contain up to 10% flavanoids by weight [6]. The consumption of cacao products can ameliorate hypertension in animal models and in humans [5]. This effect has been ascribed to the ability of cacao flavonoids to increase NO levels via eNOS activation. The vascular effects of cacao can be reproduced by EPI. EPI induces the relaxation of pre-constricted aortic rings and stimulates NO production via eNOS activation [7].
Studies in animals have demonstrated a critical role for NO in mediating cardioprotection. It has been demonstrated that EPI pre-treatment of rats limits infarct size in the setting of myocardial ischemia-reperfusion injury or with a permanent coronary occlusion [8,9]. In humans, the sustained consumption of chocolate is associated with a decrease in mortality post-myocardial infarction. The above described effects of EPI may be accounted for by the acute and/or chronic effects on eNOS. However, the signaling pathways by which the acute administration of EPI induces eNOS activation in endothelial cells are unclear.
In this study, we identified the intracellular signaling pathways by which EPI acts on eNOS to stimulate NO production using cultures of HCAEC. Our results suggest the participation of multiple pathways and the likely presence of a unique cell surface acceptor-effector for EPI.
METHODS
Cell Culture
HCAECs were obtained from Cell Applications, Inc. using three different lots. The company data sheet indicated that the cells were obtained from 14-, 40- and 60-year-old normal male donors in apparent good health.
Cells were maintained in a humidified atmosphere at 37°C with 5% CO2 and 95% O2 in HCAEC growth medium. Sixteen hours prior to experiments, HCAECs were washed with phenol red free Hank’s salts solution and kept in phenol red free M-199 supplemented with 200 mmol/L of L-glutamine and 1% antibiotic mix. Treatments were typically applied to confluent cell cultures.
NO measurements
NO levels were measured using a fluorometer (FLx800 Bio-Tek Instruments Inc.) at excitation and emission wavelengths of 360 nm and 430 nm, respectively. EPI was diluted in water (which served as vehicle for the control cells). EPI-induced NO time and dose response curves were generated. For time response curves, cells were treated with 1 μmol/L EPI, and culture media samples were collected at 0, 5, 10, 20, 30, 60, 120, 180 and 240 min. For concentration response curves, cells were treated with EPI [0.1 nmol/L–100 μmol/L] for 10 min (peak time of NO response).
Please see http://hyper.ahajournals.org for other methodologies
Results
Time-course of EPI-induced NO production
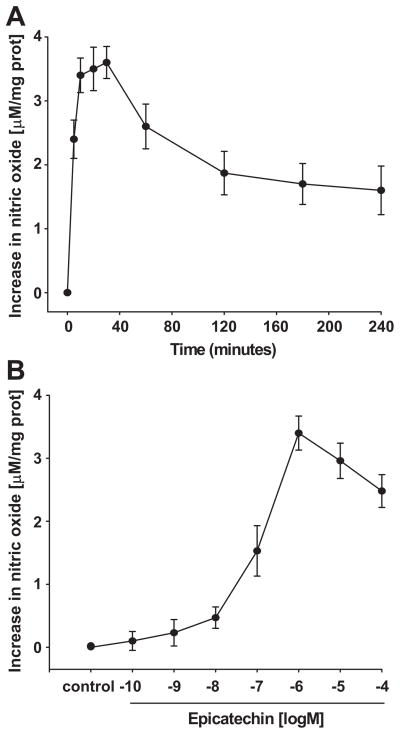
A time-course for EPI-induced NO production was performed using an arbitrarily selected concentration of 1 μmol/L. Results are shown in Figure 1A. Increases in NO were detected as early as 5 min after treatment, with a maximal effect occurring at 10–30 min. Given the comparable levels attained at these time points, all subsequent NO production experiments were carried out using 10 min of incubation. We performed EPI concentration-response curves on NO production. Results indicate that a maximal effect was obtained with 1 μmol/L EPI (Figure 1B). Subsequent experiments used this concentration and a 10-min time frame.
Figure 1. EPI-induced NO synthesis.
(A), Time-course of EPI [1 μmol/L]-induced NO synthesis in HCAECs. NO concentration levels show no statistical difference amongst 10, 20 and 30 minutes (ANOVA). (B), EPI dose-response curve on NO synthesis. HCAECs were treated with EPI [0.1 nmol/L – 100 μmol/L] for 10 min. Data are expressed as mean ± SD (n=3).
eNOS activation by EPI
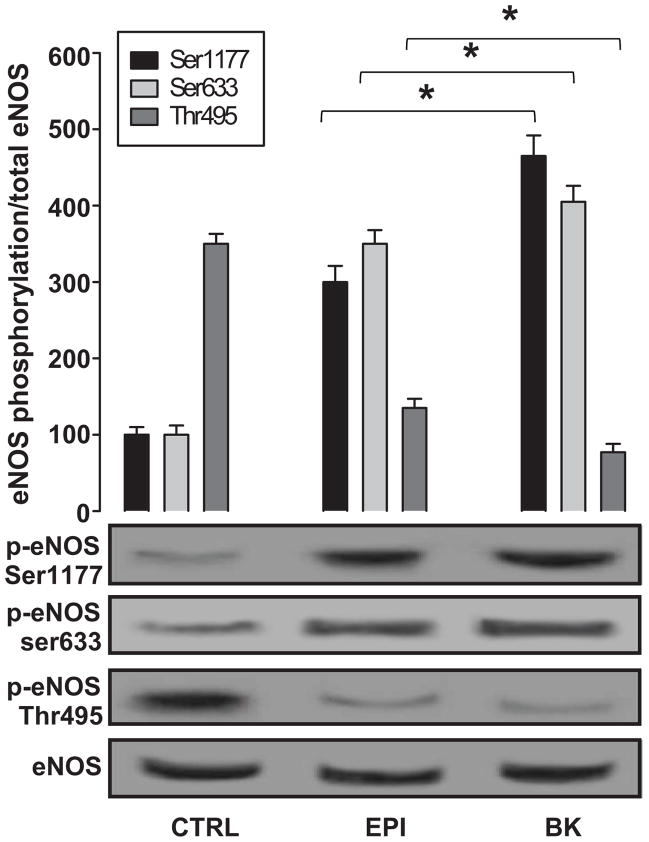
We examined the effects of EPI on eNOS activity by immunoblots, comparing the relative amount of phosphorylation at the activation residues Ser-1177 and Ser-633 and at the inactivation residue Thr-495. Total eNOS levels were used to normalize phospho-eNOS values. eNOS activity was measured under basal conditions (control) and with EPI. BK (1 μmol/L), a known eNOS activator, was used as a positive control. Untreated cells have basal levels of phosphorylation of Ser-1177, Ser-633 and Thr-495 (Figure 2). As can be observed, a relatively higher phosphorylation status of Thr-495 exists. In contrast, with EPI or BK treatment, a significant change in relative levels of the phosphorylation of Ser-1177 and Ser-633 and dephosphorylation of Thr-495 was induced.
Figure 2. EPI-induced eNOS activation via Ser-1177 and Ser-633 phosphorylation and Thr-495 dephosphorylation.
EPI [1 μmol/L] and BK [1 μmol/L] induced increases in the phosphorylation of residues Ser-1177 and Ser-633 and the dephosphorylation of Thr-495, as compared to control cells. Data are expressed as mean ± SD of n=3, in triplicate. Densitometric mean values of the phosphorylation of eNOS Ser-1177 and Ser-633 in controls were normalized to total eNOS densitometric values, and the results were set arbitrarily to a value equal to 100. EPI- and BK-induced effects were plotted as changes over control. EPI and BK values were statistically different from control. (*p<0.05 EPI vs. BK).
PI3K role in EPI induced eNOS activation
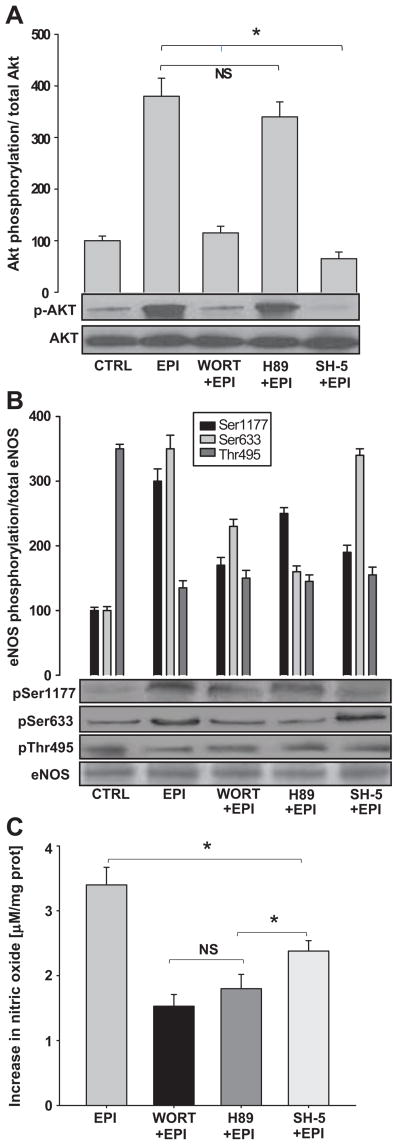
eNOS phosphorylation can occur via the PI3K pathway. Two downstream effectors of PI3K are PKA and Akt that, when activated, modulate eNOS activity through the selective phosphorylation of activation residues. PKA regulates Ser-1177 and Ser-633, while Akt only phosphorylates Ser-1177. WORT- and SH5-treated cells demonstrate less EPI-induced Akt phosphorylation, but no effect was seen with the PKA-inhibitor H89 (Figure 3A). In turn, the EPI-induced phosphorylation of eNOS Ser-1177 and Ser-633 was partially blocked with WORT and H89 pre-incubation. Phosphorylation of eNOS Ser-1177 was diminished but Ser-633 phosphorylation did not decrease with the use of SH5, as Akt does not phosphorylate this residue (Figure 3B). We also explored the effect of EPI on NO production in the presence of the inhibitors. Results demonstrate that EPI-induced NO synthesis was partially blocked when WORT, SH5 and H89 were used (Figure 3C).
Figure 3. PI3K signaling pathway mediates EPI-induced eNOS activation.
HCAECs were treated with WORT or H89 or SH-5 (see text for details) and stimulated with EPI [1 μmol/L] for 10 min. Western blots were probed with specific antibodies against Akt, p-Akt, eNOS, p-eNOS Ser-1177, Ser-633 and Thr-495; NO was measured as stated in the text. (A), EPI-induced AKT phosphorylation; this effect was blocked by WORT and SH5 pre-treatment. Normalized control densitometric levels were arbitrarily set to 100 in all cases. (B), eNOS activity was measured by its phosphorylation on Ser-1177 and Ser-633 and dephosphorylation on Thr-495. Pre-treatment with WORT and H89 leads to a decrease in eNOS phosphorylation on Ser-1177 and Ser-633. SH5 treatment decreased Ser-1177 phosphorylation. (C) The effects of WORT, H89 and SH-5 on EPI-induced NO synthesis. Control values were arbitrarily set to zero. Statistical analysis shows no difference between WORT and H-89. SH-5 effects were significantly different vs. H89. Data are expressed as mean ± SD (n=3). (*p<0.05).
Caveolin and calmodulin role in EPI-induced eNOS activation
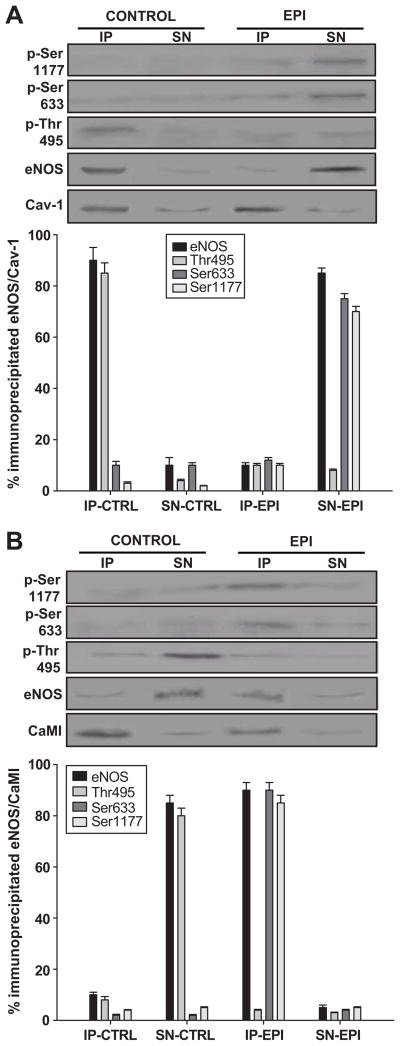
Given the reported role of Cav-1 and CaMI on eNOS signal transduction, we analyzed the interaction of eNOS with these proteins under EPI treatment. Untreated and EPI treated cells were immunoprecipitated using anti-Cav-1 or anti-CaMI antibodies, and the immunoprecipitated (IP) phase, as well as the supernatant (SN), were used for immunoblots of total eNOS protein and for the phosphorylation levels of Ser-1177, Ser-633 and Thr-495. Results are summarized in Figure 4A and B. Control cells demonstrated an “inactive” eNOS protein bound to Cav-1, as evidenced by the appearance of the eNOS protein in the IP phase and the relative low phosphorylation levels of Ser-1177 and Ser-633 vs. Thr-495. The SN showed negligible amounts of eNOS. With EPI treatment, only Cav-1 is observed in the IP phase, suggesting the uncoupling of eNOS. The presence of activated eNOS was seen in the SN phase with increased phosphorylation of Ser-1177 and Ser-633 and low levels of Thr-495 (Figure 4A and B). Results from immunoprecipitation with anti-CaMI antibodies demonstrate that under basal conditions the IP phase only contains the CaMI protein. With EPI treatment, immunoprecipitation with anti-CaMI demonstrates the coupling of activated eNOS to CaMI (Figure 4C and D).
Figure 4. EPI-induced eNOS decoupling from the caveola.
HCAECs were treated with EPI [1 μmol/L] for 10 min, lysed and immunoprecipitated with anti-Cav-1 or -CaMI antibodies. Both the immunoprecipitated (IP) and supernatant (SN) phases were analyzed for phosphorylation of Ser-1177, Ser-633 and Thr-495 as well as Cav-1 or CaMI and eNOS by immunoblots. (A) Control HCAECs demonstrate inactive eNOS associated with Cav-1. EPI treated HCAECs show only Cav-1 in the IP and activated eNOS in the SN phase. (B), Control HCAECs demonstrate no association between the active or inactive forms of eNOS and CaMI, and the SN phase demonstrates the inactive form of eNOS. EPI treated HCAECs demonstrate the activated form of eNOS linked to the immunoprecipitated CaMI, whereas the SN phase in EPI-treated HCAECs show only negligible amounts of eNOS. Data are expressed as mean ± SD (n=3).
CaMKII role on EPI-induced eNOS activation
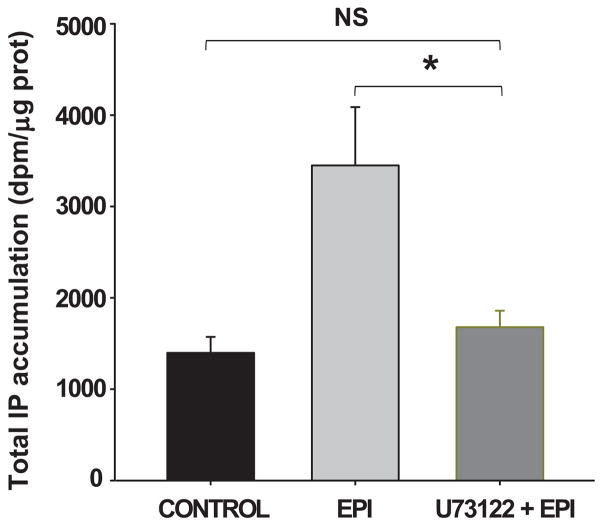
We determined if EPI could also stimulate calcium-dependent eNOS-related intracellular signaling pathways. Experiments were initially performed to determine if EPI treatment induces the accumulation of IP3 in cells. Results are summarized in Figure 5 and demonstrate that 10 min after treatment there is a significant accumulation of IP3 in cells. Bradykinin [1 μmol/L] was used as a positive control. The EPI- and BK-induced increase in IP3 was blocked with the pre-incubation with U73122.
Figure 5. EPI-induced increase in intracellular IP3 levels.
HCAECs were incubated with EPI [1 μmol/L] for 10 min. Total intracellular [3H]IP3 accumulation was measured. EPI-treated HCAECs demonstrate greater IP3 levels than the control. BK [1 μmol/L] was used as a positive control. IP3 levels decreased with the pre-incubation with U73122. Data are expressed as mean ± SD (n=3). (*p<0.05).
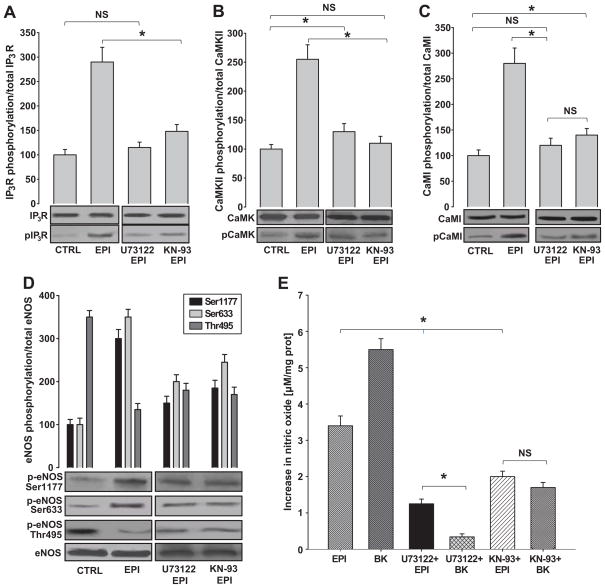
To further characterize the EPI-induced calcium-dependent activation of intracellular signaling pathways, we examined the extent of phosphorylation/activation on IP3R, CaMKII and CaMI using the inhibitors U73122 and KN-93, which block the activation of PLC and CaMKII, respectively. As shown in Figure 6A, EPI treatment induced higher levels of IP3R phosphorylation vs. controls. EPI-induced phosphorylation of IP3R was partially blocked with the pre-incubation of cells with U73122 or KN-93. To evaluate EPI effects on downstream effectors of the CaMI signaling pathway, we evaluated the phosphorylation/activation of CaMKII and CaMI. As expected, the levels of phosphorylation were higher in EPI-treated vs. control cells. The addition of each of the inhibitors led to decreased levels of phosphorylation of CaMKII (Figure 6B) and CaMI (Figure 6C).
Figure 6. EPI-induced eNOS activation is mediated by the CaM/CaMKII pathway.
HCAECs were pretreated with U73122 or KN-93 and stimulated with EPI 1 μmol/L for 10 min. Western blots were probed with specific antibodies for IP3, p-IP3, CaMKII, p-CaMKII, CaMI, p-CaMI, eNOS, p-eNOS Ser-1177, Ser-633 and Thr-495. NO was measured as stated in the text. (A) Phosphorylation of IP3R, (B) CaMKII and (C) CaMI was observed in EPI-treated HCAECs, and it was diminished in the three proteins with U73122 and KN-93 inhibitors. (D) EPI-induced eNOS Ser-1177 and Ser-633 phosphorylation and Thr-495 dephosphorylation was blocked by U73122 and KN-93. (E) EPI-induced increases in NO synthesis were diminished by U73122 and K-93 pre-treatment. BK [1 μmol/L] was used as a positive control. Data are expressed as mean ± SD (n=3) (*p<0.05).
We also assessed eNOS activity by measuring the phosphorylation of Ser-1177, Ser-633, Thr-495 and total eNOS in the absence or presence of U73122 and KN-93 (Figure 6D). Pre-incubation with U73122 induced a decrease in the phosphorylation of Ser-1177 and Ser-633. Interestingly, Thr-495 remained partially phosphorylated. The same effect was observed after incubation with the inhibitor KN-93, where Ser-1177, Ser-633 and Thr-495 were all phosphorylated (Figure 6D). Similarly, the synthesis of NO was diminished after the addition of these inhibitors to EPI or to bradykinin [1 μmol/L] (Figure 6E).
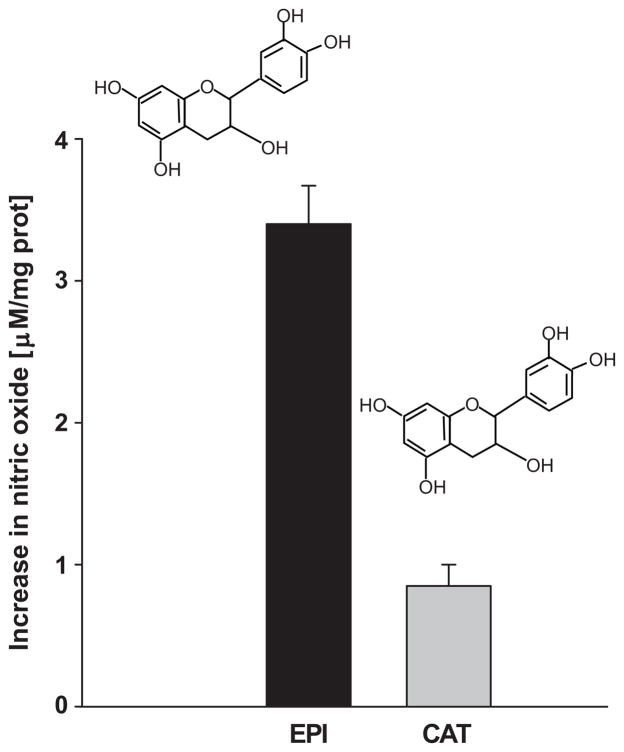
Catechin effects on NO production
Figure 7 summarizes the effects of 1 μmol/L catechin (a structural stereoisomer of EPI) in comparison with EPI. The stimulation of cells with catechin induced only ~25% of the EPI effect on NO production. In fact, co-incubation with catechin displaces the EPI-induced concentration-dependent increases in NO curves to the right (data not shown).
Figure 7. NO measurements in HCAECs treated with EPI or catechin.
NO production was measured in HCAECs treated with EPI or catechin [1 μmol/L]. EPI induces a ~4-fold increase in NO vs. catechin. Data are expressed as mean ± SD (n=3). (*p=<0.05).
DISCUSSION
In an effort to identify intracellular signaling pathways involved in eNOS activation induced by EPI and gather information relevant to vascular and cardioprotective effects, we pursued experiments utilizing human coronary artery endothelial cell cultures. Time-course and dose-response experiments were performed to determine the peak time and concentration at which maximal EPI stimulation would be exerted. Peak stimulation of NO occurred within 10–30 min after treatment. This time-frame of NO stimulation is well within those reported for physiological stimulators of eNOS, such as BK or estrogens [10,11]. Concentrations of EPI as low as 0.1 nmol/L stimulated NO production with a maximal effect observed at 1 μmol/L. The amount of NO stimulation observed at 1 μmol/L, while significant (~100% increase over control), was only about 75% of that observed for BK at similar concentrations.
We believe that the in vitro effects generated with 1 μmol/L EPI correlate to reported vasodilatory and cardioprotective actions in animals and humans. For example, in a study performed in rats, the administration of ~0.5 g/kg of cocoa powder or EPI at 1 mg/kg (by gavage) yielded ~1 μmol/L plasma levels that peaked at ~1 h after consumption. The 1 μmol/L concentration of EPI is also equivalent to peak blood levels observed at 2 h in humans who consume flavanol-rich cocoa-containing products [12,13,14]. Recently, we reported on the cardioprotective effects of 1 mg/kg/day EPI in rats subjected to myocardial ischemia reperfusion injury [8]. Observations from these studies suggest that the dose of EPI used is within the range of concentrations that can be achieved readily in animals and humans.
Results indicate that the EPI-induced synthesis of NO was dependent on the activation of eNOS, as observed by increases in the phosphorylation of Ser-1177 and Ser-633 and the dephosphorylation of the Thr-495 residues. These results are consistent with known effects of physiological NO dependent vasodilators (such as BK or estrogens) on the phosphorylation/dephosphorylation of eNOS residues, leading to NO production in endothelial cells [15]. In an effort to identify intracellular pathways involved in the EPI-induced effects, we examined the involvement of the PI3K pathway, which has been described as importantly involved in the physiological modulation of eNOS activation and NO production [16]. The kinases Akt and PKA are of particular importance as they are known members of the PI3K pathway and can phosphorylate eNOS [16]. Akt is known to phosphorylate eNOS at Ser-1177, while PKA phosphorylates eNOS Ser-1177 and Ser-633 [17]. By using specific Akt and PKA inhibitors, we identified a role for these kinases in EPI-induced eNOS activation. Inhibition of Akt with SH-5 diminishes NO synthesis by ~25% with respect to maximum EPI-induced effects. Treatment with SH-5 prevents eNOS Ser-1177 phosphorylation by ~50%; however, Ser-633 phosphorylation is not affected, which is in agreement with well-described Akt effects on eNOS phosphorylation [18]. This result evidences the partial involvement of Akt on EPI-induced eNOS activation.
The inhibition of PKA decreases the activity of eNOS to ~50% of EPI-induced maximal effects. In our experiments, we observed that the inhibition of PKA with H89 partially blocked the phosphorylation of eNOS Ser-1177 and Ser-633, which correlates with the magnitude of the decrease observed in NO production. Altogether, the results evidence the participation of the PI3K pathway in EPI-induced eNOS activation. Interestingly, a notable difference is observed in eNOS activation when EPI effects are contrasted with agents, such as acetylcholine, BK and black/red tea flavonoids [15,19,20]. These compounds mainly activate eNOS via Akt leading to the phosphorylation of Ser-1177, while EPI-induced eNOS activation is mediated through both Ser-1177 and Ser-633 phosphorylation. The incomplete inhibition gained by blocking the PKA/Akt pathway suggests that EPI-induced eNOS activation also utilizes other complementary signaling pathways.
It is well established that when eNOS is inactive, it is bound to Cav-1 on the cytoplasmic side of the cellular membrane. Increases in intracellular Ca2+ activate CaM, which in turn couples to eNOS, leading to its release/solubilization [21]. Our results indicate that EPI treatment stimulates the dissociation of eNOS from the cell membrane. As with other physiological vasodilators, these results suggest that EPI-induced eNOS activation is mediated via Ca2+/CaMI/CaMKII. To further test this hypothesis, we proceeded to demonstrate the upstream involvement of PLC, which is known to stimulate the release of Ca2+ from intracellular stores via IP3 production. Treating cells with EPI induced significant increases in IP3 production as well as the phosphorylation of IP3R in the endoplasmic reticulum. These two effects were blocked when the PLC inhibitor U73122 was used. Thus, calcium-dependent kinases, such as CaMKII, can be activated that in turn activate eNOS [22]. Indeed, we further demonstrated this, since inhibiting CaMKII with KN93 induced a decrease in eNOS phosphorylation and activity as well as the phosphorylation of IP3R. Based on these results, we speculated that EPI exerts its effects via cell membrane associated events. To evaluate the specificity of NO responses, we treated cells with a stereoisomer of EPI, catechin. Catechin has the same chemical composition as EPI but differs in the spatial orientation of one of its rings (Figure 7). The comparison of effects led to a notable difference in the levels of NO production with catechin, which, at the same concentrations, only stimulated ~25% of maximal EPI effects. In fact, catechin displaced the EPI concentration–response curve to the right, suggesting an antagonist-like effect.
PERSPECTIVES
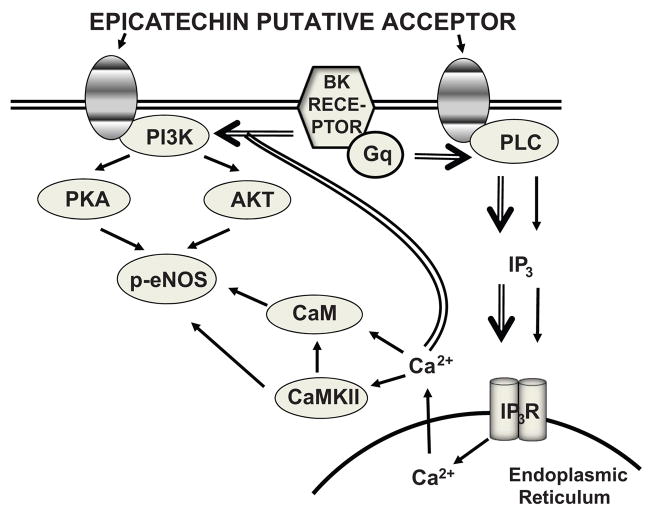
The results generated by this study highlight important differences in the activation of selected signaling pathways as induced by EPI versus those of known classical stimulators of vascular endothelium, such as BK (Figure 8). These differences suggest a distinct modulation of these pathways by EPI. On the basis of the results gathered and the observations noted above, it is reasonable to suggest the presence of an EPI cell membrane acceptor-effector, which harbors a “high” specificity given that the stereoisomer catechin only acts as a weak partial agonist or as an antagonist to EPI effects. It is very likely that this “acceptor-effector” is distinct from those described for other “cardioprotective” agents, such as resveratrol, as EPI does not compete for resveratrol receptor binding [23,24] or for estrogen receptors because the use of ICI182,780 (a selective estrogen receptor antagonist) did not block EPI effects on NO synthesis in our experiments (data not shown). Our results also suggest consideration towards a more direct therapeutic approach to limit CVD using EPI because commercial cacao products typically contain many other natural and added ingredients (EPI, catechin, quercetin, theobromine, sugar, flavorings, etc.) and usually have a high caloric content. The possible presence of a specific acceptor-effector for EPI is likely; however, there are many other mechanistic possibilities that will need to be explored, such as the following: 1) the interaction with receptors at allosteric sites, 2) the modulation of signaling pathways in a receptor-independent manner and 3) the binding to intracellular receptors/molecules. The results reported here only reflect the acute effects of EPI; hence, chronic actions must be further explored, as they may also importantly account for vascular and cardioprotective effects. Thus, much more work is required to further explore these interesting possibilities.
Figure 8. EPI-induced eNOS activation in HCAEC.
Schematic representation of signaling pathways involved in EPI-induced eNOS activation. BK receptor activated pathways are included (double arrows) to demonstrate similarities.
Supplementary Material
Acknowledgments
Sources of Funding: The work was supported by NIH HL-43617 to F. Villarreal, I. Ramirez-Sanchez, (post-doctoral fellowship #93759) and G. Ceballos (sabbatical fellowship) from CONACYT, Mexico.
Footnotes
Disclosures: NONE
References
- 1.Tarride JE, Lim M, DesMeules M, Luo W, Burke N, O’Reilly D, Bowen J, Goeree R. A review of the cost of cardiovascular disease. Can J Cardiol. 2009;25:195–202. doi: 10.1016/s0828-282x(09)70098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Räthel TR, Samtleben R, Vollmar AM, Dirsch VM. Activation of endothelial nitric oxide synthase by red wine polyphenols: impact of grape cultivars, growing area and the vinification process. J Hypertens. 2007;25:541–549. doi: 10.1097/HJH.0b013e328013e805. [DOI] [PubMed] [Google Scholar]
- 3.O’Riordan E, Chen J, Brodsky SV, Smirnova I, Li H, Goligorsky MS. Endothelial cell dysfunction: the syndrome in making. Kidney Int. 2005;67:1654–1658. doi: 10.1111/j.1523-1755.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 5.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res. 2009;53:322–331. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- 7.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G, Villarreal F. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H761–H767. doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki KG, Taub PR, Barraza-Hidalgo M, Rivas M, Zambon A, Ceballos G, Villarreal F. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling following permanent coronary occlusion. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2010.01.055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 11.Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdos EG. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- 12.Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 13.Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 14.Cienfuegos-Jovellanos E, Quinones Mdel M, Muguerza B, Moulay L, Miguel M, Aleixandre A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J Agric Food Chem. 2009;57:6156–6162. doi: 10.1021/jf804045b. [DOI] [PubMed] [Google Scholar]
- 15.Yang SW, Lee WK, Lee EJ, Kim KY, Lim Y, Lee KH, Rha HK, Hahn TW. Effect of bradykinin on cultured bovine corneal endothelial cells. Ophthalmologica. 2001;215:303–308. doi: 10.1159/000050879. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 19.Venema RC. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int Immunopharmacol. 2002;2:1755–1762. doi: 10.1016/s1567-5769(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz M, Urban J, Engelhardt U, Baumann G, Stangl K, Stangl V. Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic Res Cardiol. 2009;104:100–110. doi: 10.1007/s00395-008-0759-3. [DOI] [PubMed] [Google Scholar]
- 21.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43:532–541. doi: 10.1016/s0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 22.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 23.Lin HY, Lansing L, Merillon JM, Davis FB, Tang HY, Shih A, Vitrac X, Krisa S, Keating T, Cao HJ, Bergh J, Quackenbush S, Davis PJ. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–1744. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 24.Han YS, Bastianetto S, Dumont Y, Quirion R. Specific plasma membrane binding sites for polyphenols, including resveratrol, in the rat brain. J Pharmacol Exp Ther. 2006;318:238–245. doi: 10.1124/jpet.106.102319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.