Summary
Respiratory viral infections are the cause of severe diseases in humans. The outcome of the infection depends on the interaction of the pathogen with the immune system. The bone marrow is the primary site of hematopoiesis and releases large numbers of leukocytes in response to inflammation. Here we show that during infection with influenza or Sendai virus the lung communicates with the sterile bone marrow through type I interferons. While in the bone marrow, leukocytes exposed to type I interferons activate an anti-viral transcriptional program and become resistant to infection with different viruses. The protected bone marrow leukocytes are capable of migrating to the infected lung and contribute to virus clearance. These findings show that appropriate instruction of cells during their development in the bone marrow is needed for the effective innate control of infection.
Introduction
Infections with viruses that replicate in the respiratory tract trigger a robust inflammation of the lung. The inflamed lung produces cytokines, chemokines, and growth factors that promote a massive infiltration of cells. This infiltrate is composed by a large number of granulocytes, monocytes (Aldridge et al., 2009; Lin et al., 2008), natural killer cells (NK) (Gazit et al., 2006), plasmacytoid dendritic cells (pDCs), conventional DCs (cDCs) (Grayson et al., 2007; Wolf et al., 2009) and other cell types critical for the clearance of the infection and for the initiation of adaptive immunity (GeurtsvanKessel et al., 2008; Kim and Braciale, 2009; Moltedo et al., 2009). Much work has been performed to identify the chemokines that act to promote lung leukocytes infiltration during infection, to sub-classify these cells, and to study their role during anti-viral immunity. However, the effect that the lung cytokine milieu has on leukocytes in distal hematopoietic and lymphoid organs has been overlooked and whether such effect has implications in immunity to virus infection is unknown.
Among the cytokines produced in the infected lung are the type I interferons (IFNs) which have been extensively studied due to their potent anti-viral properties. Type I IFNs are produced by infected epithelial cells (Jewell et al., 2007), pDCs, alveolar macrophages, and other cells (Kumagai et al., 2007). Binding of type I IFNs to their receptor induces the transcription of a number of IFN stimulated genes (ISGs) establishing an anti-viral state (Samuel, 2001). ISGs include genes coding for sensors of viral RNA like retinoic acid inducible gene (RIGI) and melanoma differentiation-associated gene-5 (MDA5), signaling proteins such as the interferon regulatory factor 7 (IRF7) and proteins involved in general resistance to viral infection such as myxovirus resistance 1 (Mx1) and ISG15. The importance of type I IFNs as a major anti-viral mechanism is evident as virtually all pathogenic viruses encode for antagonists of type I IFN production and/or function (Randall and Goodbourn, 2008; Weber et al., 2004). Additionally, type I IFNs affect the development of the immune response by promoting DC maturation (Luft et al., 1998), NK cell activation (Biron et al., 1999), CD4+ Th1 bias (Murphy et al., 2000), and antigen specific T cell proliferation (Kolumam et al., 2005).
Studies conducted in vitro have shown that treatment of leukocytes such as monocytes (Gerlini et al., 2008) and monocyte derived DCs (Osterlund et al., 2005; Phipps-Yonas et al., 2008) with type I IFNs prior to virus infection limits the ability of the virus to replicate in these cells while improving their ability to stimulate T cells (Gerlini et al., 2008). We hypothesized that leukocyte priming by type I IFNs also occurs in vivo, and that leukocytes educated in the periphery by type I IFN signaling are recruited to the lung during infection to improve the efficiency of virus clearance.
The bone marrow (BM) is the major site of hematopoiesis in the adult. In the steady state, millions of leukocytes, erythrocytes and hematopoietic stem cells (HSCs) are released daily from the BM in order to replenish the hematopoietic compartment throughout the organism. Inflammation in any organ of the body significantly changes the environment and cell composition of the BM, leading to the release of monocytes (Serbina and Pamer, 2006; Tsou, 2007), neutrophils (Navarini et al., 2009; Wengner et al., 2008), NK cells (Bernardini et al., 2008) and HSCs (Elena Nardini, 2005; Ping Zhang, 2008) to the blood. The BM is therefore the most likely main supplier of cells to inflamed tissues. Studies using non-infectious inflammatory models, bacterial infections, or models of systemic infection have helped to elucidate some of the events occurring in the BM during inflammation. However, very limited research has been done with regard to the events occurring in the BM during a distal viral infection and the function of released BM cells in the resolution of a respiratory infection has not been investigated.
Infection with Sendai virus (SeV) or with most strains of influenza virus in mice does not lead to viremia since these viruses replicate exclusively in the respiratory tract (Scheid and Choppin, 1974; Tashiro et al., 1992). We chose SeV and influenza virus to study the systemic effect of cytokines produced in the lung during infection without the complicating contribution of direct viral infection of cells outside the respiratory tract. Here we demonstrate that infection of the lung is sensed systemically and that cells in distal organs including the BM are instructed to generate an improved response to the pathogen when they are recruited to the infection site. Overall our findings suggest that the communication between the infected lung and leukocytes developing in the BM is an integral part of the immune response to respiratory virus infections. This previously overlooked mechanism may provide useful insight for the development of novel medical strategies for the treatment of lethal virus infections and other immune disorders that involve BM leukocytes.
Results
SeV infection induces a systemic anti-viral response
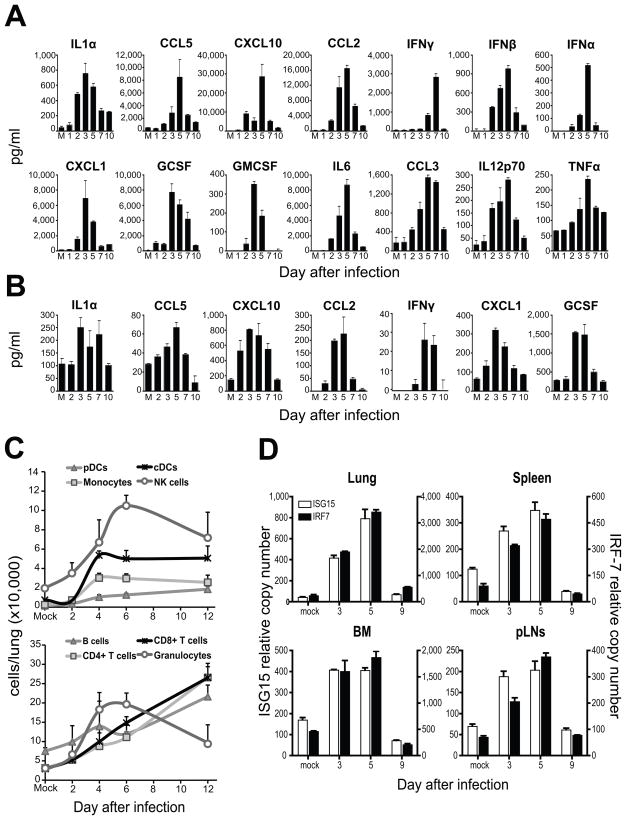
To characterize the inflammatory response induced by SeV infection, we quantified the levels of multiple cytokines and chemokines in the lung and blood of mice at different times post infection (Figures 1A and 1B). Similar to the kinetics of inflammation reported for influenza virus infection (Moltedo et al., 2009), cytokines and chemokines began to be detected in the lung on the second day after infection, reaching high levels at days 3–5 of infection. The same kinetics was closely followed in the blood (Figure 1B). While type I IFNs (IFNα and IFNβ) were detected in the lung, we were not able to detect them in the serum using conventional ELISA. As expected, the induction of chemokines in the infected respiratory tract correlated with a robust infiltration of leukocytes into the lung (Figure 1C).
Figure 1.
Lung inflammation correlates with the systemic transcription of anti-viral genes.
Cytokines from (A) fresh lung homogenates and (B) sera obtained from mice infected with SeV and sacrificed at different days post inoculation. Mock (M) infected animals were used as controls. (C) Lung cells count after tissue digestion. Cell numbers for specific cell population were extrapolated from flow cytometry analysis. The cells were pre-gated on live (PI−) CD45+ cells. B cells (CD11b−mPDCA−B220+), pDCs (CD11b−mPDCA+B220+), monocytes (Ly6c+Ly6g−CD115+), Granulocytes (Ly6c+Ly6g+), NKs (NK1.1+CD11b+), cDCs (CD11c+MHCII+CD11b+), CD4+ T cells (CD3+CD4+CD8−), CD8+ T cells (CD3+CD4−CD8+). (D) ISG15 and IRF7 mRNA levels in the lung, pLNs, BM and spleen at different days after infection with SeV. mRNA was normalized to the house keeping genes α-tubulin, rps11 and β-actin of each sample analyzed. Error bars indicate the standard deviation of triplicate measurements in a representative experiment. See also Figure S1.
We then assessed the expression of anti-viral genes in lung, spleen, BM and peripheral lymph nodes (pLNs) of infected animals. Transcription of ISG15, IRF7, Mx1, RIGI and MDA5 was observed in all the organs tested (Figure 1D and data not shown) following the same kinetics as the lung inflammatory response (Figure 1A). The enhanced anti-viral gene expression observed in the BM was not due to a relative increase in the pDC population which constitutively express IRF7 (Izaguirre et al., 2003) (Figure S1).
Lung-derived type I IFNs trigger the BM anti-viral response
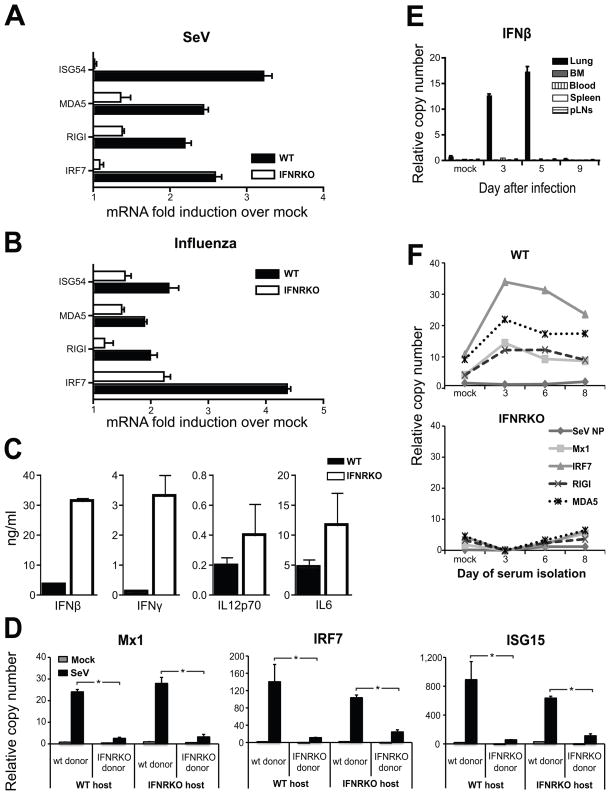
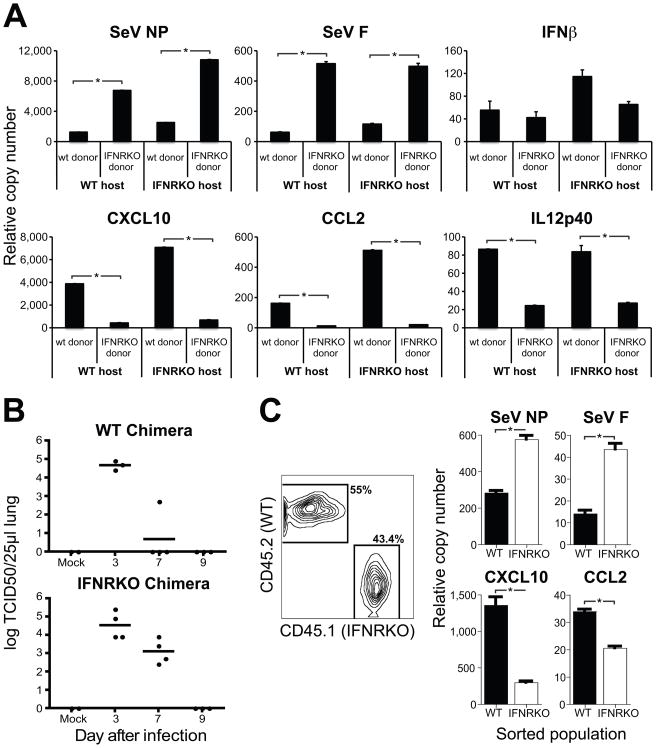
Because the genes induced in the periphery are known targets of type I IFN signaling, we investigated whether these cytokines were responsible for the induction of anti-viral genes in the BM. We infected type I IFN receptor deficient (IFNRKO) mice with SeV or influenza virus and compared their gene expression the BM to that of WT controls. Infected IFNRKO mice exhibited impaired induction of anti-viral genes compared to controls (Figure 2A and 2B). The inflammatory cytokines IL12p70, IL6, IFNβ and IFNγ were produced to high levels in the lungs of SeV infected IFNRKO mice, demonstrating that the impairment observed in the BM of these mice was not due to reduced cytokine production in the lung (Figure 2C). In addition, in chimeric mice, WT donor hematopoietic cells upregulated the transcription of anti-viral genes in response to SeV infection while donor IFNRKO hematopoietic cells did not, regardless of the host responsiveness to type I IFN (Figure 2D). These results indicate that type I IFNs provide the primary signal for the induction of anti-viral genes in BM cells.
Figure 2.
Transcription of anti-viral genes in the BM is induced by type I IFNs transported in the blood.
(A–B) Gene expression in BM of wild type mice or type I IFN receptor KO mice infected with (A) SeV two days after infection or (B) influenza PR8 virus three days after infection. (C) Cytokines in lung homogenates obtained from WT and IFNRKO mice 3 days post-infection. (D) Gene expression in the BM of complete chimeric mice infected for five days with SeV (n=4 mice in each group; *p< 0.05). Error bars represent means±S.D. (E) IFNβ expression in the lung, BM, blood, peripheral lymph nodes (pLN) and spleen of wild type mice infected with SeV. See also figure S2. (F) Gene expression in naive BM cells isolated from WT (top panel) or IFNRKO (bottom panel) mice after a 6 h incubation with serum obtained from wild type mice at different times after infection with SeV. (n=3 mice in each group; *p< 0.05).
To more closely examine the source of the type I IFNs that activate the BM during infection, we measured the level of IFN α and β mRNAs in the lung, spleen, pLNs, blood and BM cells isolated from mice infected with SeV (Figure 2E and Figure S2A). Transcription of type I IFNs was only detected in the lung of infected mice. In addition, we were unable to detect expression of IFNs or any other cytokines (IL28α, IFNγ, CCL2, IL-6, G-CSF) in any discrete BM cell population (Figure S2B and data not shown), indicating that the source of IFNs activating the BM is solely the site of infection.
We then hypothesized that during infection type I IFNs were transported through the blood to peripheral organs at levels undetectable by commercially available ELISAs. To examine whether type I IFNs were present in the serum, we used a sensitive bioassay. In this assay, serum obtained at different times after infection with SeV was incubated ex-vivo with BM cells isolated from non-infected mice. The expression of anti-viral genes by the BM cells was then determined by qRT-PCR. BM cells isolated from uninfected mice and incubated with serum isolated from infected mice showed increased anti-viral mRNA transcripts (Figure 2F), similar to what we had observed in vivo. However, IFNRKO BM cells incubated with the same serum were unresponsive, indicating that type I IFNs present in the blood of infected mice were are least partially responsible for the observed gene expression. Altogether, these results show that type I IFNs originated in the infected lung are distributed systemically through the blood and instruct cells in the BM to confront the infection.
Induction of anti-viral programming of the BM does not result from direct virus infection
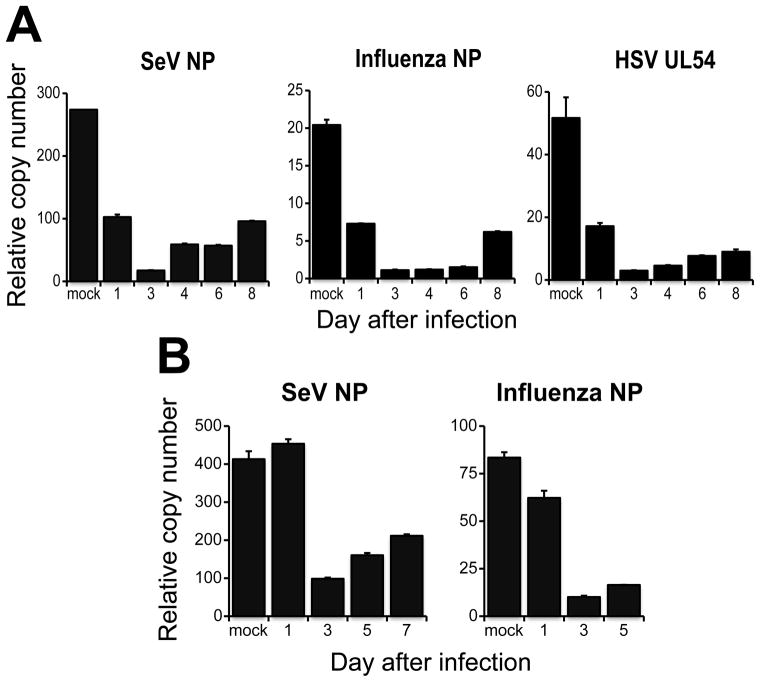
We next confirmed that in our system virus replication was restricted to the lung. Viral nuclear protein (NP) mRNA was detected at high levels in the lungs of mice infected with either SeV or influenza virus but was not detected in their blood or BM (Table 1). Furthermore, we did not detect replicating SeV in the BM and blood of infected mice at any time analyzed, while infectious virus was generated in the lung to high titers (Table 1 and data not shown). Additionally, the expression of viral proteins and virus replication were significantly higher in the lungs of IFNRKO mice than in those of WT mice. Despite the higher level of virus in IFNRKO lung, viral mRNA was not detected in the BM of these mice confirming that SeV and influenza virus do not replicate in the blood or BM of infected animals. These results validate the use of these viruses to study the effects of a localized virus infection that does not reach the BM compartment.
Table 1.
Virus titers upon infection with SeV and Influenza virus
| WT | IFNRKO | ||||
|---|---|---|---|---|---|
| Viral NPa | Virus titerb | Viral NP | Virus titer | ||
| SeV | Lung | 5557±36 | 5±0.36 | 16003±265* | 5.7±0* |
| BM | ND | ND | ND | NM | |
| Blood | ND | ND | ND | NM | |
| Influenza | Lung | 3035±59 | 5.7±0 | 3401±68 | NM |
| BM | ND | NM | ND | NM | |
| Blood | ND | NM | ND | NM | |
Viral NP was measured on day 4 post infection by qRT-PCR. Expressed as relative copy numbers. n=3.
Viral titers in the lung measured on day 4 post infection. BM and blood titers measured on day 5 after infection. Expressed as log TCID50/25ul Lung. n=4.
p<0.05 compared to WT
ND-not detected
NM- not measured
BM cells from infected mice are protected from viral replication
We reasoned that the expression of anti-viral genes confers protection to leukocytes against virus infection while in the BM or when recruited to the infected lung. We therefore investigated if BM cells isolated from infected mice were resistant to virus infection. To do so, BM cells isolated at different times after infection with SeV (Figure 3A) or influenza virus strain X-31 (Figure 3B) were infected ex-vivo with either SeV, influenza PR8 virus, or Herpes Simplex virus (HSV) and viral replication was determined through the expression of viral mRNA. BM cells isolated from infected mice resisted infection by all three viruses. These results indicate that leukocytes in the BM of infected mice are programmed to limit virus replication.
Figure 3.
BM cells of mice undergoing a respiratory infection are protected from viral replication.
Expression of viral genes in BM cells isolated at different days after infection with (A) SeV or (B) influenza virus strain X31 after 6 h of infection ex-vivo with SeV, influenza strain PR8 or HSV at a MOI of 2. n=4 mice in each group. Error bars represent means±S.D
B cells, pDCs and monocytes in the BM are instructed by type I IFN to confront a viral infection
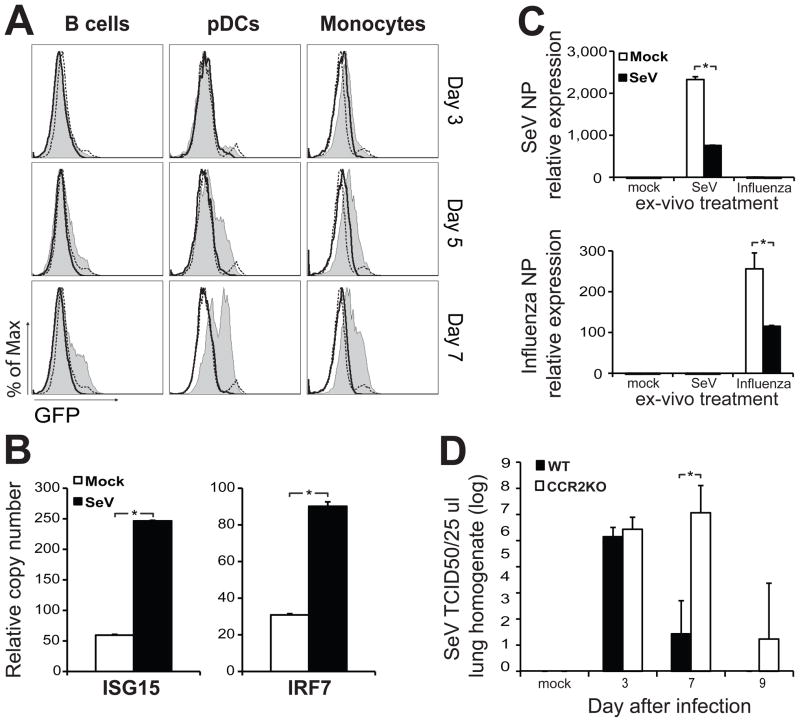
To determine which leukocytes in the BM responded to type I IFNs produced in the infected lung, we performed flow cytometry analysis on cells obtained from the BM of Mx-Cre x Rosa26-stopfloxEGFP reporter mice. The activation of the type I IFN sensitive Mx promoter in these mice leads to the expression of Cre recombinase that removes a stop cassette upstream of the floxed reporter leading to EGFP expression (Kuhn et al., 1995; Mao et al., 2001). In these reporter mice, cells that are activated by type I IFN express EGFP permanently. BM pDCs, B cells and monocytes isolated from infected mice showed increased expression of EGFP compared to mock infected or Rosa26-EGFP littermates that do not express Mx-Cre (Figure 4A). Sorted cells from the BM of infected mice demonstrated an enhanced transcription of anti-viral genes compared to control mice demonstrating that these cells were instructed while residing in the BM (Figure 4B and Figure S2B).
Figure 4.
Monocytes primed in the BM participate in the control of virus replication in the lung.
(A) EGFP expression in BM B cells (PI−SSloB220+CD11b−mPDCA−), pDCs (PI−SSloB220+CD11b−mPDCA+) and monocytes (PI−SSloCD115+CD11b+Ly6c+) from Mx-Cre x Rosa26-stopfloxEGFP mice infected for 3, 5 or 7 days with SeV (tinted), mock infected (black dashed line) or Mx-Cre(neg)Rosa26-stopfloxEGFP litter mates (black line). (B) Gene expression in BM monocytes (Ly6c+Ly6g−CD115+) sorted from mock or SeV infected mice at day 4 post-infection (n=6 mice in each group; *P < 0.05). Error bars represent means±S.D. (C) Viral gene expression in BM monocytes isolated from mice four days post infection with SeV and after ex-vivo treatment with either SeV or influenza PR8. Mock infected animals were used as controls (n=6 mice in each group; *P < 0.05). Error bars represent means±S.D.(d) SeV titers in lung homogenates of wild type or CCR2KO mice after infection with SeV. 50% Tissue culture infectious dose (TCID50). n=3–4 mice in each group; *P < 0.05 Error bars represent means±S.D. See also figure S3
Monocytes recruited from the BM have been proposed to contribute to immunity against respiratory viruses (Aldridge et al., 2009; Lin et al., 2008). In our system, monocytes from infected mice that were infected ex-vivo were less permissive to both SeV and influenza virus replication than monocytes from control animals (Figure 4C) similarly to total BM cells (Figure 3). To determine the role of these cells during the resolution of SeV infection, we infected CCR2 deficient mice (CCR2KO) that show impaired recruitment of monocytes in other infection models (Aldridge et al., 2009; Lin et al., 2008; Navarini et al., 2009; Serbina and Pamer, 2006; Tsou, 2007). As expected, CCR2KO mice had a reduced number of monocytes in the blood than WT mice. These animals also exhibited impaired recruitment of monocytes to the lung during the course of SeV infection while these cells accumulated in the BM (Figure S3A, S3B and S3C). In addition, CCR2KO mice showed delayed clearance of SeV as compared to WT controls (Figure 4D) suggesting that monocytes recruited from the BM and instructed during infection play a role in the clearance of SeV from the lung. The failure to promptly clear the virus from CCR2KO mice was independent of recruitment of T cells to the lung, as similar numbers of CD8+ virus specific T cells were present in the lung at various time points after infection (Figure S3D).
Type I IFN priming of cells newly recruited to the lung is needed for rapid viral clearance
We examined whether exposure of BM cells to type I IFN affected their function upon recruitment to the lung during infection. To do this, we determined the extent of viral replication and cytokine transcription in hematopoietic cells (CD45+) purified from the infected lung of BM transplant recipients. IFNRKO CD45+ cells showed higher level of both SeV NP and F mRNA than WT CD45+ cells regardless of the host’s IFN responsiveness (Figure 5A). However, despite exhibiting a lower level of viral mRNA, WT donors showed higher transcription levels of the pro-inflammatory genes IL12p40, CCL2 and CXCL10. SeV titers of whole lung homogenates corroborated the essential role of hematopoietic cells primed by type I IFN in controlling virus replication in vivo (Figure 5B).
Figure 5.
Type I IFNs arm hematopoietic cells against viral replication in vivo.
(A) Gene expression on CD45+ cells isolated by magnetic beads from the lung of complete chimeric WT or IFNRKO mice after five days of infection with SeV. (B) SeV titers in lung homogenates of chimeric mice at different days after infection. (C) Viral gene expression analysis of CD45.2+ (WT) and CD45.1+ (IFNRKO) cells isolated from the lung of mixed chimeric mice after four days of infection with SeV. (n=3 mice in each group; *P < 0.05). Error bars represent means±S.D
Notably, in type I IFN receptor deficient mice whole lung homogenates showed enhanced cytokine production compared to WT mice (Figure 2C), differently from the response observed in pure hematopoietic cell preparations (Figure 5A). It is possible that the contribution of different lung cell types to the cytokine response is altered in the absence of type I IFN. To rule out the possibility that the differences between WT and IFNRKO cells recruited to the lung were due to the different virus burden and cytokine environment that these cells were exposed to in the lung, we generated mixed chimeric mice transplanted with a 1/1 ratio of WT and IFNRKO donor BM cells in a WT host. In these animals both WT and IFNRKO cells were exposed to the same environment. Analysis of gene expression in cells sorted from the lungs of infected mixed chimeras (Figure 5C) confirmed that responsiveness to type I IFN protects hematopoietic cells from infection in the lung and allows for their maximal contribution to the inflammatory process through the production of pro-inflammatory molecules.
BM leukocytes from infected mice are recruited to the lung and are protected from virus infection
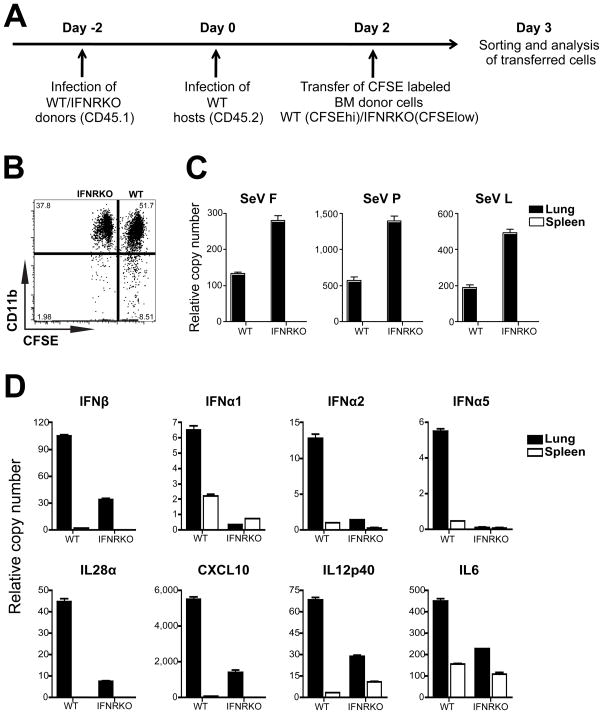
To determine if BM leukocytes are recruited to the lung during infection, we followed the migration of adoptively transferred BM leukocytes in an infected animal. To do this, we isolated BM cells from CD45.1+ WT or IFNRKO mice four days after infection with SeV (Figure S4). The cells were labeled with high and low levels of carboxyfluorescein diacetate succinimidyl ester (CFSE) respectively, and adoptively transferred to CD45.2+ WT mice that had been infected for two days with SeV (Figure 6A). This timing was selected to ensure that the appropriate cytokine and chemokine environment had been established in the lung of host mice and that newly recruited cells had not yet saturated the lung. One day after the adoptive transfer CD45.1+ cells from the lung and spleen of recipient mice were enriched and sorted based on their CFSE content. A slightly higher proportion of WT than IFNRKO donor cells were found in the infected host lung (Figure 6B). Most of the cells of either type found in the lung were of myeloid origin as indicated by their expression of CD11b.
Figure 6.
Primed BM leukocytes are recruited to the lung and are protected from infection.
(A) Experimental design for adoptive transfer of primed BM leukocytes. CD45.1+ WT or IFNRKO BM cells from mice infected with SeV for 4 days were labeled with 3.5μM or 0.5μM CFSE respectively, mixed at a 1/1 ratio and injected into WT CD45.2+ mice infected for 2 days with SeV. (B) Expression of CD11b by CD45.1+ CFSE high and low cells in the lungs of recipient mice. (C-D) qRT-PCR analysis of (C) viral mRNA and (D) cytokine mRNA in adoptively transferred BM WT and IFNRKO cells isolated from the lung and spleen.
Adoptively transferred BM cells that infiltrated the lungs but not the spleen expressed viral mRNA (Figure 6C), supporting the evidence that replication of SeV was restricted to the lung. Similarly to what we observed in chimeric animals (Figure 5), the level of virus replication was higher in IFNRKO cells present in the lung compared with WT cells. Additionally, transferred IFNRKO BM cells demonstrated impaired production of type I IFNs and other cytokines in the lung of host animals compared with WT transferred cells (Figure 6D). Cytokine induction was only observed in the cells infiltrating the lung, demonstrating that although the cells are primed in the BM they will only produce cytokines once they sense the virus in the lung. These data show that leukocytes primed in the BM can infiltrate the infected lung and become efficient cytokine producers while protected from virus infection.
Discussion
A number of different cell types are engaged in combating the pathogen in the lung during the days that immediately follow infection, before the activation of the adaptive immune response (Kohlmeier and Woodland, 2009). Since the BM is the major site of hematopoiesis in the adult mammal (Kondo et al., 2003) it is likely to contribute to replenishing and supplying most of the cells recruited to the lung during infection.
Infections with different viruses including human immunodeficiency virus (Alexaki and Wigdahl, 2008; Prost et al., 2008), vaccinia virus (Pratibha Singh et al., 2008), lymphocytic choriomeningitis virus (Binder et al., 1997), murine cytomegalovirus (Crane et al., 2009) and bacterial infections have a documented affect on the BM (Feng et al., 2008; Navarini et al., 2009; Serbina and Pamer, 2006; Tsou, 2007; Ueda et al., 2005). In addition, treatment of mice with Toll like receptors agonists was shown to drive differentiation of hematopoietic stem cells in the BM towards monocytes or DCs (Nagai et al., 2006; Welner et al., 2008). These models illustrate the ability of cells in the BM to respond to a systemic stimulus. However, the immune response to an infectious agent is tremendously complex. These studies do not discriminate between the direct detection of the pathogen that can breach the site of infection and the sensing of the pro-inflammatory environment derived from the primary infection site. SeV and most strains of influenza virus do not disseminate from the respiratory tract to the blood or other peripheral organs. These models permit the study of the BM responsiveness to the systemic inflammatory signals that originates in the infected lung without the complicating effect of direct viral infection of the cells in the BM.
Based on recent studies pointing out the responsiveness of dormant HSCs to type I IFNs (Essers et al., 2009; Sato et al., 2009), it is reasonable to hypothesize that due to the cytokine storm that follows infection with SeV and influenza virus, HSC activity in the BM is also influenced leading to the differentiation and release of cells specifically prepared to resist and fight infection. Interestingly, we were unable to detect transcription of IL6, IL12p40, CCL2 and type I IFNs (or other cytokines mRNA) in leukocytes from the BM of infected mice at any time post infection. Restricted cytokine production in the infected organ may avoid the risk of overwhelming the BM compartment with cytokines that will either over-drive cell proliferation or cause cell death leading to BM exhaustion.
The rate-limiting step for anti-viral induction in the BM appears to be the production of inflammatory cytokines at the site of infection (Moltedo et al., 2009). While the anti-viral activity of type I IFNs is extensively documented, it is usually considered to signal over short distances with a short half-life (Peleg-Shulman et al., 2004; Pepinsky et al., 2001). We have demonstrated that during a respiratory infection type I IFNs signal over long distances and that type I IFNs present in the serum are sufficient to instruct cells in the BM. Our data does not eliminate the possibility that other mechanisms complement the transport of type I IFN to the BM during infection. However, we were unable to detect transcription of type I IFNs in the BM, spleen, pLNs, thymus or blood at any time before or after infection. Although it has been suggested that type I IFNs are continually produced in the steady state (Lienenklaus et al., 2009; Wang et al., 1995) our results show that induction of anti-viral genes in non-infected tissues occurs only after type I IFNs are produced at the site of infection.
It has been reported that the BM B cells are reduced during influenza infection suggesting that cells residing in the BM respond to the lung inflammation (Sedger et al., 2002). Our data demonstrates that acute infection of the lung is sensed, mainly through type I IFN signaling, by different cells in the BM. This signal educates BM cells for their future encounter with viral cues making them resistant to virus infection and hyperresponsive to viral danger signals. These data demonstrate that the reported enhancement of cytokine production in response to virus infection of DCs and monocytes previously exposed to type I IFN in vitro also occurs during the context of infection in vivo (Gerlini et al., 2008; Osterlund et al., 2005; Phipps-Yonas et al., 2008). The need for cells to be recruited from the BM to the lung in order to fight infection efficiently is highlighted by the delayed clearance of SeV virus from the lungs of CCR2KO mice in which monocytes do not efficiently infiltrate the site of infection. It stands to reason that recruitment of primed cells is even more advantageous than that of cells that did not acquire the anti-viral state. Future studies will need to address how various cell subtypes respond in the BM to the inflammatory environment and what are their relative contributions to virus control.
Viruses that employ distinct mechanism and strategies for entry, replication and antagonism failed to replicate in primed BM cells indicating that the anti-viral effect observed in the BM is broad and not specific to the original infectious agent. Immunosuppressed individuals, for example patients receiving BM transplants, are extremely prone to viral infections (Styczynski et al., 2009). These patients are currently treated with specific anti-viral drugs to avoid infection or reactivation of chronic viruses. Based on our studies it is reasonable to propose that priming of BM cells with low doses of type I IFNs might provide these patients with protection against unrestrained virus infection.
Experimental procedure
Mice and Viruses
C57BL/6 (CD45.2+) and B6.SJL (CD45.1+) mice were obtained from Taconic Farms. IFNRKO mice (C57BL/6 background) were a kind gift from Dr. Wilson CB (Department of Immunology, Washington University) and were backcrossed to a B6.SJL (CD45.1+) background. CCR2KO from Jackson laboratories (Boring et al., 1997), Mx-Cre mice (Kuhn et al., 1995) were crossed to a reporter strain in which the Rosa26 promoter is upstream of a floxed stop cassette and the EGFP gene (Mao et al., 2001). Age and sex matched animals were used in all experiments. The animals were bred and housed in pathogen-free conditions and the experiments were performed according to institutionally approved protocols. SeV strain 52 and influenza virus strains PR8 and X31 were grown in 10-day embryonated chicken eggs (SPAFAS; Charles River Laboratories). Egg’s allantoic fluid was snap frozen in ethanol-dry ice bath and stored at −80°C. HSV (Kos1.1 strain) was propagated and titrated on Vero 2.2 cells.
Mice infection and lung virus titration
Anesthetized mice were infected intranasally with 10 to 20 50% infectious doses (ID50) of SeV-52 in phosphate-buffered saline (PBS) or by aerosol (Glass-Col Corp, Model A4212) with influenza strain PR8 or X31 solution consisting of 107.9 virus particles/12 ml PBS for 30 min. For virus titration, the lungs were extracted, homogenized in PBS-gelatin (0.1%), and frozen in dry ice-ethanol for preservation. The presence of infectious SeV and influenza virus particles was evaluated by infecting LLCMK2 or MDCK cells (respectively) with 1:10 dilutions of the lung homogenates at 37°C. After 1 h of infection, 175 μl of medium containing 2 μg/ml trypsin was added and the cells were further incubated for 72 h at 37°C. A total of 50 μl of medium was then removed from the plate and tested by hemagglutination of chicken red blood cells (RBCs) for the presence of virus particles. Viruses at 1:4 dilutions in 0.5% chicken RBCs were incubated for 30 min at 4°C. The hemagglutination of RBCs indicated the presence of virus particles.
RNA isolation and qRT-PCR
Lungs, spleen, pLNs and thymus were harvested at different times after infection and homogenized in 3ml TRIzol reagent (Invitrogen) using GentleMACS Dissociator M tubes (Miltenyi). BM from femurs and tibias was flushed with cold PBS and resuspended in TRIzol. Cells infected in vitro were resuspended in 500ul of TRIzol. RNA was isolated from TRIzol according to the manufacturer’s protocol. Total mRNA was analyzed by qRT-PCR as previously described (Yount et al., 2008). See supplemental experimental procedure for list of primers.
Flow cytometry
Lungs were flushed with cold PBS containing 0.5mM EDTA and immediately grinded and digested with collagenase (Liberase Blendzymes, Roche, Indianapolis, IN) in RPMI supplemented with 0.5% FCS at 37°C for 45 min. EDTA was added to the samples for resuspension. BM was flushed with PBS supplemented with 2% FBS using a 25 gauge syringe. RBCs were lysed with lysis buffer (BD bioseciences). Single-cell suspensions were incubated with anti–mouse CD16-32 (BD Biosciences) for 10 min at 4°C. The following antibodies were used in different staining and magnetic cell separation protocols: B220 (RA3-6B2), CD3 (145-2C11), CD19 (1D3), NK1.1 (PK136), CD49b (DX5), Ter119, CD11b (M1/70), CD11c (HL3), CD45.1 (A20), CD45.2 (104), CD115 (AFS98), Ly6C (AL-21), Gr-1 (RB6-8C5) Ly6g (1A8), IA/IE (M5/114.15.2), SiglecH (eBio440c) from BD Biosciences or eBioscience. mPDCA-PE (JF05-1C2.4.1) was obtained from Miltenyi Biotec. Immediately before reading the samples, propidium iodide (PI) was added to exclude dead cells. PE conjugated SeV NP specific tetramer was purchased from Proimmune. Flow cytometry was performed in a Cytomic FC500 Coulter station (Beckman Coulter) and analyzed by the FloJo software. See supplemental experimental procedure for cell enrichment and sorting procedure.
Cytokine detection in serum and lung
Whole lung was grinded in 1.8 ml 0.1% gelatin/PBS. Cytokine concentration in the lungs and serum was analyzed by 22-plex multiplex ELISA (Milipore). IFNβ and IFNα were measured by ELISA (PBL biomedicals).
Ex vivo infections and Serum treatment
BM cells isolated from infected mice were incubated in RPMI containing gentamycin, β mercaptoethanol, and 2% normal mouse serum, and infected with SeV 52, influenza PR8 or HSV at MOI of 2. Cells were incubated for additional 6 h prior to collection for RNA extraction. Serum was isolated from infected mice at different times post-infection. Naive BM cells from WT or IFNRKO mice were incubated in media containing freshly isolated serum for 6 h at 37°C.
Irradiation and BM transplantation
Eight-week-old recipient mice were lethally gamma-irradiated with 1,300 rads delivered in two doses of 650 rads each, three hours apart. Mice were injected intravenously with 2–3×106 BM cells. Hematopoietic engraftment was analyzed by measurement of blood chimerism four weeks after transplantation by flow cytometry.
Adoptive transfer and isolation of cells
Donor WT (n=5) and IFNRKO (n=5) CD45.1+ mice were infected for four days with SeV. BM WT and IFNRKO cells were isolated and labeled with 3.5μM or 0.5μM of CFSE respectively. Donor WT and IFNRKO BM cells were evenly mixed and 107 cells were injected intravenously to recipient WT CD45.2+ mice infected with SeV for two days (n=35). One day after the transfer CD45.1+CFSEhi/low cells were separated from the lung and spleen of recipient mice by negative magnetic bead separation against CD45.2 followed by cell sorting for high and low CFSE content. mRNA was extracted and amplified by WT-Ovation™ RNA Amplification System (NuGEN, San Carlos CA) according to the manufactures instructions prior to its analysis by qRT-PCR
Statistical analysis
We expressed the results as means±s.d. We determined the statistical significance of differences between two groups using one-sided Student’s t tests. Values of at least P < 0.05 at α=0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors wish to thank Karla Tapia for designing the graphical abstract and for technical support. We also thank Luis Muñoz, Olga Herrera, Sharon Czelusniak and Madhu Kumar for excellent technical support, the Mount Sinai School of medicine flow cytometry and the irradiator shared resource facilities for their service. This work was supported by NIH/NIAID grants A1083481 and A1083284 to CBL and grants AI041111 and AI082970 to TM.
Footnotes
Author contributions
TH designed and executed the experiments and wrote the manuscript. BM helped with discussion and execution of experiments. TM discussed and financed experiments. CBL designed, discussed and financed experiments, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JR, Jr, Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4:e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini G, Sciume G, Bosisio D, Morrone S, Sozzani S, Santoni A. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood. 2008;111:3626–3634. doi: 10.1182/blood-2007-08-106203. [DOI] [PubMed] [Google Scholar]
- Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced Transient Bone Marrow Aplasia: Major Role of Interferon-alpha/beta during Acute Infection with the Noncytopathic Lymphocytic Choriomeningitis Virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane MJ, Hokeness-Antonelli KL, Salazar-Mather TP. Regulation of Inflammatory Monocyte/Macrophage Recruitment from the Bone Marrow during Murine Cytomegalovirus Infection: Role for Type I Interferons in Localized Induction of CCR2 Ligands. J Immunol. 2009;183:2810–2817. doi: 10.4049/jimmunol.0900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena Nardini DM, Aiello Piera, Besusso Dario, Calcaterra Claudia, Mariani Luigi, Palazzo Marco, Vecchi Annunciata, Paltrinieri Saverio, Menard Sylvie, Balsari Andrea. CpG-oligodeoxynucleotides induce mobilization of hematopoietic progenitor cells into peripheral blood in association with mouse KC (IL-8) production. Journal of Cellular Physiology. 2005;204:889–895. doi: 10.1002/jcp.20360. [DOI] [PubMed] [Google Scholar]
- Essers MAG, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFN[agr] activates dormant haematopoietic stem cells in vivo. Nature. 2009 doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Feng CG, Weksberg DC, Taylor GA, Sher A, Goodell MA. The p47 GTPase Lrg-47 (Irgm1) Links Host Defense and Hematopoietic Stem Cell Proliferation. 2008;2:83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- Gerlini G, Mariotti G, Chiarugi A, Di Gennaro P, Caporale R, Parenti A, Cavone L, Tun-Kyi A, Prignano F, Saccardi R, et al. Induction of CD83+CD14+ Nondendritic Antigen-Presenting Cells by Exposure of Monocytes to IFN-{alpha} J Immunol. 2008;181:2999–3008. doi: 10.4049/jimmunol.181.5.2999. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MAM, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for Lung Dendritic Cell Maturation and Migration during Respiratory Viral Infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre A, Barnes BJ, Amrute S, Yeow W-S, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr, Bakaletz LO, Durbin RK, Flano E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007;81:9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Braciale TJ. Respiratory Dendritic Cell Subsets Differ in Their Capacity to Support the Induction of Virus-Specific Cytotoxic CD8<sup>+</sup> T Cell Responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar Macrophages Are the Primary Interferon-± Producer in Pulmonary Infection with RNA Viruses. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Lienenklaus S, Cornitescu M, Zietara N, Lyszkiewicz M, Gekara N, Jablonska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, et al. Novel Reporter Mouse Reveals Constitutive and Inflammatory Expression of IFN-{beta} In Vivo. J Immunol, jimmunol. 2009:0804277. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ Monocyte-Derived Dendritic Cells and Exudate Macrophages Produce Influenza-Induced Pulmonary Immune Pathology and Mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DNJ, Trapani J, Cebon J. Type I IFNs Enhance the Terminal Differentiation of Dendritic Cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Moltedo B, Lopez CB, Pazos M, Becker MI, Hermesh T, Moran TM. Cutting Edge: Stealth Influenza Virus Replication Precedes the Initiation of Adaptive Immunity. J Immunol, jimmunol. 2009:0900091. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci U S A. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene Expression and Antiviral Activity of Alpha/Beta Interferons and Interleukin-29 in Virus-Infected Human Myeloid Dendritic Cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Shulman T, Roisman LC, Zupkovitz G, Schreiber G. Optimizing the binding affinity of a carrier protein: a case study on the interaction between soluble ifnar2 and IFN beta. J Biol Chem. 2004:M400033200. doi: 10.1074/jbc.M400033200. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, LePage DJ, Gill A, Chakraborty A, Vaidyanathan S, Green M, Baker DP, Whalley E, Hochman PS, Martin P. Improved Pharmacokinetic Properties of a Polyethylene Glycol-Modified Form of Interferon-beta -1a with Preserved in Vitro Bioactivity. J Pharmacol Exp Ther. 2001;297:1059–1066. [PubMed] [Google Scholar]
- Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-β Pretreatment of Conventional and Plasmacytoid Human Dendritic Cells Enhances Their Activation by Influenza Virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Zhang SN, Bagby Gregory J, Siggins Robert, II, Shellito Judd E, Welsh David A. The Lineage-c-Kit+Sca-1+ Cell Response to <I>Escherichia coli</I> Bacteremia in Balb/c Mice. Stem Cells. 2008;26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Pratibha, Yao Yongxue, Weliver Abigail, Broxmeyer Hal E, Hong Soon-Cheol, Chang Cheong-Hee. Vaccinia Virus Infection Modulates the Hematopoietic Cell Compartments in the Bone Marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost S, Le Dantec M, Auge S, Le Grand R, Derdouch S, Auregan G, Deglon N, Relouzat F, Aubertin AM, Maillere B, et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARgamma/STAT5 signaling pathway in macaques. J Clin Invest. 2008;118:1765–1775. doi: 10.1172/JCI33037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral Actions of Interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Scheid A, Choppin PW. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57:475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Sedger LM, Hou S, Osvath SR, Glaccum MB, Peschon JJ, van Rooijen N, Hyland L. Bone Marrow B Cell Apoptosis During In Vivo Influenza Virus Infection Requires TNF-{alpha} and Lymphotoxin-{alpha} J Immunol. 2002;169:6193–6201. doi: 10.4049/jimmunol.169.11.6193. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, Ljungman P, Engelhard D. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–770. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Yokogoshi Y, Tobita K, Seto JT, Rott R, Kido H. Tryptase Clara, an activating protease for Sendai virus in rat lungs, is involved in pneumopathogenicity. J Virol. 1992;66:7211–7216. doi: 10.1128/jvi.66.12.7211-7216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou C-L. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. The Journal of Clinical Investigation. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–484. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Weber F, Kochs G, Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AI, Buehler D, Hensley SE, Cavanagh LL, Wherry EJ, Kastner P, Chan S, Weninger W. Plasmacytoid Dendritic Cells Are Dispensable during Primary Influenza Virus Infection. J Immunol. 2009;182:871–879. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- Yount JS, Gitlin L, Moran TM, Lopez CB. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai Virus defective interfering particles. J Immunol. 2008;180:4910–4918. doi: 10.4049/jimmunol.180.7.4910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.