Abstract
Microalgae provide various potential advantages for biofuel production when compared with ‘traditional’ crops. Specifically, large-scale microalgal culture need not compete for arable land, while in theory their productivity is greater. In consequence, there has been resurgence in interest and a proliferation of algae fuel projects. However, while on a theoretical basis, microalgae may produce between 10- and 100-fold more oil per acre, such capacities have not been validated on a commercial scale. We critically review current designs of algal culture facilities, including photobioreactors and open ponds, with regards to photosynthetic productivity and associated biomass and oil production and include an analysis of alternative approaches using models, balancing space needs, productivity and biomass concentrations, together with nutrient requirements. In the light of the current interest in synthetic genomics and genetic modifications, we also evaluate the options for potential metabolic engineering of the lipid biosynthesis pathways of microalgae. We conclude that although significant literature exists on microalgal growth and biochemistry, significantly more work needs to be undertaken to understand and potentially manipulate algal lipid metabolism. Furthermore, with regards to chemical upgrading of algal lipids and biomass, we describe alternative fuel synthesis routes, and discuss and evaluate the application of catalysts traditionally used for plant oils. Simulations that incorporate financial elements, along with fluid dynamics and algae growth models, are likely to be increasingly useful for predicting reactor design efficiency and life cycle analysis to determine the viability of the various options for large-scale culture. The greatest potential for cost reduction and increased yields most probably lies within closed or hybrid closed–open production systems.
Keywords: microalgae, algae, biofuel, biorefinery, biodiesel, green diesel
1. Introduction and background
Microalgae, a large and diverse group of unicellular photo- and heterotrophic organisms (figure 1), have attracted much global attention in recent years for the valuable natural products they produce, their ability to remediate effluents and for their potential as energy crops. Modern microalgal culture techniques owe their origins to pioneering nineteenth century microbiologists, who first developed methods for the isolation and axenic culture of single phytoplankton species using inorganic salt solutions, leading to initial attempts to rear marine animals on algae-based food chains as long ago as the early 1900s (Anderson 2005; Huntley & Redalje 2007). Mass cultivation of microalgae dates back to the mid-twentieth century, from which time all of the methodologies currently in use can be identified, including open ponds, shallow raceways and enclosed photobioreactors (PBRs). Today, microalgae cultivation is a key process in marine fish and shellfish aquaculture, providing a direct source of nutrition for larval stages of bivalve molluscs and crustacea, and as a zooplankton feed for marine fish larvae (Muller-Feuga et al. 2003). Several taxa of microalgae are also mass cultured for the production of specific extracts, such as β-carotene from Dunaliella salina. Recent developments in bioprocess engineering and increased understanding of algal physiology have paved the way for current initiatives to mass culture microalgae for bioenergy applications.
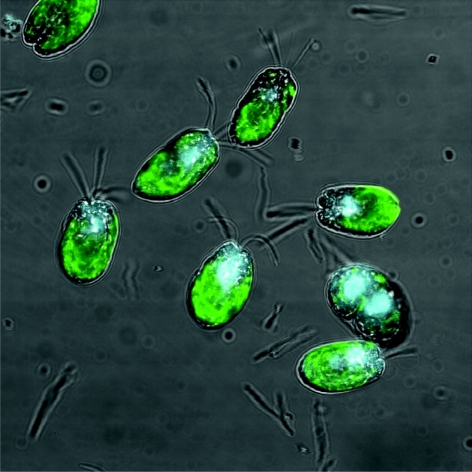
Figure 1.
Confocal microscope image of the microalgae species Tetraselmis suecica, provided courtesy of Dr Emily Roberts, Swansea University.
The demand for sustainable (bio-)fuels that are not derived from fossil oil reserves has escalated in the last few years, and this trend seems set to continue for microalgae despite periodic adverse publicity (typically associated with failed businesses who were over-optimistic in projections of algal productivity and culture process design). The reasons behind interest in microalgal biofuels are multifarious, and may not necessarily be the obvious ones. One major driver for a change to biofuels, and indeed, for making biofuel production viable, is the volatility in the price, and generally increasing cost, of crude oil. The price of crude oil was in the region of $20 per barrel (bbl) in the 1990s, but in a decade has increased to unparalleled highs, in mid-2008, in the region of $140 bbl−1. Such fluctuations in price come mainly from the actual and perceived threats to the security of oil supply owing to global events (Butler 2006).
However, there is another pressing reason to promote the use of carbon neutral fuels to replace fossil fuels, that of climate change or global warming. The International Panel on Climate Change (IPCC) reports that global greenhouse gas (GHG) emissions have grown since pre-industrial times, with an increase of 70 per cent between 1970 and 2004. The IPCC states ‘CO2 is the most important anthropogenic GHG. Its annual emissions grew by about 80 per cent between 1970 and 2004’. As a result of climate change, legislation is being introduced globally to drive the production of sustainable and alternative fuels. Climate change also has had a significant impact on water resources, while increased demand has also led to water quality problems, typically associated with eutrophication. Climate change causes problems for agriculture, with decreased yields through droughts, heavy precipitation and high winds. This has resulted in increased food prices: the food price index of the Food and Agriculture Organisation of the United Nations increased by 14 per cent in 2006 and by a further 36 per cent in 2007.
In a study of 26 biofuels (Zah 2007), the use of 21 fuels was shown to decrease GHG emissions by more than 30 per cent compared with gasoline. Twelve, however, had greater overall environmental impacts than gasoline production and use owing to practices such as deforestation associated with their manufacture. Biofuel production from terrestrial crops (e.g. rapeseed, sugar cane) is inherently limited by supply of water and land. Such crops are relatively low yielding, with only one or two harvests per year, placing a threshold above which they cannot produce enough biofuel without threatening food supplies and/or native biodiversity. Furthermore, terrestrial biofuels can have greater cumulative environmental costs compared with fossil fuels. For example, deforestation of carbon-rich forests to grow sugar cane for ethanol production causes large GHG emission increases, negating the alleged benefits of the biofuel (Scharlemann & Laurance 2008). Other adverse impacts include effects on scenery and tourism, decreases in hydrological functioning and soil protection and secondary land-use effects (displacement of previous activities to another site).
However, while terrestrial crop production and harvesting have many centuries of developmental history, algal biofuels technology is at best described as incomplete. Much of the background work on microalgal biofuel production was carried out at the US National Renewable Energy Laboratory (NREL) under the auspices of the Aquatic Species Programme from the 1970s to the 1990s (Sheehan et al. 1998). The main conclusion was that, in 1996, it was not economically viable to produce biodiesel from microalgae because, even when using the best-case scenarios of photosynthetic productivity, the price would still be twice as high as the price of a similar quantity of petroleum diesel. Since 1996 petroleum diesel prices have more than doubled; biofuels derived from microalgae production should on this basis now be viable. However, this assumes that other factors have remained relatively constant, which of course they have not; for example, the price of fertilizers has also increased markedly over this period.
2. Algae lipids, profiles and biosynthesis
One of the great biological challenges associated with algal biofuels research will be the identification and bringing into culture of species with ‘optimal’ attributes. Optimal is defined in this context as a species with a favourable combination of the following characteristics: high growth rate, high lipid content and ease of harvest and extraction. Most probably, several compromises on one or two of these parameters will need to be made when screening natural strains.
Part of the screening for an optimal microalgae species will be based on the lipid content, composition and fatty acid profile. The challenges associated with lipids in microalgae are threefold: analytical, chemical and biochemical. Inconsistencies in the reported analytical methodology for lipid analysis make it difficult to compare species and select one species over another. The challenge is to deal with the large variation in chemical composition of the lipids extracted and the lack of information on how these complex lipids behave in a catalytic upgrading process to biodiesel. An associated biochemical challenge concerns the environmental and developmental influence (e.g. nutrient stress) on lipid content and composition. The impact of the biochemical variation leads to the question as to the exact biomass harvesting conditions. We do not aim to give a comprehensive review of the literature on lipid production and yields in algae, as this has been extensively discussed elsewhere (Hu et al. 2008b). Instead, in this section, we will discuss algal lipid composition and characterization and the biochemical and metabolic aspects of the lipid biosynthesis pathways.
2.1. Lipid definition
The definition of the term ‘lipids’ is a surprisingly vague concept. Traditionally lipids were defined as the biochemical compounds not soluble in water but soluble in organic solvents instead. This definition has been the basis for the quantification of the ‘total lipid’ fraction of algae, as the total quantity of compounds soluble in a chloroform : methanol solvent mixture (based on an original method described by Bligh & Dyer (1959)). It is clear from the diversity of the published lipid contents of microalgae and inconsistencies in reported methodology that this loose definition needs to be addressed. Different research groups have reported considerably different lipid contents, after using different organic solvent mixtures. For example, Guckert et al. (1988) have shown that lipid classes are selectively extracted with variations in experimental conditions and polarity of the solvents used. This aspect of lipid research is important and has largely been overlooked. As the reported total lipid contents have served as a basis on which a large number of techno-economic models for algal biofuels have been built, current and future algal researchers will need to take the varying lipid contents into consideration and address the need for a more robust and generally applicable lipid quantification methodology.
2.2. Algal lipids
Microalgae have long been known to be rich in lipids; depending on the species, they produce many different kinds of lipids, tri- and diglycerides, phospho- and glycolipids, hydrocarbons and others (Chisti 2007; Hu et al. 2008a,b), as illustrated in figure 2. Historically, much research has focused on the lipid (specifically the fatty acid) composition from either a taxonomic or a nutritional perspective. Cultured microalgae are commonly used as feed for aquaculture applications because of the desirable fatty acid content of the algae, in particular servicing the need for essential polyunsaturated fatty acids as dietary supplements. An alternative motivation for microalgal culture was the production of high-value by-products such as pigments (e.g. the food colourant and antioxidant astaxanthin from Haematococcus pluvialis). The renewed interest in the use of algal lipid-derived biofuels, biodiesel in particular, has refocused research on algal lipids and lipid metabolism.
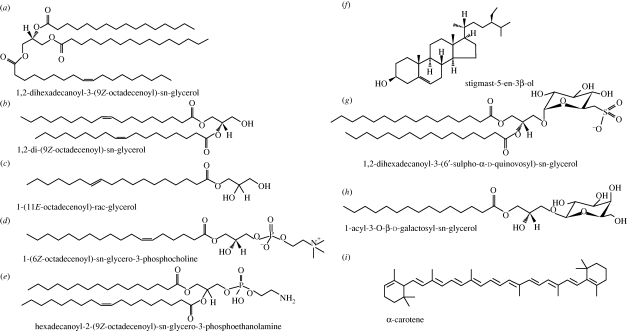
Figure 2.
Overview of the chemical structures of the most common representatives from seven lipid classes: (a) triacylglycerides; (b) diacylglycerides; (c) monoglycerides; (d,e) phospholipids; (f) sterols; (g) sulpholipids; (h) glycolipids; (i) carotenoids. Structures from www.LipidMAPS.org.
The physico-chemical properties of biodiesel, defined as the alkyl esters of fatty acid constituents of lipids, are largely determined by the structure of the constituent acyl chains (Knothe 2005). The most important fuel characteristics, according to the ASTM (American Society for Testing and Materials) D6751-09 biodiesel standard, are ignition quality, cold-flow properties and oxidative stability. For example, the level of saturation will have a great impact on the stability of the resulting fuel, with polyunsaturated fatty acyl chains being susceptible to oxidation and hence deterioration of the fuel properties.
2.3. Types and function of lipids in microalgae
Lipids are traditionally subdivided in two main classes, polar and neutral (also referred to as simple, or non-polar) lipids, based on their chemical characteristics (Christie 2003). Neutral lipids include the tri-, di- and monoglycerides, waxes and isoprenoid-type lipids (e.g. carotenoids); these are the least polar of the lipids. Polar lipids include phospholipids (e.g. phosphatidylinositol, phosphatidylcholine, phosphatidylethanolamine) and glycolipids (as combinations of oligosaccharides and lipids) as shown in figure 2. An important subcategory of polar lipids is the glycolipids (e.g. monogalactosyl diglyceride), esters of fatty acids and glycerol in which one of the hydroxyl groups of the glycerol is combined with a sugar molecule (in this case galactose) to form ester linkages with fatty acids.
This distinction in the main lipid classes is important for the subsequent conversion of microalgal oils to biofuels (§6), as the composition of the lipid feedstock affects the efficiency and yield of fuel conversion by catalytic routes. Existing technology for converting seed oils to biodiesel is optimized for a lipid feedstock comprising greater than 95 per cent triglycerides. The relative composition of algal lipids depends greatly on the species used and the nutrient, environmental and developmental conditions in which the cells are cultured and harvested. For example, it has been shown that the composition of algal lipids varies considerably with the growth cycle, under nutrient limitation and during a diurnal light dark cycle (Shifrin & Chisholm 1981; Cho & Thompson 1986; Sukenik & Carmeli 1990; Ekman et al. 2007). It remains to be seen how the composition of the lipids affects the efficiency of biofuel conversion.
In general, it appears that algal cells synthesize triglycerides at times when the energy input, through carbon assimilation, exceeds the immediate metabolic needs of the cell. It has been shown that microalgae increase the proportion of triglycerides produced upon nutrient starvation and other environmental stresses, such as temperature and essential nutrients like silicon for diatoms (Shifrin & Chisholm 1981; Roessler 1988, 1990). Cells accumulate lipids as the growth and division of the cells is put on halt. For example, in the data that Shifrin & Chisholm (1981) presented, there appears to be a trend of a decreasing growth rate with an increase in lipid content. This apparent inverse relationship could have important implications on the economics of algal biofuels. In Nannochloropsis sp. (Sukenik et al. 1989), triglycerides accumulate during the day and are subsequently rapidly mobilized in the dark to supply the energy needed for cell division. Triglycerides may serve as a sink for free fatty acids (FFAs), to remove these from the cytoplasm and thereby avoiding lipotoxicity (Kurat et al. 2006); they may act as energy and electron sinks during stress conditions (Roessler 1990; Hu et al. 2008a,b).
2.4. Lipid biosynthesis
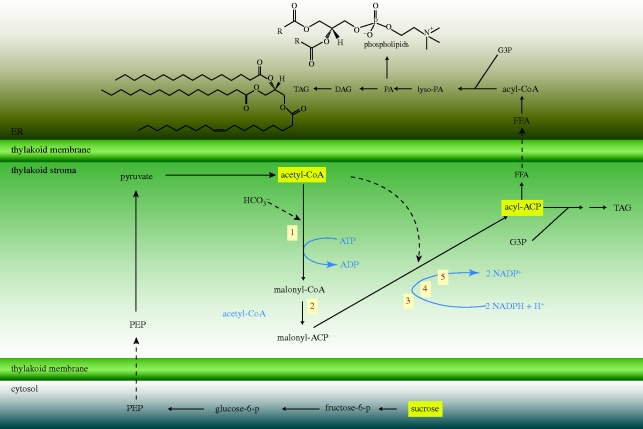
Owing to the diversity of lipid molecules, there are a large number of metabolic pathways involved in their biosynthesis. The pathway that has been studied in detail is the de novo synthesis (Hu et al. 2008a,b; for an overview, see Roessler 1988) and is shown in detail in figure 3. In brief, the first step in the pathway is catalysed by acetyl-CoA-carboxylase (ACCase) and combines CO2 (dissolved as HCO3−), ATP and acetyl-CoA to form malonyl-CoA. This step is assumed to be the committed (first irreversible) step in fatty acid synthesis (Roessler 1988; Ohlrogge & Browse 1995). The malonyl moiety formed in this reaction is subsequently used for the elongation of the acyl group as the central carbon donor (Ohlrogge & Browse 1995). The next step is a trans-acylation step involving a protein cofactor, acyl carrier protein (ACP) and the two acyl-CoA molecules. Whereas the enzyme complex malonyl-CoA/ACP is highly specific, acetyl-CoA/ACP is less so and can react with other low-molecular-weight acyl-CoA compounds, such as propionyl-CoA, and give rise to an uneven carbon number and branching in fatty acids. Although uneven carbon number and branched fatty acids are common in bacterial lipids, they are rare in microalgae. The fatty acids produced are incorporated into lipid components in either the chloroplast or the endoplasmic reticulum (discussed in detail in Riekhof et al. 2005). These pathways use glycerol-3-phosphate as a ‘scaffold’ for the sequential addition of fatty acids supplied by acyl-CoA.
Figure 3.
Overview of the fatty acid synthesis pathway (Kennedy pathway) from acetyl-CoA via ACCase. Enzymatic reactions involved: (1) ACCase; (2) malonyl-CoA : ACP transferase; steps 3–5, subsequent condensation reactions catalysed by (3) 3-ketoacyl ACP reductase; (4) 3-hydroxyacyl ACP dehydrase and (5) enoyl ACP reductase. ACP, acyl carrier protein; FFA, free fatty acid; G3P, glycerol-3-phosphate; lyso-PA, lyso-phosphatidic acid; TAG, triacylglycerides; DAG, diacylglycerides. Adapted from Ohlrogge & Browse (1995) and Riekhof et al. (2005).
The origin and fate of acetyl-CoA for this reaction is an important point of regulation for this part of the lipid synthesis pathway. Acetyl-CoA can be derived from either cytosolic or plastidial glycolysis, or directly from dihydroxyacetone phosphate from the Calvin cycle in the light. Cytosolic glycolysis in plant cells is intimately connected to sucrose degradation, producing fructose-6-phosphate and glucose-6-phosphate and ultimately phosphoenolpyruvate (PEP), which can be transported into the chloroplast. Inside the chloroplast, PEP can enter into a variety of central metabolic pathways, one of which is de novo fatty acid biosynthesis. A detailed discussion of the regulation and control of plant fatty acid synthesis can be found in Post-Beittenmiller et al. (1992), where the availability of acetyl-CoA and malonyl-CoA in the chloroplast is discussed.
Another metabolic pathway for lipid accumulation in cells is through the recycling of existing fatty acids from other cell components, e.g. membranes. The decision on whether lipids are synthesized from newly assimilated carbon, or recycled carbon, is strongly dependent on the metabolic status of the cell. In fast growing cells, lipid metabolism is focused around membrane lipids biosynthesis to support the growing membranes. Most lipids in these cells are synthesized de novo. For example, under stress conditions, just over half of the increased lipids during stress originate through de novo fatty acid synthesis, whereas the rest of the accumulated lipids result from recycling of existing fatty acids, e.g. those found in the cell or organelle membranes (Roessler 1988; Kurat et al. 2006).
Overall triglyceride metabolism is complex and is regulated at several levels, based on signals reflecting nutritional and environmental signals. For example, Cho & Thompson (1986) reported that the activity of a membrane lipid (galacto-lipid) specific acyl hydrolase was increased in nitrogen-deficient D. salina cells. The authors suggested the subsequent import of the fatty acids into triglycerides. Interestingly, a similar biochemical pathway has been reported in yeast, in which triglyceride-degrading lipases were downregulated upon nutrient starvation, suggesting that the fatty acids were preferentially retained and accumulated as triglycerides, only to be mobilized quickly upon exit from the nutrient starvation (Kurat et al. 2006). There are few data available concerning the control and regulation of this metabolic re-direction, but modulation of it through genetic modification would appear to warrant investigation.
Genetic engineering of lipid biosynthesis could allow for an increased triglyceride concentration or an increased proportion of triglycerides versus polar lipids in the cell. There are, however, still aspects of metabolic engineering of organisms that are little understood, though some theoretical work has started to address this, for example an analysis using mechanistic models of phytoplankton (Flynn 2001) suggests that manipulations of factors affecting photosynthesis (slope of the photosynthesis–irradiance curve, and the maximum organismal Chl : C ratio) may be expected to increase production approximately fivefold. Not all species or strains selected will be amenable to genetic modification, owing to the lack of genomic information or difficulties in genetic transformation of the cells. Furthermore, one has to keep in mind the delicate metabolic balance between core metabolic pathways and energy storage pathways. For example, it has been shown that increased lipid content of algal cells generally corresponds to a reduction in growth rate and cell division; in the engineering of the lipid biosynthesis pathway through over-expression of ACCase, demonstrated by Dunahay et al. (1996), higher lipid contents did not result. This could be partly explained by a disturbed metabolic equilibrium in the cells; the available carbon from assimilation pathways is limited, and there is a whole range of metabolic pathways that depend on this carbon. A detailed characterization, metabolic and growth analysis will have to be set up for any species that show promise for genetic modification. A detailed discussion on the potential of genetic engineering of microalgae falls outside of the scope of this review and can be found elsewhere (León-Bañares et al. 2004).
3. Microalgal production methods
The principles of microalgae cultivation in shallow open ponds, or engineered raceways, and in closed PBRs were in place by the 1950s (Preisig & Andersen 2005). These have been refined in the intervening decades involving cross-disciplinary research and technological development encompassing biology, process engineering, mathematics and physics. The potential of culturing microalgae for the purposes of effluent bioremediation and biofuel production has been reviewed by Chisti (2007). However, existing commercial applications remain limited to relatively low-volume/high-value markets for speciality food and feed ingredients (Spolaore et al. 2006), whether as whole cell preparations (e.g. Arthrospira sp., Chlorella sp.), or extracts such as β-carotene and astaxanthin.
3.1. Using open ponds and raceways to culture microalgae
By volume of production, most commercial microalgae production is for R-select species such as Chlorella sp., or extremophile species, such as Arthrospira sp., D. salina and H. pluvialis; these are grown in shallow fertilized ponds or raceways (Sheehan et al. 1998). Raceways (as shown in figure 4) typically consist of independent closed-loop recirculation channels in which paddle wheel-generated flow is guided around bends by baffles placed in the flow channel; such systems can yield productivities of greater than 10 g ash-free dry weight m−2 d−1 (Sheehan et al. 1998). Engineering designs and operating procedures for cultivating these organisms in unmixed ponds and stirred raceways have been much studied (Borowitzka 2005). Shallow water depths of 0.2–0.3 m are typically used, while areal dimensions range from 0.5 to 1 ha for raceway or central pivot ponds (circular ponds incorporating centrally pivoted rotating agitator), to greater than 200 ha for extensive ponds used in Australia for D. salina production. Water management procedures vary according to the intensity of operation and may include direct CO2 addition under automated pH-stat control in shallow raceways. The microalgal biomass may be harvested by flocculation or centrifugation (del Campo et al. 2007).
Figure 4.
Image of large-scale Seambiotic Nannochloropsis sp. culture ponds. Image courtesy of Nature Beta Technologies Ltd, Eilat, Israel, subsidiary of Nikken Sohonsha Co., Gifu, Japan.
While microalgal productivities will inevitably be submaximal in open raceways, it is generally envisaged that such systems will form the basis of microalgae production on the large scale required for biofuels, owing to their simplicity and low costs (Sheehan et al. 1998). However, raceway configuration and operating procedures have not yet been optimized for those microalgal species short listed for oil production (Rodolfi et al. 2009). Of particular concern is avoidance of culture contamination and population crashes. Current research seeking to address these problems includes integrating open raceways with large enclosed PBRs, to provide sufficiently large, clean inoculants to enable short-cycle duration culturing in outdoor raceways, thereby lessening opportunities for adverse events.
3.2. Using closed photobioreactor systems to culture microalgae
Closed microalgae bioreactors offer theoretical advantages in terms of avoiding contamination, yielding higher culture densities and providing closer control over physico-chemical conditions. Numerous PBR designs have been described in the scientific literature (Carvalho et al. 2006; Eriksen 2008) and in patents, only a small proportion of which have been commercialized to date. These mainly involve photoautotrophic production using natural or artificial lighting, although conventional stirred fermenters can be used to culture some microalgae species heterotrophically at high densities, without light (Harel & Place 2003).
Complete PBR systems typically incorporate the following integrated components:
— the culture vessel containing the microalgal culture, usually a light permeable vessel designed to present a short optical path under external illumination (see reviews by Carvalho et al. 2006; Eriksen 2008),
— the light delivery system typically consisting of, in the case of artificially illuminated reactors, banks of fluorescent or metal halide lamps that provide photosynthetically active radiation (λ = 400–700 nm) to the culture, while outdoor reactors use natural incident light or solar collection devices of varying complexity,
— the gas exchange system that delivers carbon dioxide and removes photosynthetically generated oxygen that may inhibit metabolism or otherwise damage the microalgae if allowed to accumulate, and
— the harvesting system that is involved in concentrating the microalgae for downstream processing and product recovery.
Such closed PBRs may be operated entirely manually, or, increasingly, incorporate automated monitoring and feedback subsystems to keep the internal culture conditions more stable. As with other types of bioreactors, PBRs may be operated in batch, semicontinuous or continuous (chemostat) modes.
Simple vertical tubular PBRs are widely used in commercial aquaculture to produce live microalgae as a feed source for larvae of marine finfish, crustacea and bivalve molluscs (Muller-Feuga et al. 2003). The most common design is a semienclosed transparent column manufactured from polyethylene tubing or fibreglass (unit operating volume up to approx. 500 l, diameter of approx. 0.4 m), bubbled from the base with CO2-enriched air and illuminated externally via natural solar irradiation or artificial lighting. Such systems offer a robust method of producing live microalgae at sufficient scale and with a suitable cost structure for commercial aquaculture hatcheries worldwide.
More sophisticated closed PBRs are designed to offer shorter optical paths under external illumination (as shown in figure 5), mainly achieved using tubular or flat plate vessel configurations manufactured from transparent materials (reviews by Carvalho et al. 2006; Eriksen 2008). These designs are intended to minimize light attenuation between the wall and the centre of the culture vessel, with typical tube diameters/plate thicknesses of ca 0.05 m. Tubular PBRs vary in their configuration, including horizontal, vertical, helical and α-shaped designs, whereas flat plate PBRs are typically thin rectangular chambers oriented vertically or inclined towards the sun. Flat plate PBRs may be partitioned into a series of internal channels (alveoli) to provide structural rigidity and to enable efficient flow of the culture medium. More novel methods of PBR illumination include solar collection devices such as light guides and Fresnel lenses (Zijffers et al. 2008; Masojidek et al. 2009) and energy-efficient, monochromatic light-emitting diodes (Gordon & Polle 2007; Wang et al. 2007). Currently, the relatively high construction and operating costs and complexity of operation of closed PBRs limit the number of large-scale commercial systems operating globally to high-value production runs. It is widely considered that closed PBRs alone will be incapable of cost effectively producing microalgal biomass on the large scale required for biofuel production, but that they will be required to produce contaminant-free inocula for large open raceways in a two-phase production process (Huntley & Redalje 2007; Rodolfi et al. 2009).
Figure 5.
Tubular PBRs in operation. Such systems have a small path length ensuring high volumetric production coupled with a small footprint. Photograph courtesy of Varicon Aqua Solutions Ltd, UK.
Despite their narrow dimensions, studies have demonstrated rapid light attenuation in high-density closed PBRs within just several millimetres of the vessel wall, owing to a combination of mutual shading and light scattering by microalgal cells and light absorption by their pigments (review by Eriksen 2008). Researchers have sought to explain the complexities of light distribution within PBRs using radial or diffuse light distribution models (e.g. Fernandez et al. 1998), enabling system productivity to be predicted in some cases (Eriksen 2008).
3.3. Design considerations
Physical mixing of the culture liquid is required to ensure that microalgal cells are moved appropriately through the illuminated zone to distribute nutrients, metabolites and heat and to transfer gases across gas–liquid interfaces (reviewed by Carvalho et al. 2006). In shallow open raceways, mixing and circulation are accomplished using rotating paddle wheels (Borowitzka 2005), while closed PBRs are mixed by mechanically pumping the culture liquid and/or by pneumatic displacement using bubble columns or airlifts (Carvalho et al. 2006). Mixing may be assisted by installing stationary devices such as baffles or discs to improve turbulent flow, or inert particles that circulate around the PBR system. Avoidance of shear stress during mixing is an important design consideration for those microalgal cells particularly susceptible to such forces. Recent studies on this aspect have compared the relative importance of mechanical- versus pneumatic-induced shear stress and the use of surfactants to lessen cell damage (review by Eriksen 2008).
Efficient gas transfer is a critical aspect of PBR design and operation, both to provide sufficient CO2 as a source of inorganic carbon for cell growth (especially for freshwater species) and to remove photosynthetically generated O2 that can inhibit photosynthetic efficiency or be directly toxic to microalgae at high concentrations (Carvalho et al. 2006). Introduction of CO2 to the PBR may be either (i) passively, via highly gas permeable or microporous membranes or (ii) by active transfer, most frequently by bubbling CO2-enriched air via porous or perforated stones/pipes positioned at the base of the culture vessel; alternatively by introducing gas into the culture medium via a gas exchanger (Carvalho et al. 2006). It is common to incorporate a pH-based monitoring and control system to regulate CO2 delivery, either involving a simple on–off dosing approach, or model-based predictive control that may incorporate prediction of photosynthesis rate (Carvalho et al. 2006).
Removal of O2 is important for closed PBRs, with their high surface area to volume ratios. This is particularly important in horizontal tubular (HT) designs, which are prone to strong axial gradients of CO2 (depletion) and O2 (saturation; Carvalho et al. 2006). Such gradients limit the physical dimensions of individual HT PBR units and necessitate multiple CO2 injection and O2 degassing points. This problem is avoided in bubbled vertical column PBRs in which oxygen leaves the culture medium at the surface; however, light penetration is less efficient in such designs owing to their longer optical paths. The internally illuminated column PBR aims to combine efficient illumination at high culture densities with effective gas transfer.
4. Harvesting and processing of biomass fractions
Harvesting and isolation of products from microalgae cultures is one of the most problematic areas of algal biofuel production technology. This is largely due to the process recovery cost from relatively dilute solutions, and up to 50 per cent of the final product costs can be from downstream processing. At present it is considered that extensive methods of microalgae production using natural lighting are the most cost effective and then only relatively low concentrations of microalgae are produced, when compared with industrial heterotrophic fermentation of yeast and bacteria, and typically concentrations of the order of 0.5–5 kg dry weight m−3 of growth medium are attained. Oil recovery from such systems represents a considerable challenge and even using the most productive microalgae species, containing around 50 per cent dry weight as oil, production amounts to only about 1–2 kg oil m−3 of culture volume. Other important considerations for downstream processing involve the reuse of water and nutrients from these systems to decrease their environmental impact and the isolation of valuable materials apart from biofuels (figure 6). How to achieve this recovery process economically is one of the greatest challenges for biofuel production from microalgae.
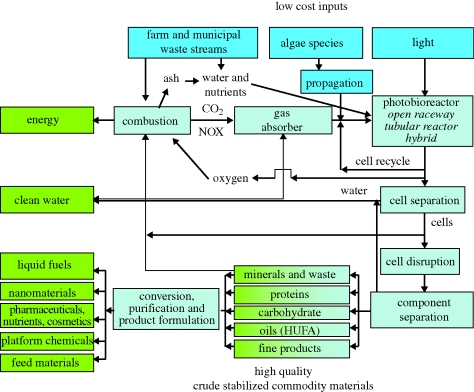
Figure 6.
Schematic of a biorefinery concept based upon the production of several products using algae from waste materials allowing their complete utilization to products such as liquid fuels, commodity chemicals and materials for high-value formulated products. This concept has little or no waste products and allows for residual energy capture, recycling of unused nutrients, water purification and recycling. This system would mean low environmental impact and maximization of the value of products from the system.
There are a number of options and processes that have been used for harvesting microalgae. In the past little consideration has been given to developing harvesting techniques that maximize the value of products (biorefining) obtained from the microalgae, since their focus has been on removal of algal cells from drinking water (Henderson et al. 2008) or aimed at a single product (Lorenz & Cysewski 2000). Oils are only one out of potentially many valuable products. In fact, the choice of which organism to cultivate has to involve the valuation of all the potential materials produced. A biorefinery approach to production should thus be considered. Figure 7 illustrates such a concept in which oil is produced together with other valuable extracts and where nutrients and process water are recycled. Products derived from microalgal biomass can include commodity materials destined for a range of chemical products such as pharmaceuticals and platform chemicals including other fuels (by conversion to ethanol and methane); highly unsaturated fatty acids such as docosahexaenoic acid (Molina Grima et al. 2003); proteins and carbohydrates, which can be used as gross nutrients (Knuckey et al. 2006); specific compounds such as pigments (Lorenz & Cysewski 2000); or silica derived from diatom cell walls (Gordon et al. 2009). Each of these components could have considerable value; many could be simultaneously harvested, with contributions between them adjusted by modulation of growth conditions.
Figure 7.
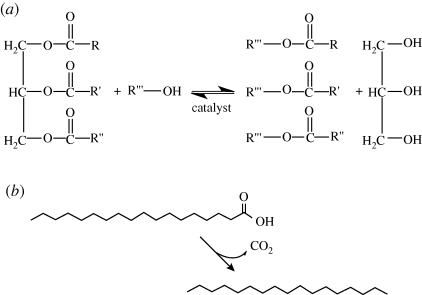
Schematic illustrating biofuel production routes from algae lipids. (a) Trans-esterification of a triglyceride. Methanol or ethanol is normally used, together with an acid or base catalyst. This is a three step reaction, proceeding via the tri-, di- and monoglycerides to the alkyl esters which can be used as biodiesel. (b) A decarboxylation process.
Another important general consideration in the processing of microalgal cells, as with handling all biological materials, is that it should take place as rapidly as possible so preserving the value of materials in the source cells.
4.1. Provision of other nutrients and its problems
Apart from water, light and carbon dioxide, additional nutrients are also required to cultivate microalgae. On the large scale required for biofuel production, sources of low cost N and P are vital for a successful process and in some cases silica will also be required (Lebeau & Robert 2003). The average elemental composition for microalgae is given by Oswald (1988) as CH1.7O0.4N0.15P0.0094. The nitrogen content of microalgae varies but is typically between 4 and 8 per cent of the cells on a dry weight basis depending on physiological state and nutrient limitation. The content of phosphate is lower at around 0.1 per cent dry weight while the content of S is ca 0.5 %w/w. The provision of S is generally not a problem in fresh or marine water; however, N and P are typically limiting materials in both marine and fresh water environments. Fertilizers, principally N and to a lesser extent P, are significant inputs of energy and finite resources if waste water is not used as a source of nutrients. Between 4 and 8 per cent of dry microalgae is N. N on the world market is of the order of $1.4 kg−1 and, more importantly, produces about 2 kg of CO2 kg−1 of N. Fertilizer production is linked directly to the cost of natural gas—the main energy source for fixing N to ammonia and then to nitric acid. In Europe (EU 25), the production of N fertilizer (109 kg) is falling, while it is rising in Russia, Norway, Saudi Arabia, Iran and China because of cheap energy. In a study of the energy of algae oil production by Chisti (2008), it was reported that over 45 per cent of energy input is in the form of nitrogen in fertilizers and about 9 per cent of the energy input into the production process is in the harvesting and recovery of the oil.
Since approximately 2 kg of CO2 is required per kilogram of microalgal biomass and the N requirement is 80 g kg−1 (160 g CO2), then 160/2000 or 8 per cent of the fixed CO2 in the microalgae is released just to supply the N. Another interesting calculation is that, assuming all fossil fuel oil in Europe (i.e. of the order of 600 × 109 kg oil per year) were to be replaced by renewable sources, this would need 2.5 × 1012 kg microalgal biomass (assuming 25% oil content) and 200 × 109 kg N. In comparison, the total volume of waste sludges (sewage and agricultural) in the EU could yield ca 2–3 × 106 kg N if recovered (Mueller 2007). The question remains as to from where to source the remaining N (ca 105 kg) that is needed. Complete supply of transport fuels accounts for 35 per cent of this (200 × 109 kg oil; 64 × 109 kg N). If annual American oil consumption for transport fuel is of the order of 66 × 109 gal, around 249 × 109 l or 200 × 109 kg (800 × 109 kg algae), then 64 × 109 kg N will be required. American capacity is currently at 13 × 109 kg N yr−1. It should also be noted that, at present, N is worth more than oil ($1.4 kg−1), with current prices at some $0.4 kg−1 for oil. Nitrogen fixation can be carried out by a number of micro-organisms, including some of the photosynthetic cyanobacteria (blue-green algae). Using these organisms is possible, but one would be restricted to a limited number of organisms, and many of these do not produce good quantities of oil. Energetically, nitrogen fixation consumes a large amount of the energy captured by these bacteria, and the efficiency of oil production would be reduced considerably. The blue-green algae are a good target if microbes were to be genetically engineered for the microalgae oil production process. In summary, N recycling, and therefore N recovery, becomes critical for large-scale microalgal production to be cost effective and environmentally sustainable.
In a biorefinery concept, the recycling of nutrients must also be an important consideration especially if large quantities of nitrogen and phosphorus are removed from the overall process as shown in figure 7. The provision of nitrogen and phosphorus can be via nutrient wastes, the most common of which originate from waste water treatment (Hoffmann 1998; de-Bashan et al. 2002), anaerobic digester fluids (Mulbry et al. 2008a,b) or even mineralized by-products of algae after the oils have been extracted (Sialve et al. 2009), and combustion systems such as incinerators or power stations where gases can provide sources of N and S and microalgae can use these gaseous forms (Yun et al. 1997; Doucha et al. 2005). The production of algal biofuels therefore has potential for integration with other environmentally sustainable technologies such as carbon sequestration (Yun et al. 1997; Benemann 1999), emissions clean-up from industrial and agricultural wastes and the purification of water (Munoz & Guieysse 2006). Microalgal farms could thus be naturally aligned with wastes from intensive animal farming, municipal waste treatment processes and power generation from combustion of gases from anaerobic digestion or pyrolysis of biomass or of solid biomass materials (Hoffmann 1998; Cantrell et al. 2008).
Inconsistent nutrient composition of wastes presents a challenge for microalgal cultivation, requiring careful reformulation to provide a consistent, and so reproducible performance. Waste streams can generally provide nitrogen in the form of ammonia or nitrate; to be used successfully, they would need to be converted to a concentrated form to allow easy formulation. Relatively high concentrations of ammonia (less than 1 g l−1) and phosphate (less than 50 mg l−1) are typically found in digester water or interstitial waters of sludge materials (Cantrell et al. 2008). Phosphate is usually highly bound to organic matter or is tied up in inorganic insoluble materials and may not therefore be easily bioavailable.
The recycling of materials and use of wastes as sources of these key elements are not without problems. Within sludge and ashes, there is an accumulation of heavy metals that may prove toxic and will be readily absorbed by the microalgae, potentially rendering the produced biomass as hazardous (Munoz & Guieysse 2006).
4.2. Physical properties of microalgae
The physical properties of microalgae are important when considering processing options. To date, most consideration on species selection has gone into the composition and yield of the product, rather than considering selecting species based on ease of recovery. There are few data available that give detailed descriptions of the physical properties of microalgae and how these are affected by growth and environmental conditions. However, there are some new techniques to apply to this topic, such as atomic force microscopy, that have already been applied to characterize the cell wall and other physical properties of bacteria and fungi (Bowen et al. 2000, 2002; Hamm et al. 2003).
Key physical properties that are useful in designing methods to separate cells from water are density difference, particle size, cell-surface properties and how these may vary according to cell physiological state and life cycle stage (e.g. spines, extracellular polymers). Size and shape variation of microalgae is considerable, ranging from ca 2 to 200 µm for individual cells, such as spheres, rods or filaments (Henderson et al. 2008). The density of microalgae varies from being buoyant (e.g. gas-vacuolated cyanobacteria) to about 1150 kg m−3 for diatoms. Surface charge at physiological conditions is typically negative, with zeta potential ranging from −5 to −40 mV. High negative charge creates an inter-particular repulsive force that maintains the microalgal cells in suspension. Circumventing these forces is the basis of flocculation technology. The control and understanding of inter-particle interactions between microalgal cells and with surfaces is important in both reactor design and primary separation technology, and the manipulation of the surface characteristics of the cells and the process environment should be a major target of research.
Pre-treatment of microalgae just prior to harvesting has been shown to have a significant effect on the cell-surface properties and is typically used in the removal of algae from reservoir water (Molina Grima et al. 2003; Henderson et al. 2008). Here, a combination of oxidation (typically ozonation), a flocculating agent and dissolved air flotation (DAF) is used to separate the microalgae from water. The first three operations rely on the movement of the particles through the fluid, which are most simply described by equation (4.1). Sedimentation of the microalgae depends on the physical properties of these materials; the sedimentation velocity (up) in ideal dilute conditions (spherical non-interacting particles) can be described by the following equation (Svarovsky 1990):
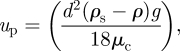 |
4.1 |
where up is sedimentation velocity of the particle (m s−1), d is particle diameter (m), ρs is particle density (kg m−3), ρ the density of the fluid (kg m−3), g is the acceleration due to gravity (m s−2) and μc is the viscosity of the suspension (Ns m−2).
In this equation, the forces of gravity and relative density difference between the particle and the suspending medium are important factors, but most critical is the diameter of the particles, which affects the drag of the particles in the fluid. Therefore, if effective particle size can be enhanced by cell flocculation, then separation speed can be enhanced considerably. There are several types of flocculent that can be used. The simplest form of flocculation is that induced by change of pH. By raising the pH to 8.5 and above, the cell-surface negative charge becomes decreased, and may become positive causing auto-flocculation. The most common additives are soluble aluminium or iron salts, but additions of these may be undesirable, affecting further downstream processing. Organic polyelectrolytes can also be used but are expensive; a possible advance comes from biologically produced flocculants such as proteins or polymer such as chitosan (Oh et al. 2001; Strand et al. 2002, 2003). Flocculation processes are well suited to fresh water environments; however, they are not so efficient in saline environments where the high ionic strength masks the cell-surface charge (Molina Grima et al. 2003). Exploitation of hydrophobic interactions may be a way forward for marine organisms, especially if the appropriate genetically manipulated organisms are used (Jameson 1999).
Other flocculation processes have been suggested such as electro-flocculation, which is achieved at charged electrode surfaces; preliminary results suggest that 1 kW h can treat a volume of 1 m3 (Poelman et al. 1997). Clearly, there is scope to optimize this process with manipulation of process pH.
The most effective and useful methods of product recovery and separation are from disrupted microalgal cells, as intact cell walls and membranes hamper all but the most extreme extraction processes. Key to cell disruption are the mechanical properties of the cells, which are functions of both cell strength and elasticity; these are factors when considering types of disruption, disruption treatment and the recovery of fragmented intracellular materials. It is desirable for the cells to be able to withstand the considerable stresses associated with fluid handling during growth and harvesting but on the contrary should be weak enough to be easily disrupted. It has been demonstrated in bacteria that control of cell wall synthesis and the composition of the suspending medium can have strong repercussions on the strength of cells. By weakening the cells in the harvest, or the pre-breakage environment, disruption and extraction costs could be significantly decreased. In general, therefore, a clear understanding of the chemical and physical chemistry of cell walls and surfaces and how these factors can be controlled, together with the manipulation of the physical environment in which the cells are suspended, could significantly enhance the ease of harvesting and post-processing. This is especially true if these physiological changes were programmed to occur in the early stages of harvesting.
4.3. Cell harvesting
The costs of harvesting microalgal biomass can be a major component of production, accounting for up to 20–30% of the total cost (Molina Grima et al. 2003). The key harvesting and dewatering operations currently used are sedimentation in gravity field, centrifugation, flotation and filtration. The first challenge is to concentrate cells from relatively dilute solutions of ca 0.5–5 kg m−3 dry weight to solutions between 20 and 100 per cent more concentrated than the starting material. In a concentrated state, with 7–10% volume as cells, the rheology of the packed microalgae starts to become non-Newtonian and handling of the cells becomes problematic. At about 15–20% solids, the systems are no longer fluid and not amenable to pumping, which makes handling even more difficult. It is generally preferable to maintain the system as liquid slurry showing Newtonian behaviour to facilitate efficient handling for further downstream processing using pumps. Although sedimentation is a simple process, it is very slow (0.1–2.6 cm h−1; Choi et al. 2006), and in high-temperature environments, much of the biomass produced will deteriorate during such a harvesting process. In consequence, sedimentation alone is largely dismissed as a viable harvesting method. However, flocculation caused by alkaline adjustment has been used to effectively remove Dunalliella testolata (Horiuchi et al. 2003) and Chaetoceros sp. from fluids (Csordas & Wang 2004; Knuckey et al. 2006).
4.3.1. Centrifugation.
Centrifuges can be used to separate and concentrate microalgal cells. Noting equation (4.1) above, if the gravitation field to which the cells are subjected is increased, then cell separation may be achieved more rapidly. In most large-scale centrifuges, a centrifugal force equivalent to 5000–10 000 g is possible, and this can achieve over 95 per cent removal under the correct operational conditions with large algal cells (Molina Grima et al. 2003). However, at a large scale, the use of centrifuges becomes more problematic as the capital costs increase with scale. This, together with the specialized materials of construction (high strength, corrosion-free alloys) and high maintenance costs required to operate in saline environments, means that these separations are expensive. Energy costs of about 1 kW h m−3 have been quoted for centrifuges (Molina Grima et al. 2003). Membrane filtration technology becomes increasingly attractive for the equivalent duty, because capital, maintenance and management costs are lower (Wang et al. 2006).
4.3.2. Flotation.
Flotation is a commonly used approach to remove microalgae from reservoir water prior to its use as drinking water. It is a well developed and mature set of processes. Typically, the water is initially ozonated, after which the sensitized cells are then treated with about 10 ppm polyelectrolyte salts (typically salts of aluminium and iron or formulations of charged organic polymers) prior to being subjected to DAF. DAF involves the generation fine bubbles produced by a decompression of pressurized fluid. The fine bubbles less than 10 µm adhere to the flocs making them very buoyant and causing them to rise rapidly to the surface of a separation tank. The resultant concentrated cell foam (7–10% dry weight) is then removed as slurry. These processes work well in fresh water and are capable of dealing with the large volumes required in a commercial scale plant (greater than 10 000 m3 d−1; Crossley et al. 2002), where additions of ozone and flocculant are made. The main disadvantage of this approach is the contamination of the materials with the floc agent, which may significantly decrease their value (Molina Grima et al. 2003). Although these methods have also not been proved in saline environments on a large scale, the integration of flotation into the bioreactor has been demonstrated. Using an integrated reactor and foam fractionator, under appropriate conditions up to 90 per cent of a Chaetoceros sp. could be removed (Csordas & Wang 2004).
4.3.3. Filtration.
There are many modes of filtration that can be used to concentrate microalgal cells, the most simple of which is dead-end filtration. Dead-end filtration of large quantities of dilute algal suspension can only be achieved using packed bed filters (mixed media or sand). This type of filtration is limited by the rheological properties of the microalgae as these form compressible cakes that easily blind filters. This technique again has been used successfully in the removal of algae from reservoirs where their concentration is relatively low. The amount of water that can be processed is severely limited by the characteristics of algal materials, e.g. compressible cakes, and the presence of extracellular fouling materials. These processes involve relatively low energy consumption, although the frequency of washing with loading increases energy costs and decreases filter productivity. Pressure or vacuum filtration can be used, but concentration of the microalgae is required for these processes to be effective. Power consumptions for these operations are of the order of 0.3–2 kW h m−3, not dissimilar to those required for centrifugation (Molina Grima et al. 2003).
To avoid problems in dead-end filtration, cross-flow filtration can be used; several studies have been published and demonstrate that high concentrations of microalgal cells can be attained of up to 100 kg m−3. The advantages of such filtration systems are their ease of scale-up, with rapid advances being made in their use and operation. Several laboratory scale studies have shown that these systems are capable of concentrating microalgae and can be used in downstream fractionation (Rossignol et al. 1999; Vandanjon et al. 1999; Rossi et al. 2004). Decreasing the process volume by at least a factor of 100 significantly lowers the costs of disruption and fractionation stages downstream. Although a definitive study on large-scale algal harvesting has yet to be published, work has shown that the cost of microfiltering river water can be as low as 0.2 kW h m−3 of water processed (Lazarova et al. 2006). Several variables associated with the choice of membranes and type of organisms could increase this cost, and there is considerable scope for optimization of this process. As a guide to potential improvement, the costs of desalination by reverse osmosis, where a far higher pressure process is used, have fallen dramatically (85%) over the past decade to give a total production cost of about $1 m−3 and with desalination energy costs being as low as 3 kW h m−3. This is largely down to a better membrane technology, greater membrane longevity, increased scale of operation and better system management. Such advances might also be expected in membrane separation processes for harvesting of microalgae.
4.4. Cell disruption
To efficiently extract materials from the inside of cells, some form of cell disruption is generally required. In most cases, because of the cost and energy involved, these disruption processes are carried out in concentrated cell preparations (50–200 kg m−3 dry weight). There are many ways to disrupt microalgal cells; however, the key criterion is the maximization of the value of the materials obtained from the processes, which means that rapid and precise disruption should be used. Mechanical disruption of cells is chosen in most cases as this offers an approach that avoids further chemical contamination of the algal preparation while preserving most of the functionality of the material within the cell (Chisti & Moo-Young 1986).
A survey of the literature fails to identify systematic studies of the large-scale disruption of microalgal cells, but there are two processes proved on large-scale non-algae applications, homogenization and bead milling (Chisti & Moo-Young 1986). Cell homogenization involves the process fluid being forced through an orifice; this creates a rapid pressure change as well as a high liquid shear, which impinges on the microalgae causing disruption. The degree of disruption is dependent on the pressure applied and the strength of the algal cell walls, the strength of which is largely down to their physiological state. Stressed organisms, for example, those grown in suboptimal growth conditions, are often more physically resilient (with thicker cell walls), but P-starved microalgae, and Si-starved diatoms, tend to be more fragile. Another factor is the breakage buffer composition, where low osmotica decreases apparent cell strength. The size of cells is also a factor; larger cells for a given equivalent strength are more exposed to high liquid shear fields in the disrupter fluidics. The second approach is the use of bead mills; these are vessels packed with small glass beads that are agitated at great speed. The result is that cells are disrupted, but the level of disruption is usually dependent upon the residence time in the system (Doucha & Livansky 2008). Cell strength and the size and shape of cells will also affect the performance of these devices.
Optimization of breakage is important as this involves the use of large amounts of energy and also affects the physical and chemical nature of the end product (e.g. the extent to which lipid membranes are disintegrated). Overall, the amounts of energy used per unit amount of microalgae disrupted are dependent on the concentrations of the cells and their strength. Most effective breakage will be carried out where cell concentrations are high and where, after disruption, the components are easily separated. Cell concentrations between 100 and 200 g l−1 dry cell weight can be used. Typical energy consumption for homogenizers (operating at 100–150 MPa) is of the order of 1.5–2 kW h to give a 95 per cent protein release for 10 l of process fluid or about 1 m3 of the original microalgal culture fluid (assuming a cell concentration factor of 100 by mass). At these levels of energy dissipation, heating of the process fluid can be a problem such that significant cooling is also required. Scale-up of these devices brings about some efficiency gains based on improved pump performance. Bead mills give an equivalent performance but the design of the milling chamber and fluid mixing can have a significant effect, while disrupters requiring multiple passes are inefficient and allow poor mixing to give uneven process treatment of the cells. There is considerable scope to study these processes to find the correct cell breakage procedure, particularly to identify biological factors (reducing cell wall strength and possible pre-treatments to achieve this) that are associated with this process, and genetic modification of many of these traits should be eminently achievable (Pienkos & Darzins 2009).
4.5. Fractionation and oil recovery
Once the microalgal cells have been disrupted, fractionation of the material can be carried out. Generally, the principles of separation of materials from disrupted cells are well established, and it is only the development of microalgae-specific optimized protocols that are now required. The main need here is for fractionation schemes that recover all fractions of the microalgae at the maximum value. Many specific one-product protocols exist, for example, the use of solvents (such as hexane) has been developed for the extraction of oils from the whole cells with, or without, disruption. However, such an approach has disadvantages in that cellular materials are often denatured by the solvent, and it is very difficult to decontaminate such material as a result. The use of solvents at a large scale also requires additional costs owing to the very high standard of plant design criteria because of the risk of fire and explosion hazards. Similarly, salt precipitation of proteins would require a desalting/buffer exchange and so is suitable only in the later stages of purification, when the materials are concentrated and occupy a small volume of process fluid. It is for these reasons that methods of separation based upon size, charge and density should be the preferred choice. The separation and concentration of fat droplets can be achieved by microfiltration, while the soluble proteins, recovered using a diafiltration process, will pass through a microfiltration membrane creating a fat-free, soluble protein fraction that can be concentrated and dried. This material can be used as a source for further refining (into enzymes, functional proteins, etc.), or alternatively the density differences may be exploited using centrifugation, which is more effective in the absence of emulsifying soluble proteins. Further fractionation of the cell debris is also possible so that the cell wall materials (carbohydrates and silica) and other organics such as pigments and other metabolites may be isolated (figure 7).
5. Converting algal biomass and lipids to fuels
The main biofuels in use today are bioethanol from carbohydrate fermentation and biodiesel/green diesel based on plant lipid fractions, only the latter of which is covered in this review with respect to microalgae. There have been relatively few studies specifically aimed at converting microalgal biomass-derived lipids to fuel-type components; however, when using the extracted oil and/or carbohydrate fraction from microalgae, the techniques used for terrestrial plant oil and carbohydrate conversion are more-or-less applicable, especially for the processes considered herein. There are, however, several differences between the lipid composition of microalgae and higher plants (Hu et al. 2008a,b). For example, the relative proportion of polar lipids to neutral lipids (triglycerides) is significantly higher in microalgae. The other notable difference is that long-chain polyunsaturated fatty acids (greater than C18), common in microalgae, are not produced in significant quantities in higher plants. Both of these aspects will affect the efficiency of biodiesel synthesis, as well as influence the fuel properties (e.g. very low oxidative stability of highly unsaturated fatty acids).
A number of reviews have been released covering the area of biomass to transport fuels; Huber et al. (2006) have reviewed the overall area of biomass conversion for transport fuels, while Smith et al. (2009) have examined catalysis in lipid to fuel conversion and Di Serio et al. (2008) have examined the research thus far into heterogeneous catalysis in biodiesel production. Although fatty acid methyl ester (FAME) type fuels are now commonplace, this must be at best a stopgap technology for the current transport infrastructure as FAME can only be blended, typically, at a 5–10% level without engine modification and has a lower energy density, 38 MJ kg−1, than fossil diesel, 43 MJ kg−1 (Kalnes et al. 2007). In this section, we discuss technologies for producing biofuels from microalgal biomass or algal lipids, covering pyrolytic upgrading of the algal biomass, catalytic upgrading of the lipids, trans-esterification and de-oxygenation/hydrogenation approaches. We also discuss the application of a variety of different catalysts and identify the challenges associated with the potential application of the technology and existing catalysts to algal lipids.
5.1. Pyrolytic and cracking upgrading of the whole biomass
Pyrolysis is a technique used to upgrade a variety of biomass at a reasonably large scale through slow heating in the absence of oxygen to produce gaseous, oil and char products. Cracking is a technique used to break down larger hydrocarbons, and other molecules, into smaller, more desirable hydrocarbons in the presence of a size-selective catalyst and the absence of oxygen and can be used to further upgrade the oil fraction from pyrolysis processes. In a recent study, Grierson et al. (2008) investigated the pyrolysis of dried and finely ground algae biomass using a slow pyrolysis method; it was found that up to 43 per cent by volume heavy bio-oil could be produced from Tetraselmis and Chlorella species. Although the oil would be suitable for further cracking, this was not investigated. Furthermore, though it seems the process is net energy producing, it is unclear whether when the drying step is taken into account this will remain the case, and the energy-intensive nature of thermochemical processes has been commented on by Chisti (2008). Catalysts used for cracking include zeolites (Twaiq et al. 1999) and other mesoporous aluminosilicates (Twaiq et al. 2003). A number of three-dimensional structures called pillared clays containing various metals have also been investigated for their ability to crack vegetable oils such as canola oil, palm oil and sunflower oil into biofuels (Kloprogge et al. 2005). In general, pyrolysis can be a useful approach for dried or even untreated biomass and biomass residues or for use in local cofiring of biomass. It is an advantageous approach in that it requires no complex separation of the biomass fractions. However, it remains unclear whether the return in oil in any way improves the economics of the process as any high commercial value chemicals would be destroyed. As such, there may well be a place for the pyrolysis of the residual biomass after oil and high-value product extraction to maximize the yield from the biomass.
5.2. Trans-esterification for biodiesel production
Biodiesel is a term used to describe ‘a fuel comprised of monoalkyl esters of long-chain fatty acids that are derived from vegetable oils or animal fats’ (Zhao 2006), although it is also used in a more general nature (Snare & Murzin 2006). Biodiesel is produced via trans-esterification, as shown in figure 7a, where the fatty acyl chains of triglycerides present in vegetable oils are catalytically trans-esterified, usually with methanol (methanolysis), to yield the corresponding FAME and glycerol. Both homogeneous (same phase) and heterogeneous (different phase) catalysis can be used to drive this reaction. The main distinction between these two types is the possible recovery and recycling of the (solid) catalyst in heterogeneous catalysis, potentially reducing the overall conversion costs.
5.2.1. Homogeneous catalysis for biodiesel production.
Homogeneous acid or base catalysis has the advantage of the catalyst being in constant contact with the reaction mixture leading to increased rates, and is a generally used method for biodiesel production from seed oils. Vicente et al. (2004) have compared the four most common homogeneous catalysts for trans-esterification—sodium hydroxide, potassium hydroxide, sodium methoxide and potassium methoxide. At 65°C, and a 6 : 1 methanol : sunflower ratio, the use of sodium methoxide was found to give the largest yield with 99.3 per cent biodiesel purity. Using microalgal oil from Chlorella protothecoides, Miao & Wu (2006) used acid-catalysed trans-esterification to produce biodiesel, with methanol and sulphuric acid (preliminary tests suggested that alkali catalyst was not suitable possibly due to the high acid value of the microalgal oil). The best combination the authors found was with 100 per cent catalyst (based on oil weight), 56 : 1 molar ratio of methanol to oil, 30°C and a reaction time of 4 h. Note the high content of methanol required—a requirement for trans-esterification processes.
A further problem with homogeneous (acid or base) catalysts is that they suffer from the requirement of a neutralization step to remove the catalyst. Additionally, plant and algal oils can contain FFAs at non-negligible concentrations; concentrations of up to 25 per cent have been reported in the logarithmic growth phase of diatom microalgae species by Dunstan et al. (1994). If these are not pre-treated (esterified), then they can react with homogeneous base catalysts during trans-esterification and form the corresponding soaps, leading to downstream separation problems (Huber et al. 2006). FFAs may also be formed from water reacting with FAME during storage; FAME fuels have a tendency to hydrolyse or undergo oxidative decomposition, and the storage of the fuel product must be maximized for longevity (Paligová et al. 2008).
5.2.2. Heterogeneous catalysis for biodiesel production.
Efficient heterogeneous (solid) catalysts offer economic benefits in producing biofuels since, unlike homogeneous catalysts, they are easily separated after trans-esterification, and so can be readily recycled, lowering production costs. Using one type of heterogeneous catalyst, calcined layered double hydroxides (LDHs), trans-esterification of plant oils in a stirred batch reactor for 3 h at 60°C with 0.05 g catalyst has been carried out with glyceryl tributyrate, methanol and hexyl ether (Cantrell et al. 2005). An LDH with an Mg : Al ratio of 2.93 : 1 led to the highest conversion, owing to the increase in the intralayer electron density (and associated basicity) with increasing Mg content. Calcination of the LDH materials leads to the formation of mixed metal oxides (MMOs), which are usually more basic catalysts than the corresponding LDH (Cavani et al. 1991). In a recent study, MMOs that had been doped with various metal ions replacing Al3+ were tested for biodiesel production (Macala et al. 2008). Ten per cent Ga dopants lead to an increase to around 80 per cent conversion at 60°C of triacetin to the corresponding methyl esters, while Fe-based dopants lead to an even greater activity with greater than 95 per cent yield after 40 min at 60°C. The surface area for the MMO catalyst was found to be approximately 50 per cent greater than the uncalcined MgAl LDH. A solid-base catalyst of KF/Al2O3 has also been used for the conversion of plant (palm) oil to alkyl esters by Bo et al. (2007). Trans-esterification was carried out at atmospheric pressure and with an optimum temperature of 65°C; above this, the volatility of methanol became an issue leading to a decrease in the methanol : oil ratio from the desired 12 : 1. Optimization led to a triglyceride conversion efficiency of over 90 per cent after 4 h.
In another approach, a superbase was prepared by calcination of Eu(NO3)3/Al2O3 forming Eu2O3/Al2O3 with an optimal Eu content of greater than 6.75 per cent (Li et al. 2007). This was used to trans-esterify soybean oil in a fixed bed reactor. Again, reaction temperature was optimal at 70°C owing to the volatility of methanol. Water had to be removed from the oil and methanol to prevent reaction with the catalyst. A final conversion of 63 per cent at 8 h was observed. The methanol : oil ratio was 4 or more, although continually increasing methanol ratio can lead to separation problems from the prepared methyl esters. After 40 h of use, the catalyst activity had decreased (35%) conversion, thought to be due to water and FFAs. High FFA oils, such as found in some microalgae, are unsuitable for base catalysis; a heterogeneous acid catalyst is preferred. Sulphated zirconia catalyst has been found to catalyse soybean oil to biodiesel with 98.6 per cent yield (Garcia et al. 2008). Unfortunately, the catalyst is deactivated rapidly. Carbohydrate-derived heterogeneous acid catalysts have been shown to trans-esterify oils with up to 27.8 wt% FFA content to 92 per cent FAME after 8 h (Lou et al. 2008), and are exceptionally stable, still around 93 per cent active after 50 successive uses. Such advances offer potentially robust and FFA-tolerant catalysts for trans-esterification catalysts for microalgae lipid feedstocks.
In general, there are a plethora of homogeneous and heterogeneous catalysts giving good yields for the production of biodiesel from a variety of feedstocks, and there seems no reason why algal lipids should behave differently, though there can be a marked dependence on feedstock of the end product. Utilizing the carbohydrate fraction of the microalgae for bioethanol production could mitigate the large excess of methanol or ethanol required in trans-esterification reactions. However, a wider question arises as to whether, other than in the very short term for blending with and extending fossil reserves, biodiesel is the best use of algal lipid fractions.
5.3. Deoxygenating fatty acids for green diesel production
Trans-esterification results in fuels that are oxygenated, owing to the oxygen present in the acyl glycerides being retained in the products. Therefore biodiesel is not a direct substitute for diesel, which is characterized by medium- to long-chain, unbranched alkanes of 12–20 carbon atoms long. Trans-esterification suffers problems with excess alcohol needed, which is volatile at high temperatures, and the presence of FFAs and water decreases the efficiency of many catalysts. If the fuels produced can be deoxygenated, then they could be used as a direct replacement for fossil fuels. Pyrolytic cracking or hydrogenation of oils is the normal method for deoxygenating vegetable feedstock (Demirbas 2007; Maher & Bressler 2007), for example using zeolites (Demirbas 2008), but this can lead to a fuel with a lower energy content than other methods.
In recent times, attention has focused on attaining deoxygenation of oils via decarboxylation/hydrogenation reactions. Such fuels have been termed ‘green diesel’. Green diesel has been produced by catalytic saturation, hydrodeoxygenation, decarboxylation and hydroisomerization reactions (Kalnes et al. 2007). Using hydrogen at around 1.5–3.8% in the reactor produced green diesel yields between 88 and 99 per cent depending on the catalyst used. The resulting product was comparable in properties to ultra-low sulphur diesel and superior to oxygenated biodiesel. As part of the study by Kalnes et al. (2007), a life cycle analysis was undertaken; green diesels were found to have higher net energy balances than petroleum diesel and biodiesel, which translate to lower fossil fuel inputs during their production and increased sustainability—an important point often overlooked. They also lead to considerably lower greenhouse gas emissions per energy equivalent, as the sulphur content is negligible, thereby reducing sulphur oxide emissions compared with petroleum diesel, being slightly less than comparable amounts of biodiesel. These results suggest that green diesel is a superior bioenergy source, in terms of sustainability, compared with oxygenated biodiesel. No data have been published specifically on algal biodiesel; however, the literature has covered the catalytic decarboxylation of fatty acids in great detail. We will now consider the technology and discuss the published reaction conditions and catalysts.
The technology is not new; in the 1930s, Bertram (1936) used a homogeneous catalyst over selenium to decarboxylate stearic acid. Recently, using activated carbon-supported catalysts, n-heptadecane was found to be the main product when stearic acid, ethyl stearate and tristearin were deoxygenated (Kubickova et al. 2005). By controlling reaction conditions at 300°C, under 5 per cent hydrogen and at 1.7 MPa, stearic acid was found to have a higher percentage of conversion. Ethyl stearate converted into stearic acid before decarboxylating to n-heptadecane, although selectivity to n-heptadecane decreased from 300 to 360°C when aromatics started to be produced instead, which are unsuitable in diesel fuels. The reaction kinetics for ethyl stearate and stearic acid decarboxylation over palladium/carbon (Pd/C) catalyst have also been studied; a problem when using fatty acids is that the Pd/C catalyst is deactivated at high concentrations (Snare et al. 2007).
Snare et al. (2006) investigated a range of catalysts, Ir, Mo, Ni, Os, Pd, Pt, Rh and Ru (supported on carbon and metal oxides) as well as a Raney nickel catalyst. Side reactions were observed over 6 h of reaction (300°C, 600 kPa, helium) such as isomerization, dehydrogenation, aromatics and shorter hydrocarbons by cracking. It was found that some side products produced using Ru/C and Rh/C catalysts were selective towards unsaturated side products, resulting in catalyst deactivation. Out of those tested, 5 per cent Pd/C was found to be the preferred catalyst for the decarboxylation of stearic acid. Additionally, further work has led to diesel-like hydrocarbons with unsaturated renewable feedstock (Snare et al. 2008). Another method to produce desirable long-chain alkanes is the coupling of fatty acids by ketonic decarboxylation and subsequent hydrogenation reactions (Corma et al. 1998). The reaction was found to lead to 100 per cent conversion when either a Pt/MgO or a Pt/Al2O3 catalyst was employed. These findings are promising, and there is reason to believe that this technology would be readily applicable to microalgal lipids. This would yield straight chain alkanes that can be blended with existing petroleum diesel without affecting the oxygen, stability and flow properties of the resulting fuels.
6. Modelling approaches for microalgal biomass growth, optimizing lipid yield and process development
Mathematical approaches (computer models, simulations) may be used in many ways to enhance the development and exploitation of microalgae for commercial gain. The most obvious features of interest are the immense saving in time and resources that may be achieved by using models simulating algal physiology operating within bioreactors of different configuration, if only to rule out the less efficient combinations. The catch, as ever, is the need for the model to simulate reality with sufficient fidelity. Modelling techniques are of potential importance in the following areas: (i) optimization of algal growth and production of specific end products, (ii) optimization of bioreactor design and operation, (iii) production facility operation, and (iv) coupled operation and financial modelling and risk analysis.
6.1. Optimization of microalgal growth and production of specific end products
At the heart of any attempt to commercially exploit microalgae is the need to identify the optimal combination of microalgal strain and growth conditions. The permutations are enormous. The potential for models to help in at least identifying likely contenders is clear.
Traditional models of microalgal growth have been developed to describe very small-scale, 1 l flask-type, systems. There is a long and rich history of such models, stemming from the work of the likes of Droop (1968); see reviews by Flynn (2003, 2008). Such descriptions, and simpler, can readily provide the basis for bioreactor simulators (Frisch & Gotham 1979; Gotham & Frisch 1981). Mechanistic models allow comparisons between different physiological configurations of nutrient acquisition, photosynthetic capacity (as described by the initial slope of the photosynthesis–irradiance curve, α; pigment content, Chl:C; maximum fixation rate, Pmax), stoichiometric configurations (quotas of C, N, P, lipids, etc.), maximum growth rate and so on. That aside, traditional modelling methods (i.e. using overly simplistic, non-dynamic, non-mechanistic descriptions) may prove inadequate for the simulation of growth in bioreactors (Grover 1991). Mainly, this is because decades of microalgal research have not provided the types of data required to fully develop or parametrize models for commercial exploitation of microalgae. For example, there are few data series describing C-biomass and lipid content varying with combinations of light, temperature and nutrient availability.
A recognized approach for the enhancement of lipid, and potentially for the enhanced production of other products (in terms of production rate and/or proportion of yield), is the manipulation of growth conditions (e.g. Li et al. 2008; Chi et al. 2009). Control of nitrogen source availability is a well-documented form of manipulation (Flynn et al. 1992; Navarro-Angulo & Robledo 1999). In some instances, using microalgal heterotrophic potential has been shown to be of value (Xu et al. 2006; Chi et al. 2007); microalgae are useful within some fermentation systems because, being photosynthetic, they have unique biochemical pathways not present in non-carbon fixing organisms. Our understanding, and hence our ability, to model the commercial viability of such approaches is weak.
Other areas of microalgal growth and production that have attracted some modelling attention include grazing and allelopathic interactions. Algal–algal (allelopathic) interactions may be important in open-air ponds. Allelopathic interactions are only of real consequence at high cell densities, and their role in nature is unclear. In commercial systems, however, both these interactions and grazing can generate important changes in biomass structure, in some instances even being counterintuitive; Mitra & Flynn (2006) describe and model a situation where an outwardly inferior microalga grows to dominate a system. Modelling allelopathic interactions in microalgal systems has been considered by Fergola et al. (2007).
The use of genetically modified (GM) microalgae was suggested and explored over a decade ago (e.g. Roessler et al. 1994; Dunahay et al. 1996 in Cyclotella cryptica). Indeed, it is considered that the use of GM microalgae, like the deployment of GM higher plants, may be essential (Gressel 2008) in order to boost production levels beyond what can be achieved by manipulation of growth and nutrient conditions. Modification of the photosystems, to enhance photosynthesis, is an obvious target (e.g. Mussgnug et al. (2007) for Chlamydomonas reinhardtii), as is enhancement of lipid production, through the manipulation of the efficiency of the committing enzyme in lipid biosynthesis (ACCase, figure 3; Dunahay et al. 1996). Hydrogen (rather than biomass) production has also been suggested as a renewable fuel from algae (Berberoglu et al. 2007; Hankamer et al. 2007) and is another approach that requires some level of genetic modification (Melis et al. 2007) and/or careful manipulation of the growth conditions to redirect biochemical processes to perform a production that does not naturally occur to any significant extent. Such papers do not make it clear what the economic viability is for this approach, but clearly the construction of dynamic models would force the calculation of system energetics.
This whole arena is ripe for theoretical investigations using modelling approaches, not only to consider benefits, but also risks, but there do not appear to be any published studies. Some of these interactions would no doubt be of value to the biologists as well; Mussgnug et al. (2007) express concern about the potential susceptibility of GM microalgae to photodamage, while a typical bioreactor simulation would suggest that, at the biomass levels required for commercial production, light limitation (rather than saturation at damaging levels) is more likely.
6.2. Optimization of bioreactor design and operation
The choice of bioreactor is as critical as that of the organism, for it governs the conditions under which the organisms grow. The two basic options are either for an enclosed system, rather akin to an experimental biologist's culture flask, or a pond (as discussed in §3). The latter has far more in common with the natural growth of these organisms, potentially with the inclusion of uncontrolled and/or uncontrollable interferences (light, temperature, contamination, grazers); such interferences make the culture growth and behaviour more difficult to predict.
As mentioned above, traditional phycological models (i.e. Monod or Droop type) were developed using data from small-scale cultures often using chemostats, which are akin to through-flow PBRs. In contrast, the modelling of production in pond systems (Hagiwra & Mitsch 1994) may be likened to that in environmental management. While there is a large literature on the modelling of microalgal growth in lakes and reservoirs (e.g. Imteaz et al. 2003; Komatsu et al. 2006), this is invariably directed towards minimizing the growth of natural algal populations in low-nutrient systems, and understanding processes that are important to model in simulations of that growth (e.g. Wallace & Hamilton 1999). The value of such work towards maximizing the growth of specific species in (very) high biomass systems is limited, with the drivers for the work (both modelling, and logistic/financial) being very different. At the interface is the growth of microalgae in sewage ponds, for which modelling has been shown to have utility (Buhr & Miller 1983; Llorens et al. 1991). Modelling applications towards technologically similar waste effluent treatment systems involving microalgae are similarly possible (e.g. Mulbry et al. (2008a,b) for swine waste).
Models for bioreactor-type applications that are in the literature are typically (and unsurprisingly) deterministic ordinary differential equation structures. For simulations of bioreactors, deterministic approaches should be quite sufficient, provided that enough detail is known of the system; stochastic input to such models would typically be linked to meteorological descriptions. When using poorly understood systems, perhaps with microalgae with a complex life history, fuzzy logic modelling approaches may be considered (e.g. Clarkson et al. 2001).
At the other end of the computational scale, coupled fluid dynamics and biological modelling offer additional potential for the optimization of bioreactor design. The main parameters of importance here are dilution rates, optical path length, nutrient and light supply. At a higher level, modelling allows a consideration of the detailed physical design of the reactor (e.g. Cornet et al. 1992, 1995; Fernandez et al. 1998, 2000, 2001; Lin et al. 2003). Other concepts, such as vertical sheet reactors (Zemke et al. 2007), also provide an opportunity for modelling. Such explorations have as long a history as models of microalgae (e.g. Incropera & Thomas 1978; Morton 1978). However, typically modelling studies of such systems employ sophisticated physics descriptions with arguably over-simplified descriptions of the biology. Biological systems acclimate to changing conditions; models of microalgae growing bioreactors, especially in reactors with changing light and/or nutrient regimes, should be able to simulate physiological changes (Camacho Rubio et al. 2003).
Modelling in the arena of bioreactor design offers many opportunities of value for commercial optimization. For example, Merchuk & Wu (2003) used models, calibrated against experimental data, for growth of microalgae in bioreactors under different light–dark regimes, different levels of mixing and reactor design. Suh & Lee (2003a,b) used models to explore the placement of lights within bioreactors. The type of work done by Rodolfi et al. (2009), exploring the growth of different strains of microalgae under contrasting conditions, represents the types of study required to assist in model parametrization. Likewise, the growth optimization work of Zhu & Jiang (2008) could have been undertaken in the space of a few hours using models rather than experimental methods. Scale-up of bioreactor performance is a critical issue for commercial viability, and modelling offers the only realistic way of exploring very expensive alternatives, rather than actually building and testing them (Molina Grima et al. 1996, 1999). Model formulation also acts to assist in the proper design of experiments; it is very much a two-way street, but academic research projects have generally hitherto failed to take a holistic approach.
6.3. Microalgae production facility operation
The other application of models to microalgal growth is in the area of systems control. It is important, in this context, to appreciate that bioreactors for photosynthetic organisms (PBRs) are not so simple to control as are traditional fermentors; the self-induced light limitation, through shading of the densely grown organisms that is generated in a PBR, coupled with the importance of a regulated gas flow, requires a complex series of control measures (Li et al. 2003). So-called intelligent modelling systems have been deployed to control production in bioreactors (e.g. Jones et al. 2002; Hu et al. 2008a,b); such systems operate in real time to control system parameters such as pH, nutrients, light and temperature. Models have also been applied to post-harvesting subjects, such as drying of the biomass (Vega-Galvez et al. 2008).
6.4. Coupled operation and financial modelling and risk analysis
This is the ideal operations route for modelling, to not only aid as a guide to testing the viability of the commercial exploitation of microalgae under dynamic conditions, but to aid in the operation of the enterprise. This capacity would add far more to our ability to optimize production than steady-state estimates. Into such a model daily changes in irradiance (for pond systems) and even commodity prices could be entered, facilitating the optimal regime for algal growth, harvesting and downstream processing. While the goal is clear, there is no evidence that we are close to achieving it without a significant input of resources. That said, there is more than sufficient generic knowledge to construct such a model and to test it. For sure, the risk analysis will reveal large margins for error, but as more information is added, and the model refined, these margins of error will be decreased.
Recently, we (Flynn et al. submitted) have conducted an analysis of the operation of bioreactors of different optical path size, making use of a mechanistic model of algal physiology (Flynn 2001), which has been used in oceanographic scenarios (e.g. Fasham et al. 2006). We used the approach developed by Flynn (2001) to consider the best commercial configuration for production, and also the potential for using GM strains with altered photo-physiology. Assuming a maximum algal growth rate of two divisions a day (and most algae attain half this because their cell cycle is linked to the diel day–night cycle), using a typical physiological configuration for cellular photosystem operation, yields year-averaged areal production rates around 5 gC m−2 d−1 over a wide range of optical depths (0.01–1 m). The output of the most productive oceanic waters is around 100 molC m−2 yr−1 (Sarmiento & Gruber 2006). This equates to a daily rate of 3.29 gC m−2 d−1, a value quite similar to that we predict from PBRs making use of naturally occurring high growth rate phytoplankton. In comparison, rates that have sometimes been informally advertised for microalgal productivity in bioreactors have been as high as in the region of 100 gC m−2 d−1. We can only attain such values from simulations of GM organisms.
This type of analysis provides several useful insights into both the general topic of algal biofuels and the role of models. Firstly, obtaining robust data for modelling algal production is difficult. Much of the data are couched in terms of dry or wet weight, while models (and ultimately commercial production) require data for C flow through these systems. Most of the data are shrouded in commercial secrecy and unavailable for scrutiny. While one may argue that production in certain systems with selected strains is much higher than in others, it is difficult to understand how the underlying physiology of the organisms in commercial systems can be at such variance with the published scientific literature that forms the bedrock of mechanistic modelling of microalgae so as to explain a 50-fold difference between productivity reported for commercial systems versus that predicted using mechanistic models. The implications are either the unlikely scenario that physiologists have been studying microalgae using models that are grossly incorrect or that commercial claims have been somewhat exaggerated. Finally, modelling shows the potential for GM manipulation of algal physiology, and allows an analysis of its benefits and risks.
7. Conclusions
The nature of algal biofuel projects requires a cross-sector approach, with aquaculturists working with reactor manufacturers, biologists, engineers and chemists. Much work remains to be done on the basic biology of microalgae, species selection, genetic manipulation and molecular characterization of the metabolic switch for carbon sequestering and storage. Furthermore, the chemistry of biofuels synthesis requires further investigation. Although there has been much work published on the upgrading of vegetable/algal oils, this has mainly been using model compounds, such as stearic acid and its esters, rather than actual algal oil. Additionally, for decarboxylation and trans-esterification upgrading, it is usually only the relatively short-chain-length aliphatic acids that were used—a relatively small component of the total algal oils. Further investigation of catalytic decarboxylation and trans-esterification of specific algal oils or biomass is needed. Furthermore, a more efficient conversion route and a potentially economical green diesel or whole-biomass-based biofuels synthesis technology could prove to reduce the overall cost of the algal biofuels production system.
Given the limits of biological light utilization and energy conversion, the economic feasibility of algal biofuel production will depend on lowering the costs and/or increasing the efficiency of the following: (i) culturing systems, and accompanying land, water, nutrient and CO2 requirements, (ii) modification of the photosynthetic capability and productivity, (iii) the method of cell harvest, (iv) the method of cell rupture and/or subsequent lipid extraction, (v) cost of the bio/green diesel production from the crude lipid fraction, and (vi) market potential and value of the by-products and/or energetic value of the ‘waste’ fraction.
Biomass production costs will very much depend on the systems considered—at present, it still remains unclear whether an open pond approach will outcompete raceways and/or closed PBRs. The argument presented in favour of open pond systems is that this is how the majority of current microalgae culture at scale takes place, making no allowance for the development of alternative technologies. Of these factors, the one that is least often considered is that of nutrient usage and costs. Identifying low/no-cost nutrients, for example from eutrophicated water, or intensive farming effluent, and gas waste streams from industry will reduce production costs. This, however, results in a narrowing of the areas in which the microalgal production plant may be located. Given the limitations of biological productivity in these organisms, if a non-GM route is taken, the limiting factor becomes reduction of production costs per unit biomass/oil yield. At its simplest, the large-scale pond production system is already operating at near the limits of minimum cost and maximum productivity and provides a difficult environment to control, possibly GM, species. In such systems, the main gains may well be in species selection for stable culture populations with a fine balance of biomass yield, oil content and composition, and handling characteristics. Therefore, it might be argued that largest changes in cost per unit volume will come from closed systems or hybrid closed–open systems where the technology is relatively immature. The respective costs are only recently being addressed. In such systems, high volumetric productivity could potentially reduce harvesting costs and therefore influence the economics of the whole production system. The details and economic considerations remain to be demonstrated using large-scale culture facilities combined with modelling approaches.
In summary, microalgae have the potential to offer a simultaneous production technology for bio/green diesel, bioethanol and high-value chemicals. The challenge remains to close the cost gap between microalgae-derived fuels and fossil oil.
References
- Anderson R. A. 2005. Algal culturing techniques. London, UK: Elsevier Science and Technology Books, Academic Press. [Google Scholar]
- Benemann J. R. 1999. Biological processes for the mitigation of greenhouse gases. In Conf. Proc. of the 4th Int. Conf. on Greenhouse Gases Control Technologies (eds Eliason B., Riemer P., Wokaun) A., pp. 689–693. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- Berberoglu H., Yin J., Pilon L. 2007. Light transfer in bubble sparged photobioreactors for H2 production and CO2 mitigation. Int. J. Hydrogen Energy 32, 2273–2285. ( 10.1016/j.ijhydene.2007.02.018) [DOI] [Google Scholar]
- Bertram S. H. 1936. Elaidinization of oleic acid and cis–trans isomerism. Chem. Weekblad 33, 457–459. [Google Scholar]
- Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Bo X., Xiao G. M., Cui L. F., Wei R. P., Gao L. J. 2007. Transesterification of palm oil with methanol to biodiesel over a KF/Al2O3 heterogeneous base catalyst. Energy Fuels 21, 3109–3112. ( 10.1021/ef7005035) [DOI] [Google Scholar]
- Borowitzka M. A. 2005. Culturing microalgae in outdoor ponds. In Algal culturing techniques (ed. Andersen R. A.), pp. 205–218. London, UK: Academic Press. [Google Scholar]
- Bowen W. R., Lovitt R. W., Wright C. J. 2000. Application of atomic force microscopy to the study of micromechanical properties of biological materials. Biotechnol. Lett. 22, 893–903. ( 10.1023/A:1005604028444) [DOI] [Google Scholar]
- Bowen W. R., Fenton A. S., Lovitt R. W., Wright C. J. 2002. The measurement of Bacillus mycoides spore adhesion using atomic force microscopy, simple counting methods, and a spinning disk technique. Biotechnol. Bioeng. 79, 170–179. ( 10.1002/bit.10321) [DOI] [PubMed] [Google Scholar]
- Buhr H. O., Miller S. B. 1983. A dynamic model of the high-rate algal bacterial wastewater treatment pond. Water Res. 17, 29–37. ( 10.1016/0043-1354(83)90283-X) [DOI] [Google Scholar]
- Butler N. 2006. The transition from fossil fuels. Talk given at Sustainable Energy meeting, Cambridge, UK, 1 December 2006. See http://www.cambridgeenergy.com/archive/2006-12-01/cef01dec2006butler.html [Google Scholar]
- Camacho Rubio F., García Camacho F., Fernández Sevilla J. M., Chisti Y., Molina Grima E. 2003. A mechanistic model of photosynthesis in microalgae. Biotechnol. Bioeng. 81, 459–473. ( 10.1002/bit.10492) [DOI] [PubMed] [Google Scholar]
- Cantrell D. G., Gillie L. J., Lee A. F., Wilson K. 2005. Structure–reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 287, 183–190. ( 10.1016/j.apcata.2005.03.027) [DOI] [Google Scholar]
- Cantrell K. B., Ducey T., Ro K. S., Hunt P. G. 2008. Livestock waste to bioenergy generation opportunities. Bioresour. Technol. 99, 7941–7953. ( 10.1016/j.biortech.2008.02.061) [DOI] [PubMed] [Google Scholar]
- Carvalho A. P., Meireles L. A., Malcata F. X. 2006. Microalgal reactors: a review of enclosed system designs and performances. Biotechnol. Progr. 22, 1490–1506. ( 10.1021/bp060065r) [DOI] [PubMed] [Google Scholar]
- Cavani F., Trifirò F., Vaccari A. 1991. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 11, 173–301. ( 10.1016/0920-5861(91)80068-K) [DOI] [Google Scholar]
- Chi Z. Y., Pyle D., Wen Z. Y., Frear C., Chen S. L. 2007. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 42, 1537–1545. ( 10.1016/j.procbio.2007.08.008) [DOI] [Google Scholar]
- Chi Z. Y., Liu Y., Frear C., Chen S. L. 2009. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 81, 1141–1148. ( 10.1007/s00253-008-1740-7) [DOI] [PubMed] [Google Scholar]
- Chisti Y. 2007. Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306. ( 10.1016/j.biotechadv.2007.02.001) [DOI] [PubMed] [Google Scholar]
- Chisti Y. 2008. Response to Reijnders: do biofuels from microalgae beat biofuels from terrestrial plants? Trends Biotechnol. 26, 351–352. ( 10.1016/j.tibtech.2008.04.002) [DOI] [PubMed] [Google Scholar]
- Chisti Y., Moo-Young M. 1986. Disruption of cells for intracellular products. Enzyme Microbiol. Technol. 8, 194–204. ( 10.1016/0141-0229(86)90087-6) [DOI] [Google Scholar]
- Cho S. H., Thompson G. A., Jr 1986. Properties of a fatty acid hydrolase preferentially attacking monogalactosyldiacylglycerols in Dunalliella salina chloroplasts. Biochim. Biophys. Acta 878, 353–359. ( 10.1016/0005-2760(86)90243-2) [DOI] [Google Scholar]
- Choi S. K., Lee J. Y., Kwon D. Y., Cho K. J. 2006. Settling characteristics of problem algae in the water treatment process. Water Sci. Technol. 53, 113–119. [DOI] [PubMed] [Google Scholar]
- Christie W. W. 2003. Lipid analysis. Bridgewater, NJ: The Oily Press, PJ Barnes and Associates. [Google Scholar]
- Clarkson N., Jones K. O., Young A. J. 2001. Modelling of a continuous algal production system using intelligent methods. In Algae and their biotechnological potential: 4th Asia-Pacific Conf. on Algal Biotechnology (eds Chen F., Jiang Y.), pp. 93–106. London, UK: Springer. [Google Scholar]
- Corma A., Iborra S., Miquel S., Primo J. 1998. Catalysts for the production of fine chemicals—production of food emulsifiers, monoglycerides, by glycerolysis of fats with solid base catalysts. J. Catal. 173, 315–321. ( 10.1006/jcat.1997.1930) [DOI] [Google Scholar]
- Cornet J. F., Dussap C. G., Dubertret G. 1992. A structured model for simulation of cultures of the cyanobacterium Spirulina platensis in photobioreactors. 1. Coupling between light transfer and growth-kinetics. Biotechnol. Bioeng. 40, 817–825. ( 10.1002/bit.260400709) [DOI] [PubMed] [Google Scholar]
- Cornet J. F., Dussap C. G., Gros J. B., Binois C., Lasseur C. 1995. A simplified monodimensional approach for modeling coupling between radiant light transfer and growth-kinetics in photobioreactors. Chem. Eng. Sci. 50, 1489–1500. ( 10.1016/0009-2509(95)00022-W) [DOI] [Google Scholar]
- Crossley I. A., Valade M. T., Shawcross J. 2002. Using the lesson learned and advanced methods to design a 1500 Ml/day DAF water treatment plant. Water Sci. Technol. 43, 35–41. [PubMed] [Google Scholar]
- Csordas A., Wang K. 2004. An integrated photobioreactor and foam fractionation unit for the growth and harvest of Chaetoceros spp. in open systems. Aquacult. Eng. 30, 15–30. ( 10.1016/j.aquaeng.2003.07.001) [DOI] [Google Scholar]
- de-Bashan L. E., Moreno M., Hernadez J. P., Bashan Y. 2002. Removal of ammonia and phosphorus ion from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilised in alginate beads with microalgae growth promoting Azospirrilum brasilense. Water Res. 36, 2941–2948. ( 10.1016/S0043-1354(01)00522-X) [DOI] [PubMed] [Google Scholar]
- Del Campo J. A., Garcia-Gonzalez M., Guerrero M. G. 2007. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl. Microbiol. Biotechnol. 74, 1163–1174. ( 10.1007/s00253-007-0844-9) [DOI] [PubMed] [Google Scholar]
- Demirbas A. 2007. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process. Technol. 88, 591–597. ( 10.1016/j.fuproc.2007.01.010) [DOI] [Google Scholar]
- Demirbas A. 2008. New liquid biofuels from vegetable oils via catalytic pyrolysis. Energy Educ. Sci. Technol. 21, 1–59. [Google Scholar]
- Di Serio M., Tesser R., Pengmei L., Santacesaria E. 2008. Heterogeneous catalysts for biodiesel production. Energy Fuels 22, 207–217. ( 10.1021/ef700250g) [DOI] [Google Scholar]
- Doucha J., Livansky K. Y. 2008. Influence of processing parameters on disintegrations of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 81, 431–440. ( 10.1007/s00253-008-1660-6) [DOI] [PubMed] [Google Scholar]
- Doucha J., Straka F., Livansky K. Y. 2005. Utilisation of flue gas for the cultivation of microalgae (Chlorella sp.) in an indoor open thin layer photobioreactor. J. Appl. Phycol. 17, 403–412. ( 10.1007/s10811-005-8701-7) [DOI] [Google Scholar]
- Droop M. R. 1968. Vitamin B12 and marine ecology. IV. The kinetics of uptake, growth, and inhibition in Monochrysis lutheri. J. Mar. Biol. Assoc. UK 48, 689–733. [Google Scholar]
- Dunahay T. G., Jarvis E. E., Dais S. S., Roessler P. G. 1996. Manipulation of microalgal lipid production using genetic engineering. Appl. Biochem. Biotechnol. 57, 8223–8231. [Google Scholar]
- Dunstan G. A., Volkman J. K., Barrett S. M., Leroi J.-M., Jeffrey S. W. 1994. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophycae). Phytochemistry 35, 155–161. ( 10.1016/S0031-9422(00)90525-9) [DOI] [Google Scholar]
- Ekman A., Bülow L., Stymne S. 2007. Elevated atmospheric CO2 concentration and diurnal cycle induce changes in lipid composition in Arabidopsis thaliana. New Phytol. 174, 591–599. ( 10.1111/j.1469-8137.2007.02027.x) [DOI] [PubMed] [Google Scholar]
- Eriksen N. T. 2008. The technology of microalgal culturing. Biotechnol. Lett. 30, 1525–1536. ( 10.1007/s10529-008-9740-3) [DOI] [PubMed] [Google Scholar]
- Fasham M. J. R., Flynn K. J., Pondaven P., Anderson T. R., Boyd P. W. 2006. Development of a robust ecosystem model to predict the role of iron on biogeochemical cycles: a comparison of results for iron-replete and iron-limited areas, and the SOIREE iron-enrichment experiment. Deep Sea Res. Pt I 53, 333–366. ( 10.1016/j.dsr.2005.09.011) [DOI] [Google Scholar]
- Fergola P., Cerasuolo M., Pollio A., Pinto G., Della Greca M. 2007. Allelopathy and competition between Chorella vulgaris and Pseudokirchneriella subcapitata: experiments and mathematical model. Ecol. Model. 208, 205–214. ( 10.1016/j.ecolmodel.2007.05.024) [DOI] [Google Scholar]
- Fernandez F. G. A., Camacho F. G., Perez J. A. S., Sevilla J. M. F., Grima E. M. 1998. Modeling of biomass productivity in tubular photobioreactors for microalgal cultures: effects of dilution rate, tube diameter, and solar irradiance. Biotechnol. Bioeng. 58, 605–616. () [DOI] [PubMed] [Google Scholar]
- Fernandez F. G. A., Perez J. A. S., Sevilla J. M. F., Camacho F. G., Grima E. M. 2000. Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol. Bioeng. 68, 173–183. () [DOI] [PubMed] [Google Scholar]
- Fernandez F. G. A., Sevilla J. M. F., Perez J. A. S., Grima E. M., Chisti Y. 2001. Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: assessment of design and performance. Chem. Eng. Sci. 56, 2721–2732. ( 10.1016/S0009-2509(00)00521-2) [DOI] [Google Scholar]
- Flynn K. J. 2001. A mechanistic model for describing dynamic multi-nutrient, light, temperature interactions in phytoplankton. J. Plankton Res. 23, 977–997. ( 10.1093/plankt/23.9.977) [DOI] [Google Scholar]
- Flynn K. J. 2003. Modelling multi-nutrient interactions in phytoplankton; balancing simplicity and realism. Prog. Oceanogr. 56, 249–279. ( 10.1016/S0079-6611(03)00006-5) [DOI] [Google Scholar]
- Flynn K. J. 2008. Use, abuse, misconceptions and insights from quota models: the Droop cell-quota model 40 years on. Oceanogr. Mar. Biol. 46, 1–23. [Google Scholar]
- Flynn K. J., Garrido J. L., Zapata M., Öpik H., Hipkin C. R. 1992. Changes in fatty acids, amino acids and carbon/nitrogen biomass during nitrogen starvation of ammonium and nitrate grown Isochrysis galbana. J. Appl. Phycol. 4, 95–104. ( 10.1007/BF02442457) [DOI] [Google Scholar]
- Flynn K. J., Greenwell H. C., Lovitt R. W., Shields R. J. Submitted Selection for fitness at the individual or population levels: modeling effects of genetic modifications in microalgae on productivity and environmental safety. [DOI] [PubMed]
- Frisch H. L., Gotham I. J. 1979. Simple-model for periodic cyclostat growth of algae. J. Math. Biol. 7, 149–169. [Google Scholar]
- Garcia C. M., Teixeira S., Marciniuk L. L., Schuchardt U. 2008. Transesterification of soybean oil catalyzed by sulfated zirconia. Bioresour. Technol. 99, 6608–6613. ( 10.1016/j.biortech.2007.09.092) [DOI] [PubMed] [Google Scholar]
- Gordon J. M., Polle J. E. 2007. Ultrahigh bioproductivity from algae. Appl. Microbiol. Biotechnol. 76, 969–975. ( 10.1007/s00253-007-1102-x) [DOI] [PubMed] [Google Scholar]
- Gordon R., Losic D., Tiffany M. A., Nagy S. A., Sterrenburg F. A. S. 2009. The glass menagerie: diatoms for novel applications. Trends Biotechnol. 27, 116–127. ( 10.1016/j.tibtech.2008.11.003) [DOI] [PubMed] [Google Scholar]
- Gotham I. J., Frisch H. L. 1981. A simple-model for cell-volume and developmental compartments in nutrient limited cyclostat cultures of algae. J. Theor. Biol. 92, 435–467. ( 10.1016/0022-5193(81)90258-7) [DOI] [Google Scholar]
- Gressel J. 2008. Transgenics are imperative for biofuel crops. Plant Sci. 174, 246–263. ( 10.1016/j.plantsci.2007.11.009) [DOI] [Google Scholar]
- Grierson S., Stezov V., Ellem G., Mcgregor R., Herbertson J. 2008. Thermal characterization of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrol. 85, 118–123. ( 10.1016/j.jaap.2008.10.003) [DOI] [Google Scholar]
- Grover J. P. 1991. Non-steady state dynamics of algal population growth—experiments with two chlorophytes. J. Phycol. 27, 70–79. ( 10.1111/j.0022-3646.1991.00070.x) [DOI] [Google Scholar]
- Guckert J. B., Cooksey K. E., Jackson L. L. 1988. Lipid solvent systems are not equivalent for analysis of lipid classes in the microeukaryotic green algae, chlorella. J. Microbiol. Methods 8, 139–149. ( 10.1016/0167-7012(88)90015-2) [DOI] [Google Scholar]
- Hagiwra H., Mitsch W. J. 1994. Ecosystem modeling of a multispecies integrated aquaculture pond in South China. Ecol. Model. 72, 41–73. ( 10.1016/0304-3800(94)90145-7) [DOI] [Google Scholar]
- Hamm C. E., Merkel R., Springer O., Jurkojc P., Maier C., Prechtel K., Smetacek V. 2003. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843. ( 10.1038/nature01416) [DOI] [PubMed] [Google Scholar]
- Hankamer B., Lehr F., Rupprecht J., Mussgnug J. H., Posten C., Kruse O. 2007. Photosynthetic biomass and H2 production by green algae: from bioengineering to bioreactor scale-up. Physiol. Plantarum 131, 10–21. ( 10.1111/j.1399-3054.2007.00924.x) [DOI] [PubMed] [Google Scholar]
- Harel M., Place A. R. 2003. Heterotrophic production of marine algae for aquaculture. In Handbook of microalgal culture: biotechnology and applied phycology (ed. Richmond A.), London, UK: Wiley-Blackwell. [Google Scholar]
- Henderson R., Parson S. A., Jefferson B. 2008. The impact of algal properties and preoxidation on solid liquid separation of algae. Water Res. 42, 1827–1845. ( 10.1016/j.watres.2007.11.039) [DOI] [PubMed] [Google Scholar]
- Hoffmann J. P. 1998. Wastewater treatment with suspended and nonsuspended algae. J. Phycol. 34, 757–763. ( 10.1046/j.1529-8817.1998.340757.x) [DOI] [Google Scholar]
- Horiuchi J. -I., Ohba I., Tada K., Kobayashi M., Kanno T., Kishimoto M. 2003. Effective cell harvesting of the halotolerant microalgae Dunaliella tertiolecta with pH control. J. Biosci. Bioeng. 95, 412–415. ( 10.1016/S1389-1723(03)80078-6) [DOI] [PubMed] [Google Scholar]
- Hu D. W., Liu H., Yang C. L., Hu E. Z. 2008a. The design and optimization for light-algae bioreactor controller based on Artificial Neural Network-Model Predictive Control. Acta Astronaut. 63, 1067–1075. ( 10.1016/j.actaastro.2008.02.008) [DOI] [Google Scholar]
- Hu Q., Sommerfeld M., Jarvis E., Ghirardi M., Posewitz M., Seibert M., Darzins A. 2008b. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 54, 621–639. ( 10.1111/j.1365-313X.2008.03492.x) [DOI] [PubMed] [Google Scholar]
- Huber G. W., Iborra S., Corma A. 2006. Synthesis of transportation fuels from biomass: chemistry, catalysts and engineering. Chem. Rev. 106, 4044–4098. ( 10.1021/cr068360d) [DOI] [PubMed] [Google Scholar]
- Huntley M. E., Redalje D. G. 2007. CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitigat. Adapt. Strategies Global Change 12, 573–608. ( 10.1007/s11027-006-7304-1) [DOI] [Google Scholar]
- Imteaz M. A., Asaeda T., Lockington D. A. 2003. Modelling the effects of inflow parameters on lake water quality. Environ. Model. Assess. 8, 63–70. [Google Scholar]
- Incropera F. P., Thomas J. F. 1978. Model for solar-radiation conversion to algae in a shallow pond. Solar Energy 20, 157–165. ( 10.1016/0038-092X(78)90189-5) [DOI] [Google Scholar]
- Jameson G. J. 1999. Hydrophobicity and floc density on in induced-air flotations for water treatment. Colloids Surf. A 151, 269–281. ( 10.1016/S0927-7757(98)00503-2) [DOI] [Google Scholar]
- Jones K. O., Clarkson N., Young A. J. 2002. Intelligent process modelling of a continuous algal production system. In Computer applications in biotechnology. 2001 8th IFAC Int. Conf. on Computer Applications in Biotechnology (CAB8) (eds Dochain D., Perrier M.), pp. 239–243. New York, NY: Pergamon. [Google Scholar]
- Kalnes T., Marker T., Shonnard D. R. 2007. Green diesel: a second generation biofuel. Int. J. Chem. React. Eng. 5, A48. [Google Scholar]
- Kloprogge J. T., Duong L. V., Frost R. L. A. 2005. Review of the synthesis and characterisation of pillared clays and related porous materials for cracking of vegetable oils to produce biofuels. Environ. Geol. 47, 967–981. ( 10.1007/s00254-005-1226-1) [DOI] [Google Scholar]
- Knothe G. H. 2005. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 86, 1059–1070. ( 10.1016/j.fuproc.2004.11.002) [DOI] [Google Scholar]
- Knuckey R. M., Brown M. R., Dion R., Frampton D. M. F. 2006. Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquacult. Eng. 35, 300–313. ( 10.1016/j.aquaeng.2006.04.001) [DOI] [Google Scholar]
- Komatsu E., Fukushima T., Shiraishi H. 2006. Modeling of P-dynamics and algal growth in a stratified reservoir—mechanisms of P-cycle in water and interaction between overlying water and sediment. Ecol. Model. 197, 331–349. ( 10.1016/j.ecolmodel.2006.03.023) [DOI] [Google Scholar]
- Kubickova I., Snare M., Eranen K., Maki-Arvela P., Murzin D. Y. 2005. Hydrocarbons for diesel fuel via decarboxylation of vegetable oils. Catal. Today 106, 197–200. ( 10.1016/j.cattod.2005.07.188) [DOI] [Google Scholar]
- Kurat C. F., et al. 2006. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 281, 491–500. ( 10.1074/jbc.M508414200) [DOI] [PubMed] [Google Scholar]
- Lazarova V., Phillippe R., Sturny V., Arcangell J. P. 2006. Evaluation of economic viability and benefits of urban water reuse and its contribution to sustainable development. Water Prac. Technol. 1, 1–11. [Google Scholar]
- Lebeau T., Robert J.-M. 2003. Diatom cultivation and biotechnology relevant products. Part 1 Cultivation at various scales. Appl. Microbiol. Biotechnol. 60, 612–623. ( 10.1007/s00253-002-1176-4) [DOI] [PubMed] [Google Scholar]
- Leon-Banares R., Gonzalez-Ballester D., Galvan A., Fernandez E. 2004. Transgenic microalgae as green cell-factories. Trends Biotechnol. 22, 45–52. ( 10.1016/j.tibtech.2003.11.003) [DOI] [PubMed] [Google Scholar]
- Li J., Xu S., Su W. W. 2003. Online estimation of stirred-tank microalgal photobioreactor cultures based on dissolved oxygen measurement. Biochem. Eng. J. 14, 51–65. ( 10.1016/S1369-703X(02)00135-3) [DOI] [Google Scholar]
- Li X., Lu G. Z., Guo Y., Wang Y. Q., Zhang Z. G., Liu X. H., Wang Y. S. 2007. A novel solid superbase of Eu2O3/Al2O3 and its catalytic performance for the transesterification of soybean oil to biodiesel. Catal. Commun. 8, 1969–1972. ( 10.1016/j.catcom.2007.03.013) [DOI] [Google Scholar]
- Li Y. Q., Horsman M., Wang B., Wu N., Lan C. Q. 2008. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 81, 629–636. ( 10.1007/s00253-008-1681-1) [DOI] [PubMed] [Google Scholar]
- Lin Y. H., Leu J. Y., Lan C. R., Lin P. H. P., Chang F. L. 2003. Kinetics of inorganic carbon utilization by microalgal biofilm in a flat plate photoreactor. Chemosphere 53, 779–787. ( 10.1016/S0045-6535(03)00509-5) [DOI] [PubMed] [Google Scholar]
- Llorens M., Saez J., Soler A. 1991. Primary productivity in sewage pond: semiempirical model. J. Environ. Eng. ASCE 117, 771–781. ( 10.1061/(ASCE)0733-9372(1991)117:6(771)) [DOI] [Google Scholar]
- Lorenz R. T., Cysewski G. R. 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. TIBTECH 18, 160–167. [DOI] [PubMed] [Google Scholar]
- Lou W.-Y., Zong M.-H., Duan Q. 2008. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 99, 8752–8758. ( 10.1016/j.biortech.2008.04.038) [DOI] [PubMed] [Google Scholar]
- Macala G. S., Robertson A. W., Johnson C. L., Day Z. B., Lewis R. S., White M. G., Iretskii A. V., Ford P. C. 2008. Transesterification catalysts from iron doped hydrotalcite-like precursors: solid bases for biodiesel production. Catal. Lett. 122, 205–209. ( 10.1007/s10562-008-9480-y) [DOI] [Google Scholar]
- Maher K. D., Bressler D. C. 2007. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour. Technol. 98, 2351–2368. ( 10.1016/j.biortech.2006.10.025) [DOI] [PubMed] [Google Scholar]
- Masojidek J., Sergejevova M., Rottnerova K., Jirka V., Korecko J., Kopecky J., Zat'kova I., Torzillo G., Stys D. 2009. A two-stage solar photobioreactor for cultivation of microalgae based on solar concentrators. J. Appl. Phycol. 21, 55–63. ( 10.1007/s10811-008-9324-6) [DOI] [Google Scholar]
- Melis A., Seibert M., Ghirardi M. L. 2007. Hydrogen fuel production by transgenic microalgae. Adv. Exp. Med. Biol. 616, 10–21. [DOI] [PubMed] [Google Scholar]
- Merchuk J. C., Wu X. 2003. Modeling of photobioreactors: application to bubble column simulation. J. Appl. Phycol. 15, 163–170. ( 10.1023/A:1023879619535) [DOI] [Google Scholar]
- Miao X. L., Wu Q. Y. 2006. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 97, 841–846. ( 10.1016/j.biortech.2005.04.008) [DOI] [PubMed] [Google Scholar]
- Mitra A., Flynn K. J. 2006. Promotion of harmful algal blooms by zooplankton predatory activity. Biol. Lett. 2, 194–197. ( 10.1098/rsbl.2006.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina Grima E., Perez J. A. S., Camacho F. G., Sevilla J. M. F., Fernandez F. G. A. 1996. Productivity analysis of outdoor chemostat culture in tubular air-lift photobioreactors. J. Appl. Phycol. 8, 369–380. ( 10.1007/BF02178580) [DOI] [Google Scholar]
- Molina Grima E., Fernandez F. G. A., Camacho F. G., Chisti Y. 1999. Photobioreactors: light regime, mass transfer, and scaleup. J. Biotechnol. 70, 231–247. ( 10.1016/S0168-1656(99)00078-4) [DOI] [Google Scholar]
- Molina Grima E., Belarbi E.-H., Fernandes F. G. A., Robles M., Christi Y. 2003. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 20, 491–515. ( 10.1016/S0734-9750(02)00050-2) [DOI] [PubMed] [Google Scholar]
- Morton R. 1978. Model for light-scattering by algae in water. Math. Biosci. 40, 195–204. ( 10.1016/0025-5564(78)90084-6) [DOI] [Google Scholar]
- Mueller J. A. 2007. Western Europe. In Wastewater sludge: a global overview of the current status and future prospects (ed. Spinosa L.), pp. 5–8. London, UK: IWA Publishing. [Google Scholar]
- Mulbry W., Kondrad S., Buyer J. 2008a. Treatment of dairy and swine manure effluents using freshwater algae: fatty acid content and composition of algal biomass at different manure loading rates. J. Appl. Phycol. 20, 1079–1085. ( 10.1007/s10811-008-9314-8) [DOI] [Google Scholar]
- Mulbry W., Kondrad S., Pizzarro C., Kebede-Westhead E. 2008b. Treatment of dairy effluent using freshwater algae: algal productivity and recovery of manure nutrients using algal turf scrubbers. Bioresour. Technol. 99, 8137–8142. ( 10.1016/j.biortech.2008.03.073) [DOI] [PubMed] [Google Scholar]
- Muller-Feuga A., Moal J., Kaas R. 2003. The aquaculture of microalgae. In Live feeds in marine aquaculture (eds McEvoy L. A., Stottrup J. G.), pp. 206–252. London, UK: Wiley-Blackwell. [Google Scholar]
- Munoz R., Guieysse B. 2006. Algal–bacterial processes for the treatment of hazardous contaminants. Water Res. 40, 2799–2815. ( 10.1016/j.watres.2006.06.011) [DOI] [PubMed] [Google Scholar]
- Mussgnug J. H., Thomas-Hall S., Rupprecht J., Foo A., Klassen V., McDowall A., Schenk P. M., Kruse O., Hankamer B. 2007. Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol. J. 5, 802–814. ( 10.1111/j.1467-7652.2007.00285.x) [DOI] [PubMed] [Google Scholar]
- Navarro-Angulo L., Robledo D. 1999. Effects of nitrogen source, N: P ratio and N-pulse concentration and frequency on the growth of Gracilaria cornea (Gracilariales, Rhodophyta) in culture. Hydrobiologia 399, 315–320. [Google Scholar]
- Oh H. M., Lee S. J., Park M. H., Kim H.-S., Kim H. C., Yoon J. H., Kwon G., Yoon B. D. 2001. Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49. Biotechnol. Lett. 23, 1229–1234. ( 10.1023/A:1010577319771) [DOI] [Google Scholar]
- Ohlrogge J., Browse J. 1995. Lipid biosynthesis. Plant Cell 7, 957–970. ( 10.1105/tpc.7.7.957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald W. J. 1988. Microalgae and waste water treatment. In Microalgae biotechnology (ed. Borowitzka M. B. L.), pp. 254–260. Cambridge,UK: Cambridge University Press. [Google Scholar]
- Paligová J., Joríkova L., Cvengros J. 2008. Study of FAME stability. Energy Fuels 22, 1991–1996. ( 10.1021/ef7007419) [DOI] [Google Scholar]
- Pienkos P. T., Darzins A. 2009. The promise and challenges of microalgal-derived biofuels. Biofuel. Bioprod. Bioref. 3, 431–440. ( 10.1002/bbb.159) [DOI] [Google Scholar]
- Poelman E., De Pauw N., Jeurissen B. 1997. Potential of electrolytic flocculation for recovery of micro-algae. Resour. Conserv. Recy. 19, 1–10. ( 10.1016/S0921-3449(96)01156-1) [DOI] [Google Scholar]
- Post-Beittenmiller D., Roughan G., Ohlrogge J. B. 1992. Regulation of plant fatty acid biosynthesis; analysis of acyl-coenzyme A and acyl–acyl carrier protein substrate pools in spinach and pea chloroplasts. Plant Physiol. 100, 923–930. ( 10.1104/pp.100.2.923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig H. R., Andersen R. A. 2005. Historical review of algal culturing techniques. In Algal culturing techniques (ed. Andersen R. A.), pp. 1–12. London, UK: Academic Press. [Google Scholar]
- Riekhof W. R., Sears B. B., Benning C. 2005. Annotation of genes involved in glycerolipid biosynthesis in Chlamydomonas reinhardtii: discovery of the betaine lipid synthase BTA1Cr. Eukaryotic Cell 4, 242–252. ( 10.1128/EC.4.2.242-252.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolfi L., Zittelli G. C., Bassi N., Padovani G., Biondi N., Bonini G., Tredici M. R. 2009. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112. ( 10.1002/bit.22033) [DOI] [PubMed] [Google Scholar]
- Roessler P. G. 1988. Changes in the activities of various lipid and carbohydrate biosynthetic enzymes in the diatom Cyclotella cryptica in response to silicon deficiency. Arch. Biochem. Biophys. 267, 521–528. ( 10.1016/0003-9861(88)90059-8) [DOI] [PubMed] [Google Scholar]
- Roessler P. G. 1990. Environmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J. Phycol. 26, 393–399. ( 10.1111/j.0022-3646.1990.00393.x) [DOI] [Google Scholar]
- Roessler P. G., Brown L. M., Dunahay T. G., Heacox D. A., Jarvis E. E., Schneider J. C., Talbot S. G., Zeiler K. G. 1994. Genetic-engineering approaches for enhanced production of biodiesel fuel from microalgae. Enzym. Convers. Biomass Fuels Prod. ACS Symp. Ser. 566, 255–270. ( 10.1021/bk-1994-0566.ch013) [DOI] [Google Scholar]
- Rossi N., Jaouen O., Legentilhomme P., Petit I. 2004. Harvesting of cyanobacterium Arthospira platensis using organic filtration membranes. Food Bioprod. Process. 82, 244–250. ( 10.1205/fbio.82.3.244.44177) [DOI] [Google Scholar]
- Rossignol N., Vandanjon L., Jaouen O., Quemeneur F. 1999. Membrane technology for the continuous separation microalgae/culture medium: compared performances of cross flow microfiltration and ultrafiltration. Aquacult. Eng. 20, 191–208. ( 10.1016/S0144-8609(99)00018-7) [DOI] [Google Scholar]
- Sarmiento J. L., Gruber N. 2006. Ocean biogeochemical dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- Scharlemann J. P. W., Laurance W. F. 2008. How green are biofuels? Science 319, 43–44. ( 10.1126/science.1153103) [DOI] [PubMed] [Google Scholar]
- Sheehan J., Dunahay T., Benemann J., Roessler P. 1998. A look back at the US Department of Energy's aquatic species program—biodiesel from algae. Report NREL/TP-580–24190, National Renewable Energy Laboratory, Golden, CO. [Google Scholar]
- Shifrin N. S., Chisholm S. W. 1981. Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 17, 374–384. ( 10.1111/j.0022-3646.1981.00374.x) [DOI] [Google Scholar]
- Sialve B., Bernet N., Bernard O. 2009. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 27, 409–416. ( 10.1016/j.biotechadv.2009.03.001) [DOI] [PubMed] [Google Scholar]
- Smith B., Greenwell H. C., Whiting A. 2009. Catalytic upgrading of tri-glycerides and fatty acids to transport biofuels. Energy Environ. Sci. 2, 262–271. ( 10.1039/b814123a) [DOI] [Google Scholar]
- Snare M., Murzin D. Y. 2006. Reply to comment on heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 45, 6875 ( 10.1021/ie061051t) [DOI] [Google Scholar]
- Snare M., Kubickova I., Maki-Arvela P., Eranen K., Murzin D. Y. 2006. Heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 45, 5708–5715. ( 10.1021/ie060334i) [DOI] [Google Scholar]
- Snare M., Kubickova I., Maki-Arvela P., Eranen K., Warna J., Murzin D. Y. 2007. Production of diesel fuel from renewable feeds: kinetics of ethyl stearate decarboxylation. Chem. Eng. J. 134, 29–34. ( 10.1016/j.cej.2007.03.064) [DOI] [Google Scholar]
- Snare M., Kubickova I., Maki-Arvela P., Chichova D., Eranen K., Murzin D. Y. 2008. Catalytic deoxygenation of unsaturated renewable feedstocks for production of diesel fuel hydrocarbons. Fuel 87, 933–945. ( 10.1016/j.fuel.2007.06.006) [DOI] [Google Scholar]
- Spolaore P., Joannis-Cassan C., Duran E., Isambert A. 2006. Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87–96. ( 10.1263/jbb.101.87) [DOI] [PubMed] [Google Scholar]
- Strand S. P., Nordengen T., Østgaard K. 2002. Efficiency of chitosans applied for flocculation of different bacteria. Water Res. 36, 4745–4752. ( 10.1016/S0043-1354(02)00173-2) [DOI] [PubMed] [Google Scholar]
- Strand S. P., Vårum K. M., Østgaard K. 2003. Interactions between chitosans and bacterial suspensions: adsorption and flocculation. Colloids Surf. B Biointerf. 27, 71–81. ( 10.1016/S0927-7765(02)00043-7) [DOI] [Google Scholar]
- Suh I. S., Lee S. B. 2003a. Optimization of radiator position in an internally radiating photobioreactor: a model simulation study. J. Microbiol. Biotechol. 13, 789–793. [Google Scholar]
- Suh I. S., Lee S. B. 2003b. A light distribution model for an internally radiating photobioreactor. Biotechnol. Bioeng. 82, 180–189. ( 10.1002/bit.10558) [DOI] [PubMed] [Google Scholar]
- Sukenik A., Carmeli Y. 1990. Lipid synthesis and fatty acid composition in Nannochloropsis sp. (Eustigmatophyceae) grown in a light-dark cycle. J. Phycol. 26, 463–469. ( 10.1111/j.0022-3646.1990.00463.x) [DOI] [Google Scholar]
- Sukenik A., Carmeli Y., Berner T. 1989. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 25, 686–692. ( 10.1111/j.0022-3646.1989.00686.x) [DOI] [Google Scholar]
- Svarovsky L. D. 1990. Solid liquid separation, 3rd edn. London, UK: Butterworth & Co. [Google Scholar]
- Twaiq F. A., Zabidi N. A. M., Bhatia S. 1999. Catalytic conversion of palm oil to hydrocarbons: performance of various zeolite catalysts. Ind. Eng. Chem. Res. 38, 3230–3237. ( 10.1021/ie980758f) [DOI] [Google Scholar]
- Twaiq F. A., Mohamed A. R., Bhatia S. 2003. Liquid hydrocarbon fuels from palm oil by catalytic cracking over aluminosilicate mesoporous catalysts with various Si/Al ratios. Micropor. Mesopor. Mater. 64, 95–107. ( 10.1016/j.micromeso.2003.06.001) [DOI] [Google Scholar]
- Vandanjon L., Jaouen P., Rossignol N., Quemeneur F., Robert M. 1999. Concentration and desalting by membrane processes of a natural pigment produced by a marine diatom Haslea ostrearia Simonsen. J. Biotechnol. 70, 393–402. ( 10.1016/S0168-1656(99)00092-9) [DOI] [Google Scholar]
- Vega-Galvez A., Ayala-Aponte A., Notte E., de la Fuente L., Lemus-Mondaca R. 2008. Mathematical modeling of mass transfer during convective dehydration of brown algae Macrocystis pyrifera. Dry. Technol. 26, 1610–1616. ( 10.1080/07373930802467532) [DOI] [Google Scholar]
- Vicente G., Martinez M., Aracil J. 2004. Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour. Technol. 92, 297–305. ( 10.1016/j.biortech.2003.08.014) [DOI] [PubMed] [Google Scholar]
- Wallace B. B., Hamilton D. P. 1999. The effect of variations in irradiance on buoyancy regulation in Microcystis aeruginosa. Limnol. Oceanogr. 44, 273–281. [Google Scholar]
- Wang A., Lewus R., Rathore A. S. 2006. Comparison of different options for harvest of a therapeutic protein product from high cell density yeast fermentation broth. Biotechnol. Bioeng. 94, 91–104. ( 10.1002/bit.20816) [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Fu C. C., Liu Y. C. 2007. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 37, 21–25. ( 10.1016/j.bej.2007.03.004) [DOI] [Google Scholar]
- Xu H., Miao X. L., Wu Q. Y. 2006. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 126, 499–507. ( 10.1016/j.jbiotec.2006.05.002) [DOI] [PubMed] [Google Scholar]
- Yun Y.-S., Lee S.-B., Park J. M., Lee C.-I., Yang J. W. 1997. Carbon dioxide fixation by algal cultivation using wastewater. J. Chem. Technol. Biotechnol. 69, 451–455. () [DOI] [Google Scholar]
- Zah R. 2007. Energy and raw materials—the contributions of chemistry and biochemistry in the future: biofuels—which one is the most ecological one? Chimia 61, 571–572. [Google Scholar]
- Zemke P. E., Wood B. D., Dye D. J., Bayless D. J., Muhs J. D. 2007. Economic analysis of a vertical sheet algal photobioreactor for biodiesel production. In Proc. ES2007 Energy Sustainability, Long Beach, CA, 27–30 July 2007, pp. 815–820. [Google Scholar]
- Zhao Z. B. 2006. Comment on heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 45, 6874 ( 10.1021/ie060988r) [DOI] [Google Scholar]
- Zhu Y. H., Jiang J. G. 2008. Continuous cultivation of Dunaliella salina in photobioreactor for the production of beta-carotene. Eur. Food Res. Technol. 227, 953–959. ( 10.1007/s00217-007-0789-3) [DOI] [Google Scholar]
- Zijffers J. W. F., Janssen M., Tramper J., Wijffels R. H. 2008. Design process of an area-efficient photobioreactor. Mar. Biotechnol. 10, 404–415. ( 10.1007/s10126-007-9077-2) [DOI] [PMC free article] [PubMed] [Google Scholar]