Abstract
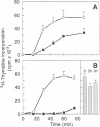
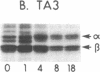
Macrophages and B cells process antigens to produce antigenic peptides that associate with class II major histocompatibility complex molecules (e.g., Ia molecules); these Ia-peptide complexes are recognized by CD4+ T lymphocytes. Processing of the antigen hen egg white lysozyme was inhibited by cycloheximide in peritoneal exudate cells (PECs, largely macrophages), but not in TA3 B-lymphoma cells. The uptake and metabolism of hen egg white lysozyme was largely intact in cycloheximide-treated PECs, implicating a blockade in other steps in the formation of Ia-peptide complexes. Turnover of Ia-peptide complexes was markedly enhanced in viable antigen-presenting cells (TA3 and PEC) as compared to such complexes studied on fixed cells or in isolated preparations of Ia and peptide. In B cells the half-life of Ia-peptide complexes was much shorter than the half-life of the Ia molecules, implying turnover of Ia-peptide complexes by dissociatin and peptide exchange. In PECs, the dissociation of Ia-peptide complexes was more limited; the enhanced Ia-peptide turnover in viable PECs reflected in part biosynthetic turnover of Ia molecules. Specific mechanisms may exist in TA3 cells to facilitate exchange of peptides bound to Ia, allowing recycling of Ia to present another antigenic peptide; such Ia recycling would explain the ability of these cells to process and present antigen in the absence of Ia synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Matsueda G., Haber E., Unanue E. R., Allen P. M. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4509–4513. doi: 10.1073/pnas.83.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Sachs D. H., Pierres M., Samelson L. E., Sharrow S. O., Paul W. E. IA mutant functional antigen-presenting cell lines. J Immunol. 1983 May;130(5):2287–2294. [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989 Jan 1;142(1):12–19. [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Jensen P. E. Protein synthesis in antigen processing. J Immunol. 1988 Oct 15;141(8):2545–2550. [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- Luescher I. F., Allen P. M., Unanue E. R. Binding of photoreactive lysozyme peptides to murine histocompatibility class II molecules. Proc Natl Acad Sci U S A. 1988 Feb;85(3):871–874. doi: 10.1073/pnas.85.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]