Abstract
Objective
To determine the incidence of cancer in a population-based cohort of patients with giant cell arteritis (GCA) compared to age- and gender-matched referent subjects.
Methods
Using the resources of the Rochester Epidemiology Project (REP), all incident cases of GCA diagnosed between January 1, 1950 and December 31, 2004 were identified. For each GCA patient, 2 subjects without GCA of the same sex, similar age and length of medical history were selected. Diagnosis of malignancy was made by histopathology. Patients were followed until death, last contact, or December 31, 2006.
Results
Our study included 204 GCA patients and 407 non-GCA subjects. The GCA cohort had 163 (79%) women and 41 (21%) men, mean age 76.0 years (± 8.2 years), median follow-up 7.7 years. The non-GCA cohort consisted of 325 (80%) women and 82 (20%) men, mean age 75.6 years (± 8.4 years), median follow-up 8.1 years. During follow-up, 46 GCA patients and 76 non-GCA subjects developed cancer (HR: 1.26; 95% CI: 0.87, 1.83). Adjustment for smoking did not alter the results. The 1, 10 and 20 year cumulative incidences of any malignancy were 5.9%, 33.6% and 50.0% among GCA patients and 2.6%, 27.0% and 47.0% among non-GCA patients. There were no differences in hematologic or solid malignancies between both groups. Colon cancer appeared more commonly in the GCA group (p=0.07). Mortality following cancer was similar in both cohorts (HR: 0.80; 95% CI: 0.52, 1.24).
Conclusion
GCA patients are not at increased risk of first cancer after diagnosis.
Keywords: Giant Cell Arteritis, malignancy
Giant cell arteritis (GCA) is the most common vasculitis in people over the age of 50 years, with a peak incidence in the 70–80 year age group [1]. Given the age restriction of this disease, immunosenescence may play a role in disease pathogenesis [2]. Immunosenescence has also been proposed as a possible mechanism by which the elderly are predisposed infections and malignancy [3, 4].
At present, there is conflicting information about malignancy risk in GCA patients. Case reports of concurrent GCA and malignancy [5–9] suggest it may be a paraneoplastic phenomenon in some cases. In a retrospective case series of 271 patients with GCA, concurrent malignancy was noted in 7.4% of GCA patients [10]. In a case-control study by Haga, et al. no overall increase in malignancy was found in patients with GCA and polymyalgia rheumatica (PMR). However, the investigators did find an increased risk of malignancy in the subset of GCA patients with a positive temporal artery biopsy (OR: 2.35; 95% CI 1.03, 5.34) [11]. A subsequent population-based study of malignancy in GCA and PMR patients found no such increased risk of cancer in GCA patients. However, only 80 of the 398 patients in this study had GCA [12]. Finally, in a population-based study from Spain, the authors evaluated mortality in GCA patients with cancer and found no increased mortality [9].
The somewhat contradictory findings of these few studies make it difficult to draw any definitive conclusions regarding risk of malignancy in patients with GCA. Clarifying the risk of cancer in GCA has prognostic implications for GCA patients. Knowledge of an increased risk of cancer could alter cancer screening practices in these patients. To address this issue, we employed a population-based incident cohort of GCA patients, to calculate and compare the incidence of malignancy in GCA patients to that of age- and gender-matched cohort of non-GCA patients from the same geographic region.
PATIENTS AND METHODS
This is a population-based historical cohort study utilizing the resources of the Rochester Epidemiology Project (REP). The REP is a unique linkage system which allows ready access to the medical records of all health care providers for the population of Olmsted County, Minnesota, including the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Group and Olmsted Community Hospital, local nursing homes and the few private practitioners [13]. Detailed indices containing all clinical and pathologic diagnoses and surgical procedures have been recorded since 1909 and are used to retrieve records [14]. The diagnoses assigned at each visit are coded and indexed continuously. The ability to review the entire medical records that cover all inpatient and outpatient care from all local health care providers is a unique strength of the REP.
The study was approved by the institutional review boards at Mayo Clinic and Olmsted Medical Center. Patients who declined authorization for review of their medical records for research were excluded from the study.
GCA cohort
Using the REP, an incident cohort of all Olmsted County, Minnesota residents diagnosed with GCA between January 1, 1950 and December 31, 2004 has been established [15, 16]. Details regarding this cohort have been previously described [15, 16]. All patients met the 1990 American College of Rheumatology classification criteria for GCA [17].
Referent cohort
For each GCA patient, 2 subjects without GCA of the same sex and similar age (+/− 1 year in 96%) and length of medical history were randomly selected from the Olmsted County population. Each non-GCA subject was assigned an index date corresponding to the date of diagnosis of GCA of the matched GCA patient.
Case ascertainment and Follow-up
The complete inpatient and outpatient medical records of all patients in both cohorts were reviewed for the time period and outcomes of interest. Ascertainment of malignancy was carried out in two stages. First, we used diagnostic codes for malignancy from chapter 2 (neoplasms) of the International Classification of Diseases, 9th Revision (ICD-9) to screen for malignancy in both groups. For cases before 1975, a classification system developed at Mayo Clinic called the Berkson index was utilized [13]. After 1975, the International Classification of Diseases: Adapted Code for Hospitals (H-ICDA) codes for cancer or suggestive of cancer were used. Data from these coding systems was then converted to ICD-9 codes. A similar method of malignancy ascertainment with medical codes has been used in previous REP studies of cancer in patients with Parkinson’s Disease and was sensitive based on their test of validity [18, 19]. In the next stage, a rheumatologist (TK) reviewed the charts of all patients who screened positive in both cohorts. Malignancy was defined based on histologic diagnosis. Clinical pathology reports were reviewed to confirm the diagnosis of malignancy. Information about the type of malignancy, including non-melanoma skin cancers, stage (metastatic or localized) and treatment was abstracted. If a patient had multiple malignancies, this information was abstracted for each cancer. Pre-cancerous lesions were excluded. Smoking status was abstracted as a binary variable (ever/never). Patients in both cohorts were followed until death, last contact, or December 31, 2006 (end of study).
Statistical analysis
Descriptive statistics were used to summarize the data. The cumulative incidence of malignancy during follow-up was estimated using Kaplan-Meier methods. Cox proportional hazard models were used to examine the influence of GCA on the development of malignancy after adjusting for age, sex and calendar year of GCA diagnosis index date. Age was used as the time scale in these models for optimal age adjustment. Adjustment for smoking was performed as a secondary analysis due to missing values in smoking status. This was done 2 ways: first, allowing patients with missing values for smoking to be excluded from the analyses, and then treating patients with missing smoking status as non-smokers.
RESULTS
There were 207 patients diagnosed with GCA in Olmsted County between January 1, 1950 and December 31, 2004. Of these, 3 (1.4%) declined research authorization and were excluded from the study. There were 408 referent subjects. One (0.2%) of the non-GCA subjects declined research authorization following selection and was excluded from the study. The final study population included 204 GCA patients and 407 non-GCA subjects.
The GCA cohort consisted of 163 (79%) women and 41 (21%) men; mean age 76.0 (± 8.2) years. Median follow-up was 7.7 years (total 1856 person-years). Temporal artery biopsy (TAB) was positive in 179 of 207 patients (86.5%) of the GCA cohort. Forty-eight patients in this cohort had PMR prior to diagnosis of GCA and 19 patients in the cohort developed PMR after GCA diagnosis. The non-GCA cohort had 325 (80%) women and 82 (20%) men; mean age 75.6 (± 8.4) years. Median follow-up in the non-GCA group was 8.1 years (total 3890 person-years). Smoking data was not available in 16 (8%) GCA patients and 28 (7%) non-GCA subjects. In those with smoking data available, 84 (45%) GCA patients were ever-smokers compared to 154 (41%) non-GCA subjects (p=0.32).
Diagnosis of malignancy was confirmed by histopathology in all but 1 subject from the referent group. This patient had a large lung mass but refused further evaluation. This subject was included based on the strong clinical suspicion of malignancy in the physician clinical notes. During follow-up, 52 GCA patients and 107 non-GCA subjects developed malignancy (HR: 1.07; 95% CI: 0.77, 1.50; p=0.22). Excluding patients with a prevalent malignancy at index date, 46 GCA patients and 76 non-GCA subjects developed malignancy during follow-up (HR: 1.26; 95% CI: 0.87, 1.83; p=0.68). While smoking was a significant predictor of malignancy, additional adjustment for smoking did not alter the comparison in these analyses (data not shown). There was no difference in malignancy risk between the two groups when stratified by age (<75 years or ≥75 years), gender or type of malignancy (solid versus hematologic) (Table 1). Diagnosis of PMR was not associated with risk of malignancy in GCA patients (HR: 0.8; 95% CI 0.4, 1.5; p=0.43). Similarly, positive TAB was not associated with risk of malignancy after GCA diagnosis (HR: 1.6; 95% CI: 0.3, 6.3; p=0.64). The most common cancer was non-melanoma skin cancer (22 patients and 52 referent subjects). Overall, no significant differences were found between the two groups with respect to different types of malignancy (Table 1). While not reaching statistical significance, there was a suggestion of increased risk of colon cancer in GCA patients (HR: 2.71; 95% CI: 0.94, 7.83; p=0.07).
Table 1.
Association between GCA and malignancy following the diagnosis of GCA
| Variable | GCA | Non-GCA | HR* (95% CI) | P-value |
|---|---|---|---|---|
| Any malignancy after index date (including patients with prevalent malignancy) |
52 / 204 | 107 / 407 | 1.07 (0.77, 1.50) | 0.22 |
| Any malignancy after index date (excluding patients with prevalent malignancy) |
46 / 159 | 76 / 282 | 1.26 (0.87, 1.83) | 0.68 |
| Any malignancy after index date (excluding non-melanoma skin cancer and prevalent malignancy other than non- melanoma skin cancer) |
33 / 181 | 55 / 322 | 1.21 (0.78, 1.88) | 0.39 |
| Male** | 9 / 35 | 19 /67 | 1.34 (0.59, 3.02) | 0.47 |
| Female | 24 / 146 | 36 / 255 | 1.18 (0.70, 1.98) | 0.55 |
| Age<75 years | 17 / 79 | 27 / 146 | 1.33 (0.72, 2.45) | 0.37 |
| Age≥75 years | 16 / 102 | 28 / 176 | 1.08 (0.57, 2.04) | 0.81 |
| Solid*** | 43 / 161 | 71 / 288 | 1.24 (0.85, 1.82) | 0.27 |
| Hematologic*** | 3 / 203 | 6 / 404 | 1.11 (0.28, 4.48) | 0.88 |
adjusted for age, sex and calendar year of index date
No significant differences were found between males and females (p=0.64) or age groups (p=0.47).
Patients with prevalent cancer of the same type were excluded
The 1-, 10- and 20- year cumulative incidences of the first cancer of any type after index date were 5.9% (±1.9%), 33.6% (±4.6%) and 50.0% (±7.0%) among the GCA patients and 2.6% (±1.0%), 27.0% (±3.2%) and 47.0% (±5.1%) among the non-GCA patients (Figure 1). The 1-, 10- and 20- year cumulative incidences of the first cancer after index date excluding non-melanoma skin cancer were 3.4% (±1.4%), 23.7% (±4.0%) and 33.4% (±6.3%) among GCA patients and 0.6% (±0.5%), 17.3% (±2.6%) and 33.3% (±4.4%) among non-GCA patients (Figure 2). There was a trend toward increased risk of malignancy in GCA patients in the first year after diagnosis. Excluding patients with prevalent cancer, 9 GCA patients and 7 referent subjects were diagnosed with cancer in the first year after index date (HR: 2.35; 95% CI: 0.87, 6.31; p=0.09). All 9 of these GCA subjects had typical cranial symptoms of GCA in addition to a positive TAB. Median time from GCA diagnosis to diagnosis of malignancy was 4 (range 0–11) months. The malignancies in the 9 GCA patients were as follows: 1 squamous cell skin cancer, 2 basal cell skin cancers, 1 metastatic adenocarcinoma of the pancreas, 2 metastatic adenocarcinomas of the breast, 1 localized adenocarcinoma of the breast, 1 metastatic adenocarcinoma of the small intestine and 1 adenocarcinoma of the appendix.
Figure 1.
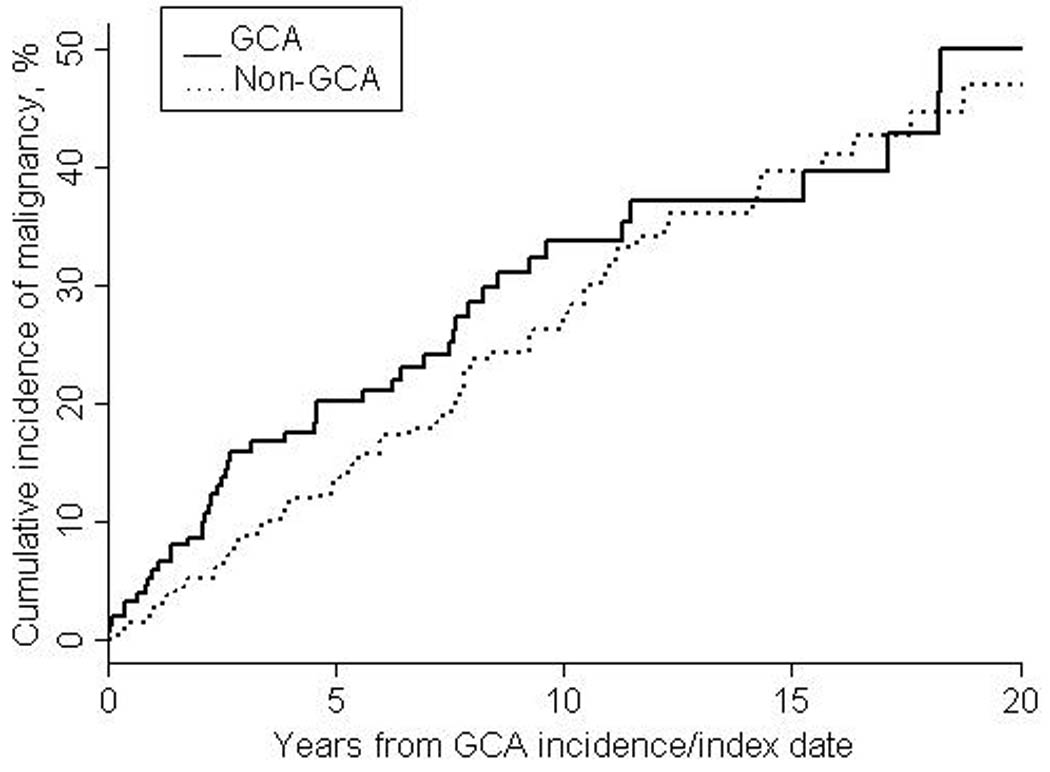
Cumulative incidence of any first malignancy after index date
Figure 2.
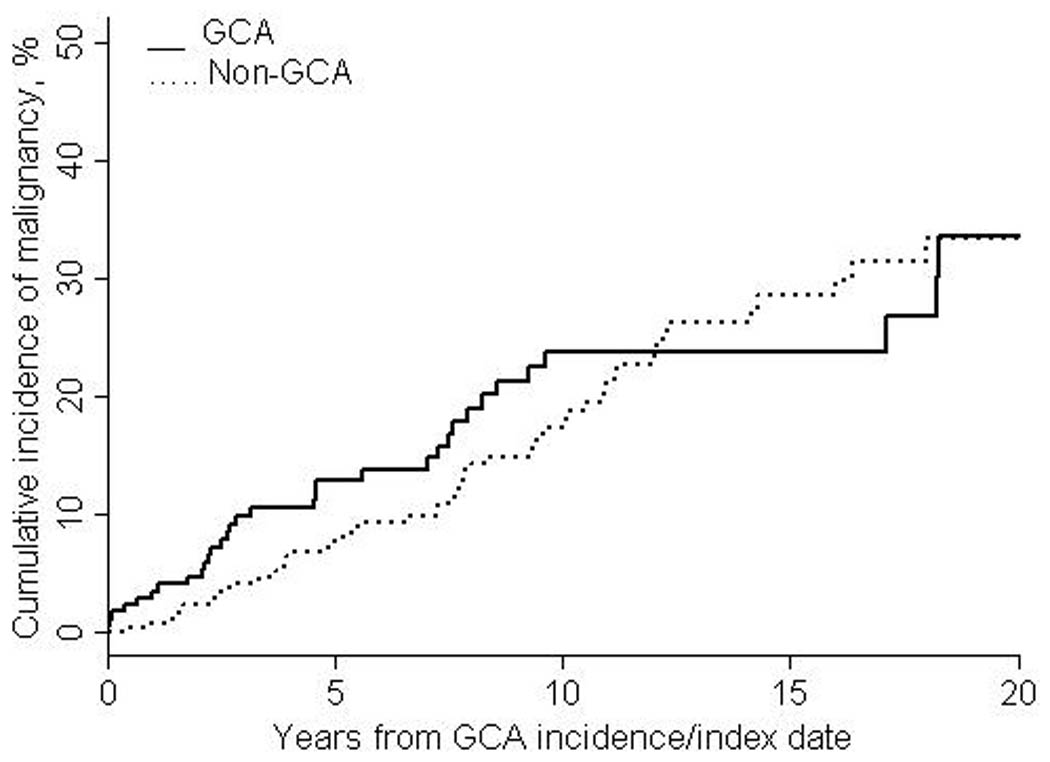
Cumulative incidence of first malignancy other than non-melanoma skin cancer after index date
During follow-up, 142 GCA and 270 non-GCA patients died. The overall mortality of the GCA and non-GCA cohorts was similar (age, sex and calendar year adjusted HR: 1.06; 95% CI: 0.86, 1.30; p=0.58). There was no increase in mortality following any first malignancy after index date in GCA patients compared to non-GCA subjects (HR: 0.80; 95% CI: 0.52, 1.24; p=0.32). Even after exclusion of non-melanoma skin cancers, there was no difference between the GCA and non-GCA cohorts in mortality following any malignancy after index date (HR: 0.71; 95% CI: 0.43, 1.19; p=0.19).
DISCUSSION
Patients with GCA do not have an increased risk of incident cancer following diagnosis compared to age- and gender-matched referent subjects in this study. The results were similar when subjects with a prevalent malignancy at index date were included.
The few other studies of malignancy risk in GCA have suggested either no, or increased risk of malignancy [9–12]. A case series of concurrent malignancy (less than 1 year before or after diagnosis) in 271 consecutive patients with GCA reported malignancy in up to 7.4% of cases [10]. In this study, the mean time between diagnosis of GCA and malignancy was 3.7 months. Another, prospective case-control study by Haga et al. suggested an increased risk of malignancy in GCA patients [11]. While the overall risk of malignancy in patients with GCA or PMR was not increased, an increased risk of malignancy was reported in the subset of GCA patients with a positive TAB [11].
Our study results support the findings of a population-based study from Norway in which there was no increase in the incidence of malignancy in GCA patients [12]. These authors speculated that the prior findings by Haga et al. may have been due to selection of cases from two hospitals rather than the general population [12]. In contrast to this Norwegian study which also included patients with only PMR, we used an incident cohort composed entirely of patients with GCA. Our study did not find increased risk of cancer in the subset of GCA patients with a positive TAB.
The 1-, 10- and 20-year cumulative incidences of any first cancer were similar between the GCA and non-GCA groups. We did note a statistically non-significant trend toward more cancer being diagnosed in GCA patients in the first year after index date. A similar finding was also noted in a population-based study of 255 consecutive patients with biopsy-positive GCA where 7 (17.9%) of the 39 cases of cancer were diagnosed within the first 12 months after diagnosis [9]. In our study, 9 patients with GCA developed an incident malignancy in the first year. Since all 9 GCA patients who were diagnosed with cancer in the first year had cranial symptoms of GCA in addition to a positive TAB, it is unlikely that there was a misclassification bias with respect to the GCA diagnosis. Three of these patients had a non-melanoma skin cancer. Both patients with small intestine adenocarcinomas and the patient with pancreatic adenocarcinoma presented with gastrointestinal symptoms which led to further evaluation and diagnosis of cancer. Two patients with breast cancer were diagnosed because of incidental findings on clinical breast examination. Another patient was noted to have an abnormal mammogram on routine screening mammography. While surveillance bias in the period after diagnosis may partially explain our findings, it is also possible that GCA may occur as a paraneoplastic phenomenon in a very small subset of patients.
Overall, the types of cancers found in the GCA and non-GCA cohorts in our study were similar. Non-melanoma skin cancers were the most common cancers in our study, accounting for over half the cancers seen after index date in both groups. In the study by Myklebust and colleagues, breast and female genital cancers were the most common followed by gastrointestinal malignancies. In their study, skin cancers including non-melanoma skin cancers accounted for only 15.5% of all malignancies noted in the cases and controls [12]. They also found clustering of prostate cancer near the time of diagnosis of GCA but this was thought to be coincidental. Our study found that colon cancer occurred more frequently in GCA patients compared to non-GCA subjects, although the difference did not reach statistical significance (p=0.07). While this finding may be related to surveillance bias, it is possible that other shared factors like immunosenescence and inflammation are important. Studies have found an association between increased plasma C-reactive protein (CRP) and development of colon cancer [20–22]. These findings suggest that chronic inflammation may play a role in the pathogenesis of cancer including colon cancer and may explain the slightly increased risk (while not significant) in GCA patients. Alternatively, shared genetic factors not yet identified may increase the risk of colon cancer in GCA patients.
Finally, we did not find any differences in overall mortality between the two cohorts. Mortality from cancer (excluding non-melanoma skin cancer) was also similar in GCA and non-GCA patients. These findings are in concordance with those of Gonzalez-Gay et al., who found no increase in mortality in GCA patients with cancer [9]. In the study by Myklebust et al. patients with GCA/PMR there was a trend to better survival of GCA patients with cancer compared to population controls, not reaching statistical significance (RR: 0.46; 95% CI 0.21, 1.02; p=0.05). Limitations of the current study include its retrospective design. However, the main outcome of interest is malignancy and utilization of REP allowed access to the entire inpatient and outpatient history of each individual. It is therefore unlikely that a diagnosis of malignancy would not be present in and abstracted from the medical record. The person abstracting the information (TK) was not blinded to the GCA status of the patients. However, ICD-9 codes were cross-referenced with the REP database to identify all potential malignancies in both groups. All charts were then reviewed systematically to confirm the diagnosis of malignancy. Using such a computerized system should reduce the likelihood of measurement bias from more careful review of charts from one group compared to the other. The study had a relatively small sample size which decreased our ability to detect a clinically relevant increase in cancer risk in the subset analyses as evidenced by the wide confidence intervals. However, we did have sufficient power to address the primary purpose of the study which was to calculate and compare the incidence of malignancy in patients with GCA to age- and gender-matched referent subjects.
We acknowledge the limitations of retrospective data abstraction of such information as smoking and cytotoxic medication use, for which reason we did not abstract information on other potential confounders such as body mass index, family history of malignancy, and hormone use. According to the 2000 US census data, 90.3% of the Olmsted County population is White. Since GCA predominantly affects individuals of Northern European descent, the results of this study should be generalizable to US patients with GCA.
Strengths of the current study include the population-based design and the REP, which allowed review of the entire individual medical record covering all inpatient and outpatient care from all local health care providers. Our study also included a referent cohort from the same population for comparison. The diagnosis of cancer was confirmed by review of the histopathology report in all except 1 subject. In contrast to other studies which have also included patients with PMR, our study population consisted of GCA patients only [11, 12]. As well, unlike previous studies, we adjusted for smoking as a potential confounder. Smoking is a known risk factor for certain cancers and studies in GCA have suggested that it may be a risk factor for developing GCA [23–25].
Based on our findings, GCA patients are not at increased risk of cancer following disease diagnosis. The risk of cancer in the first year after diagnosis may be slightly increased. In part, surveillance bias could have contributed to this finding. However, we cannot exclude the possibility that GCA may occur as a paraneoplastic phenomenon in a small subset of cases. Non-melanoma skin cancers are the most common cancer following index date. It remains uncertain whether the risk of colon cancer may be slightly increased in GCA subjects. Finally, there is no increase in overall mortality or mortality following cancer in GCA patients.
Table 2.
Type of malignancy in GCA and non-GCA patients following index date
| Specific cancer*** | GCA | Non-GCA | HR* (95% CI) | P-value |
|---|---|---|---|---|
| Melanoma skin cancer | 1 / 204 | 3 / 403 | 0.68 (0.07, 6.61) | 0.74 |
| Non-Melanoma skin cancer | 22 / 180 | 52 / 349 | 0.85 (0.51, 1.40) | 0.51 |
| Lung | 5 / 203 | 13 / 401 | 0.82 (0.29, 2.30) | 0.70 |
| Breast | 8 / 195 | 17 / 381 | 0.83 (0.34, 2.01) | 0.68 |
| Prostate/ testicular | 3 / 38 | 7 / 73 | 1.24 (0.31, 4.92) | 0.76 |
| Gynecological cancer (uterus, ovaries, fallopian tubes, cervix) |
2 / 159 | 3 / 305 | 1.25 (0.21, 7.51) | 0.81 |
| Urinary tract (kidneys, ureters, bladder) | 3 / 201 | 8 / 400 | 0.78 (0.21, 2.97) | 0.72 |
| Colon | 8 / 203 | 6 / 398 | 2.71 (0.94, 7.83) | 0.066 |
| Other GI | 2 / 203 | 4 / 405 | 1.11 (0.20, 6.07) | 0.91 |
| Other | 5 / 203 | 8 / 399 | 1.34 (0.44, 4.10) | 0.61 |
adjusted for age, sex and calendar year of index date
Patients with prevalent cancer of the same type were excluded
ACKNOWLEDGMENTS
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Grant Supports:
This study was made possible by the Rochester Epidemiology Project (Grant # R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR)
REFERENCES
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234–245. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Multisystem interactions in the pathogenesis of vasculitis. Curr Opin Rheumatol. 1997;9:3–11. doi: 10.1097/00002281-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawelec G. Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa G, Font J, Munoz-Rodriguez FJ, Cervera R, Ingelmo M. Myelodysplastic and myeloproliferative syndromes associated with giant cell arteritis and polymyalgia rheumatica: a coincidental coexistence or a causal relationship? Clin Rheumatol. 2002;21:309–313. doi: 10.1007/s100670200081. [DOI] [PubMed] [Google Scholar]
- 6.Lie JT. Simultaneous clinical manifestations of malignancy and giant cell temporal arteritis in a young woman. J Rheumatol. 1995;22:367–369. [PubMed] [Google Scholar]
- 7.Warrington KJ, Scheithauer BW, Michet CJ. Acute myeloid leukemia associated with necrotizing temporal arteritis. J Rheumatol. 2003;30:846–848. [PubMed] [Google Scholar]
- 8.Solans-Laque R, Bosch-Gil JA, Perez-Bocanegra C, Selva-O'Callaghan A, Simeon-Aznar CP, Vilardell-Tarres M. Paraneoplastic vasculitis in patients with solid tumors: report of 15 cases. J Rheumatol. 2008;35:294–304. [PubMed] [Google Scholar]
- 9.Gonzalez-Gay MA, Lopez-Diaz MJ, Martinez-Lado L, Pena-Sagredo JL, Lopez-Agreda H, Miranda-Filloy JA, et al. Cancer in biopsy-proven giant cell arteritis. A population-based study. Semin Arthritis Rheum. 2007;37:156–163. doi: 10.1016/j.semarthrit.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Liozon E, Loustaud V, Fauchais AL, Soria P, Ly K, Ouattara B, et al. Concurrent temporal (giant cell) arteritis and malignancy: report of 20 patients with review of the literature. J Rheumatol. 2006;33:1606–1614. [PubMed] [Google Scholar]
- 11.Haga HJ, Eide GE, Brun J, Johansen A, Langmark F. Cancer in association with polymyalgia rheumatica and temporal arteritis. J Rheumatol. 1993;20:1335–1339. [PubMed] [Google Scholar]
- 12.Myklebust G, Wilsgaard T, Jacobsen BK, Gran JT. No increased frequency of malignant neoplasms in polymyalgia rheumatica and temporal arteritis. A prospective longitudinal study of 398 cases and matched population controls. J Rheumatol. 2002;29:2143–2147. [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–834. doi: 10.1016/j.rdc.2004.07.010. vii. [DOI] [PubMed] [Google Scholar]
- 15.Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 16.Kermani TA, Schäfer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, Warrington KJ. Increase in Age at Onset of Giant Cell Arteritis: A Population-based Study. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.111005. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 18.Elbaz A, Peterson BJ, Bower JH, Yang P, Maraganore DM, McDonnell SK, et al. Risk of cancer after the diagnosis of Parkinson's disease: a historical cohort study. Mov Disord. 2005;20:719–725. doi: 10.1002/mds.20401. [DOI] [PubMed] [Google Scholar]
- 19.Elbaz A, Peterson BJ, Yang P, Van Gerpen JA, Bower JH, Maraganore DM, et al. Nonfatal cancer preceding Parkinson's disease: a case-control study. Epidemiology. 2002;13:157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 21.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 22.Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein levels and subsequent cancer outcomes: results from a prospective cohort study. Eur J Cancer. 2006;42:704–707. doi: 10.1016/j.ejca.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Larsson K, Mellstrom D, Nordborg E, Oden A, Nordborg E. Early menopause, low body mass index, and smoking are independent risk factors for developing giant cell arteritis. Ann Rheum Dis. 2006;65:529–532. doi: 10.1136/ard.2005.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duhaut P, Pinede L, Demolombe-Rague S, Loire R, Seydoux D, Ninet J, et al. Giant cell arteritis and cardiovascular risk factors: a multicenter, prospective case-control study. Groupe de Recherche sur l'Arterite a Cellules Geantes. Arthritis Rheum. 1998;41:1960–1965. doi: 10.1002/1529-0131(199811)41:11<1960::AID-ART10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Machado EB, Gabriel SE, Beard CM, Michet CJ, O'Fallon WM, Ballard DJ. A population-based case-control study of temporal arteritis: evidence for an association between temporal arteritis and degenerative vascular disease? Int J Epidemiol. 1989;18:836–841. doi: 10.1093/ije/18.4.836. [DOI] [PubMed] [Google Scholar]


