Abstract
Background
DAS181 (Fludase®) is a sialidase fusion protein in clinical development as a broad-spectrum anti-influenza virus (IFV) therapeutics. Previous reports by others raised the concern that desialylation of airway epithelium might increase the susceptibility to Streptococcus pneumoniae infection.
Methods
To address if DAS181 would lead to increased risk of pneumococcal infection, we tested S. pneumoniae colonization following DAS181 treatment of human A549 cells, healthy mice, and mice challenged with a lethal dose of IFV A/PR/8/34 (H1N1) or A/Victoria/3/75 (H3N2) followed by 104 cfu S. pneumoniae (D39) on day 3 or day 7. DAS181 treatment was given 24–48 hr after IFV challenge.
Results
DAS181 treatment did not increase S. pneumoniae colonization in vitro or in vivo in healthy animals. In IFV infected mice, DAS181 prevented pneumonia and significantly prolonged survival and inhibited IFV virus titer by ≥3 logs. None of the treated animals showed enhanced S. pneumoniae lung colonization. Additionally, opportunistic infections by Citrobacter ssp or Klebsiella ssp occurred only in mice receiving vehicle, not in DAS181 treated animals.
Conclusions
These data indicate that DAS181 treatment does not exacerbate secondary bacterial infection in mice. DAS181 may reduce the risk of secondary bacterial infection by inhibiting IFV.
Keywords: Sialic acid, Sialidase, Influenza virus, Pneumococcus, Pneumonia
Introduction
Secondary bacterial infection following influenza virus (IFV) infection is a major health concern due to the increased risk for mortality. Historical records suggest that a large number of influenza deaths occurring in the influenza pandemics of 1918–1919, 1957, and 1968 were the result of secondary bacterial pneumonia and not the initial influenza infection itself (1–4). Of the bacterial pathogens causing secondary infections, Streptococcus pneumoniae (pneumococcus) is the most common cause of bacterial pneumonia (5). Although vaccination against S. pneumoniae has shown great promise in preventing pneumonia associated with respiratory virus infection (6,7), serotypes not covered by the vaccine are on the rise (8), and complications caused by secondary bacterial infections are still significant cause of morbidity and mortality during seasonal influenza, and represent an even more serious threat during pandemics.
DAS181 is a 46 kDa recombinant fusion protein consisting of a sialidase functional domain fused with an amphiregulin glycosaminoglycan binding sequence that anchors the sialidase to the respiratory epithelium (9). DAS181 is intended for therapeutic and prophylactic treatment against infections caused by IFV and parainfluenza virus (PIV). By cleaving sialic acids (SAs) from the host cell surface, DAS181 inactivates the host cell receptors recognized by both IFV and PIV (10), and thereby potentially renders the host cells resistant to IFV and PIV infection.
Many different virulence factors are thought to contribute to the secondary pneumococcal infection, including viral and/or bacterial sialidase (11–13). Consequently one concern regarding safety of DAS181 is that desialylation of airway surface might increase the risk of pneumococcal infection by unmasking certain cryptic receptors for the bacteria. This concern was mainly derived from experimental observations made by McCullers and coworkers (14,15), which demonstrated that mice infected with IFV followed by a S. pneumoniae challenge 3–7 days later suffered from higher mortality from secondary pneumococcal pneumonia if the primary IFV carries higher neuraminidase (NA, a sialidase) activity. The authors hypothesized that the increased susceptibility to S. pneumoniae was due to desialylation of the airway epithelium by the viral NA activity. The role of sialidase function in pneumococcal infection also remains controversial. On the one hand, the pneumococcal sialidase gene, nanA, has been shown to enhance adhesion and colonization of pneumococci (16–21). On the other hand, a recent report demonstrated that nanA deficiency did not compromise the in vivo fitness of pneumococci (22).
In this report, we performed studies to directly assess if DAS181 would increase pneumococcal colonization in normal airway epithelium and if DAS181 treatment would exacerbate secondary pneumococcal infection following influenza. Our data indicate that DAS181 does not increase pneumococcal colonization in human epithelial cells, nor does it increase S. pneumoniae colonization in naive mice. DAS181 treatment against IFV infection did not exacerbate secondary pneumococcal infection in IFV-infected mice. Interestingly, DAS181 treatment seems to prevent certain secondary opportunistic bacterial infections in mice.
Materials and Methods
Infectious Agents and Cells
For viral challenge, mouse-adapted A/PR/8/34 (H1N1) was obtained from ATCC (#VR-95) (Manassas, VA), and mouse-adapted A/Victoria/3/75 (H3N2) obtained from Dr. Don Smee, Utah State University. Viral stocks were diluted in sterile PBS to 100 pfu and 5000 pfu per mouse for A/PR/8/34 and A/Victoria/3/75, respectively. MDCK cells were used to propagate and titer virus by plaque assay (9). Type 2 S. pneumoniae strain D39 was kindly provided by Dr. Jonathan McCullers (St. Jude Children’s Research hospital, Memphis TN). Bacteria were propagated in Todd Hewitt broth with 0.5% yeast extract or on TSAII + 5% sheep erythrocyte plates (BD, Sparks, MD). To prepare bacteria for inoculation, a freshly inoculated culture was grown to an OD600 of 0.6 and diluted in PBS to 104 cfu in 50 μL prior to use. Human lung carcinoma cell line A549 obtained from ATCC was grown at 37°C in a humidified atmosphere of 5% CO2 in DMEMF12 supplemented with 10% FBS, 1× Glutamax (Invitrogen, Carlsbad, CA), and 1× Antibiotic/Antimycotic solution (Sigma, St Louis, MO).
Quantitating Sialic Acid Level on A549 Cells
The lectin binding assay for quantitation of sialic acid level was previously described (9). In brief, confluent monolayers of A549 cells were treated with various doses of DAS181. Relative levels of either α(2,6)-linked sialic acid or α(2,3)-linked sialic acid were detected using lectins as follows. The cells were fixed with 0.05% glutaraldehyde in PBS, and blocked with 3% BSA in PBS at 4 °C overnight followed by incubation with either 2 μg/mL of biotinylated SNA lectin (Vector Laboratories, Burlingame, CA) or 20 μg/mL of biotinylated MAL-II (Vector Laboratories) for 2 h at 37°C. SNA (Sambucus nigra) is specific for Neu5Ac α(2,6)-Gal (alpha 2,6-linked sialic acids) and MAL-II (Maackia amurensis) is specific for Neu5Ac α(2,3)-Gal (alpha 2,3-linked sialic acids). Following four washes in PBS + 0.1% Tween 20 (PBST), bound lectin was detected by 5 μg/mL Streptavidin-HRP (Vector Laboratories) for 1 h at 37°C. The cells were washed five times with PBST and developed in TMB (Sigma). The reaction was terminated by the addition of 1M H2SO4. Background samples were vehicle treated samples that were incubated with streptavidin-HRP with out biotinylated lectin. Absorbance (Abs) was measured at 450 nm and the percentage of sialic acid remaining was calculated using 100% × (Abs of DAS181 treated cells — background)/(Abs of untreated cells — background).
In vitro Bacterial Adhesion Assays
These assays were performed as described previously (23). Briefly, confluent monolayers of A549 cells (approximately 5 × 105 cells per well) were incubated with 0.25 mL of 1 U/mL of DAS181 for 30 minutes at 37°C. Afterwards, the cells were washed three times with the DMEMF12 medium and then overlaid with bacteria diluted in 0.25 mL DMEMF12 medium for 1 hour at 37°C. Unbound bacteria were removed by washing three times with the same medium. The cells were then detached with 0.2 mL of TrypLE express (Invitrogen, Carlsbad, CA) containing 0.025% Triton X-100. Viable plate count assay was performed by plating 0.1 mL of serial ten fold dilutions of each sample in sterile PBS onto tryptic soy agar with 5% sheep erythrocytes (BD, Sparks, MD) for S. pneumoniae strains. The plates were grown overnight and the colony forming units (cfu) were counted to determine the number of bacteria that were bound to the monolayers. All experiments were performed in triplicate and data were normalized such that the untreated control was 100%.
Animal infection and survival
Animal studies were conducted according to the protocol approved by the NexBio, Inc Animal Care and Use Committee. 10 week-old BALB/c female mice (Charles River, Hollister, CA) were used for the in vivo infection studies. The mice were assigned to groups based on body weights (18–20 g) to achieve comparable group mean body weights. During the study animals were observed daily and clinical observations and body weights were recorded every two days. Prior to intranasal delivery of infectious agent or test article, the animals were anesthetized with 100 mg/kg ketamine.
Subsequently, the animals were held in an upright position while a total volume of 50 μl was delivered by intranasal injection using a syringe with a 22G blunt tipped needle. For S. pneumoniae colonization studies normal healthy mice were treated with PBS or DAS181 (1 mg/kg) intranasally for three days prior to intranasal inoculation with 104 cfu of S. pneumoniae. At 0, 24, 48, 72, 96 and 168 hr post challenge, mice were sacrificed and the lungs were harvested for bacterial counts. For co-infection studies, mice were infected with 100 pfu of mouse-adapted A/PR/8/34 or 5,000 of mouse-adapted A/Victoria/3/75, and received intranasal treatment at 24 or 48 hours post-infection (q.d. ×3) with 1 mg/kg of DAS181. S. pneumoniae challenge was performed intranasally on day 3 or day 7, respectively.
Lung Homogenization
Upon dissection each lung/trachea was weighed, and placed in 2 ml of DMEM-F12 media (HyClone Labs, Logan, Utah) and placed on ice. Homogenization of tissues was performed using a hand held homogenizer (Tissue Tearor, Biospec Product Inc., Bartlesville, OK) on ice (speed 15, ~20 s). Before each homogenization, the small probe-tip was rinsed in 5 successive PBS washes in 50 mL conicals, sterilized once in 70% ethanol, and rinsed one final time in PBS. Tissue homogenates were centrifuged at 4 °C in a microfuge for 10 min at 2000 × g. Supernatants from these cleared homogenates were stored at −80°C until further analysis of viral load by qRT-PCR.
Viral Copy Number Analysis
The cleared homogenate was used for quantitation of viral load by qRT-PCR assay. Viral RNA was purified from 50 μL of cleared homogenate sample using Applied Biosystem’s MagMAX-96™ Viral RNA isolation kit. The RNA was purified according to manufacturer’s instructions and eluted in 50 μl elution buffer. The cDNA was synthesized with 10 μL of the purified viral RNA using Applied Biosystem’s cDNA Synthesis kit. For the standard curve, 10 fold serial dilutions of the A/PR/8/34 or A/Victoria/3/75 M-gene were performed starting from 109 to 101 copies per quantitative reaction. A no-template control was included to control for cross contamination. The quantitative PCR reaction was carried out with 4 μL of cDNA, standard curve DNA, or H2O for the no template control. 2X Fast Universal Master Mix (Applied Biosystem’s), 900 nm of forward and reverse primers, and 225 nm of Taqman probe were added to the templates. PCR reactions were run under the conditions of one cycle of 95°C for 20 seconds, 45 cycles of 95°C for 3 seconds, and 60°C for 30 seconds on an ABI 7500 Fast Real-time PCR system. Data was collected during the 30 seconds at 60°C. The Ct threshold was determined automatically and the Ct values were plotted to form a standard curve by the Applied Biosystem’s Software. Typically, amplification with good linearity was observed in 109 to 102 copies. Copy numbers from the samples were interpolated with a fresh standard curve in each run.
Viable Bacterial Counts and Microbiology Characterization
Whole lungs were aseptically harvested and placed in 2 mL of sterile PBS on ice. The lungs were homogenized on ice with a hand held homogenizer (Biospec Products, Inc., Bartlesville, OK). Viable plate count assay was performed by plating 0.5mL of undiluted lung homogenate and 0.1 mL of serial ten fold dilutions of each sample in sterile PBS onto tryptic soy agar with 5% sheep erythrocytes (BD, Sparks, MD). The plates were grown overnight and the colony forming units (cfu) were counted and adjusted for dilution to determine the number of bacteria in each sample. Colonies with a green zone of alpha-hemolysis were denoted as S. pneumoniae and included in viable plate counts. All colonies with other morphologies were also noted, and sent to IDEXX laboratories (Sacramento, CA) for identification.
Histology and Immunostaining
Lungs for histological evaluation were perfused with PBS, and fixed in 10% buffered formalin for 24 h. Paraffin embedding and preparation of H&E slides was performed at Pacific Pathology (San Diego, CA). Sectionswere stained with H&E for histology review.
Statistical analysis
Statistical analysis of survival data was performed using Kaplan-Meier Log Rank test. Any mice that survived the observation period were assigned a value 21 days. The mean day to death data was found to follow normal distribution according to the D’Agostino &Pearson omnibus normality test, and hence analyzed using parametric test (t-test). Changes in body weight data was analyzed by Two-way ANOVA. All statistical tests were performed using GraphPad Prism version 4.02 for Windows, (GraphPad Software, San Diego CA).
Results
Desialylation of human epithelial cells in vitro
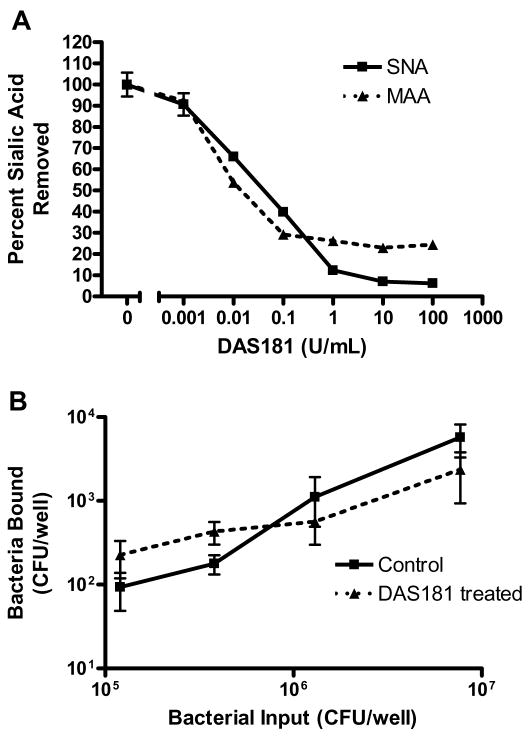
We have previously reported that DAS181 treatment did not increase colonization of well differentiated human airway epithelium (HAE) by three different strains of S. pneumoniae (24). Here, we further tested pneumococcal colonization to DAS181 treated A549 cells which are derived from human type II pneumocytes and contain high levels of both α(2,6)-linked and α(2,3)-linked SAs. DAS181 dose response curves show that at 1 U/ml of DAS181, desialylation of A549 cells approached a plateau after 30 min with approximately 90% of the α(2,6)-linked SA and 70% of the α(2,3)-linked SA removed based on SNA and MAL-II lectin binding (Fig 1A). To test the effect of desialylation on S. pneumoniae cell adhesion, confluent layers of A549 cells were treated with DAS181 (1 U/mL) for 30 min prior to adding different amounts of S. pneumoniae to the cells. The level of adherent bacteria following washing was determined by viable count analysis. As shown in Fig 1B, overall, DAS181 treatment did not increase S. pneumoniae adhesion to A549 cells. Similar to S. pneumoniae, adhesion by Haemophilus influenzae and Pseudomonas aeruginosa to A549 cells over a broad range of bacterial input was not augmented by DAS181 treatment (data not shown).
Figure 1. Cell surface sialic acid level after DAS181 treatment of human respiratory epithelial cells.
Monolayers of human epithelial A549 cells were treated with various doses of DAS181 as indicated for 30 min at 37°C. After incubation with DAS181, the cells were fixed and assayed for the surface level of sialic acids using SNA and MAL-II (A). Error bars represent one standard deviation above and below the mean of three replicate samples. After washing, the cells were incubated with S. pneumoniae (strain D39) at the indicated input amount for 1 hr at 37 °C. After washing, the adherent bacteria were quantitated by viable counts (B). Solid lines: bacterial adhesion to the cells treated with medium alone; dashed lines: bacterial adhesion to the cells treated with DAS181. Student’s t-test was used to determine P values; *=p<0.05.
Colonization of S. pneumoniae following DAS181 treatment in vivo
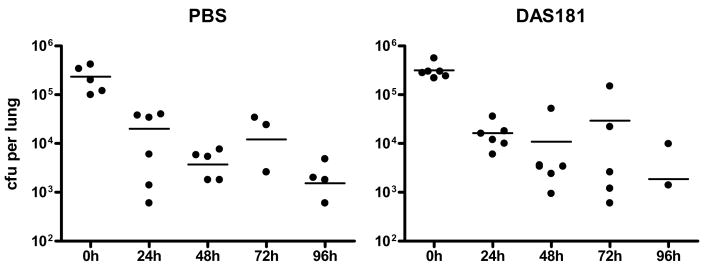
To evaluate if DAS181 would predispose normal animals to pneumococcal colonization, healthy mice were treated intranasally with DAS181 or PBS for three days and subsequently infected intranasally with 104 cfu of S. pneumoniae per mouse. This infectious dose of S. pneumoniae was chosen because it was the highest dose used by previous investigators (100–10,000 cfu per mouse) (23,25,26). Following DAS181 treatment, we harvested mouse lungs at various time points and determined bacterial counts in the lung homogenates. We found essentially identical bacterial counts in the lungs of the normal mice treated with either PBS or DAS181 during 96 hr post infection, with peak counts observed immediately after infection that approximated the total counts inoculated (Fig. 2). At 7 days post-infection, 4 out of 6 mice in the DAS181 treated groups had completely cleared the bacteria while only 2 out of 6 animals in the PBS group had cleared the bacteria from their lungs. In all of the animals, pneumococcal inoculation only caused slightly ruffled coats and mild lethargy and no mortality.
Figure 2. S. pneumoniae colonization in vivo.
Normal healthy mice were treated with PBS or DAS181 (1 mg/kg) intranasally for three days prior to intranasal inoculation with 104 cfu of S. pneumoniae. At 0, 24, 48, 72, 96 and 168 hr post challenge, six mice from each group were sacrificed and the lungs were harvested to determine bacterial counts. Statistical analysis was performed using Student’s t-test.
DAS181 treatment in mice with secondary pneumococcal infection
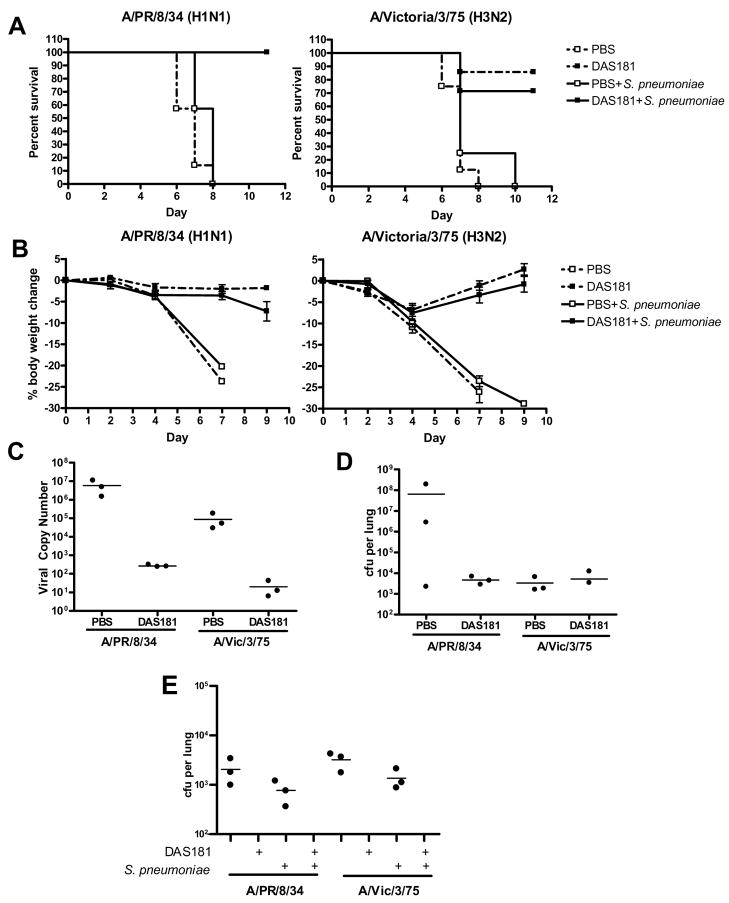
To evaluate if DAS181 treatment of IFV would predispose animals to secondary bacterial infection, mice were infected intranasally with two mouse-adapted IFV strains at lethal doses: A/PR/8/34 (H1N1) at 100 pfu/mouse or A/Victoria/3/75 (H3N2) at 5,000 pfu/mouse. These lethal infectious doses of the respective virus were determined in preliminary studies (data not shown). Following viral infection, mice were treated once daily for three days with DAS181 (1 mg/kg) with the first dose given 24 hours post-viral infection. The intranasal S. pneumoniae challenge (104 cfu per mouse) was administered 6 hours after the final DAS181 treatment dose on day 3. The timing of the pneumococcal inoculation was selected to coincide with the timing of the peak lung viral titer in the untreated animals.
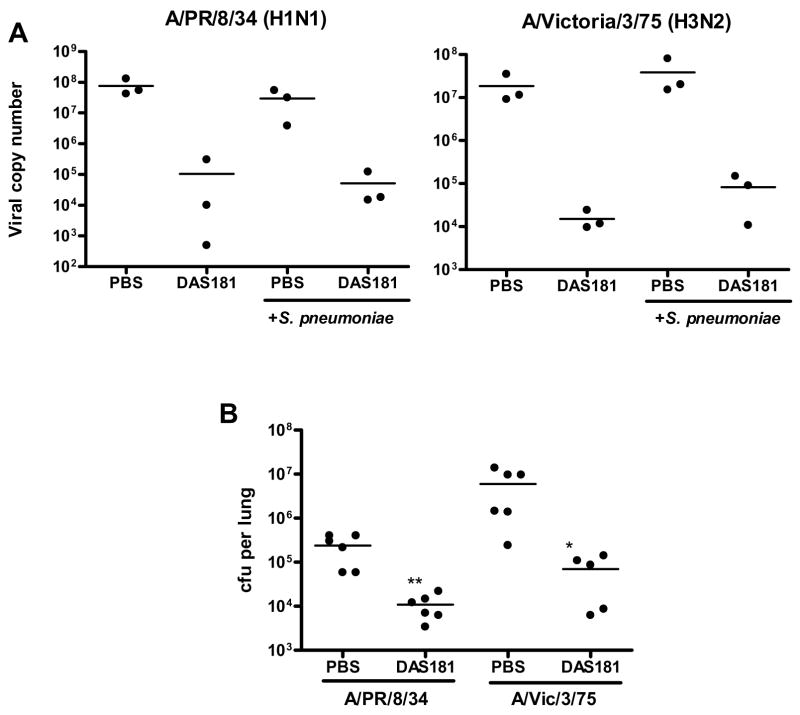
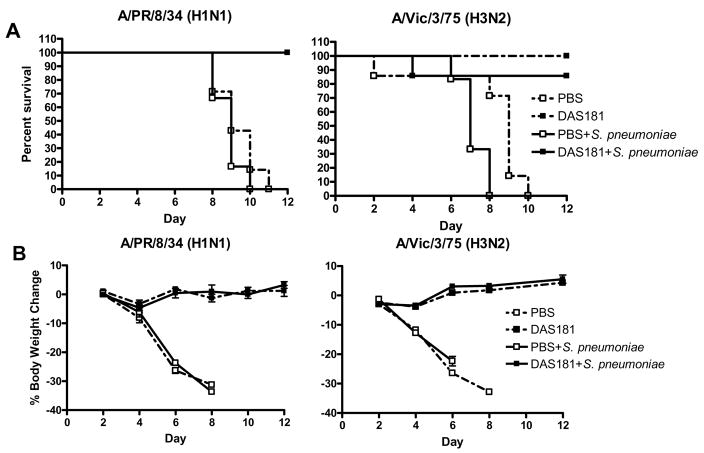
Interestingly, without DAS181 treatment, pneumococcus grew to higher titers in the IFV infected animals than in the normal animals in spite of identical inoculation dose (compare Fig 4B and Fig 2). Animals co-infected with A/Victoria/3/75 and S. pneumoniae also had shorter survival than animals infected with A/Victoria/3/75 alone (Fig 3A). These observations demonstrate that preceding IFV infection predisposes animals to an exacerbated bacterial infection. DAS181 significantly improved survival, and prevented body weight loss and clinical signs in the IFV and pneumococcus co-infected animals (p<0.001 for A/PR/8/34 and p<0.01 for A/Victoria/3/75 infected mice) (Fig. 3). The DAS181 treated animals had significantly extended mean day to death, which were in the range of ≥21 days (for A/PR/8/34) and 18.6±6.4 days (for A/Victoria/3/75), compared to that of their respective PBS treated IFV-infected control groups, which were 8.8±0.7 days and 7.2±0.7 days, respectively. As expected, DAS181 treatment inhibited IFV titer by about 3 logs in all of the treated animals (Fig 4A). Importantly, DAS181 treatment resulted in significantly lower bacterial counts in the lungs in all animals infected with either A/PR/8/34 or A/Victoria/3/75 (p<0.01) (Fig. 4B).
Figure 4. Bacterial counts and viral copy numbers of mice co-infected with IFV and S. pneumoniae.
Mice (N=3–6 per group)were infected with 100 pfu of mouse-adapted A/PR/8/34 or 5,000 of mouse-adapted A/Victoria/3/75 and 24 hours later received intranasal treatment (q.d. ×3) with DAS181 (1 mg/kg). S. pneumoniae challenge was performed intranasally on day 3. At 24 hours post bacterial challenge, lungs from three mice per group were harvested and homogenized for analysis of bacteria counts (A) and viral copy numbers (B). Statistical analysis was performed using Student’s t-test. *=p<0.05, **=p<0.01.
Figure 3. Survival and body weight change in mice co-infected with IFV and S. pneumoniae.
Mice (N=6–7 per group) were infected with 100 pfu of mouse-adapted A/PR/8/34 or 5,000 of mouse-adapted A/Victoria/3/75 and 24 hours later received intranasal treatment (q.d. ×3) with 1 mg/kg of DAS181. S. pneumoniae challenge was performed intranasally on day 3. (A) Survival was monitored daily and Graphpad Prism 4.2 was used to assess statistical differences in survival curves by the Kaplan-Meier Log Rank test. (B) Body weights were recorded every two days. Differences in body weights changes were compared by two-way ANOVA.
Lung histology further confirmed that DAS181 treatment prevented secondary pneumonia (Figure 5). The lungs from DAS181 treated animals co-infected with IFV and S. pneumoniae showed near normal histology, whereas control animals infected with eitherA/PR/8/34 or A/Victoria/3/75 virus alone or co-infected with bacteria showed obvious signs of pneumonia with multi-focal infection throughout the bronchiolar tree as well as the lung parenchyma, displaced alveolar structures, hemorrhage and infiltration of inflammatory cells. Consistent with H3N2 being more virulent than H1N1 strains, lungs from the A/Victoria/3/75 infected mice appeared to have more severe pathological changes compared to animals infected with A/PR/8/34.
Figure 5. Histopathological analysis of lungs from non-infected controls or mice infected with influenza virus and S. pneumonia.
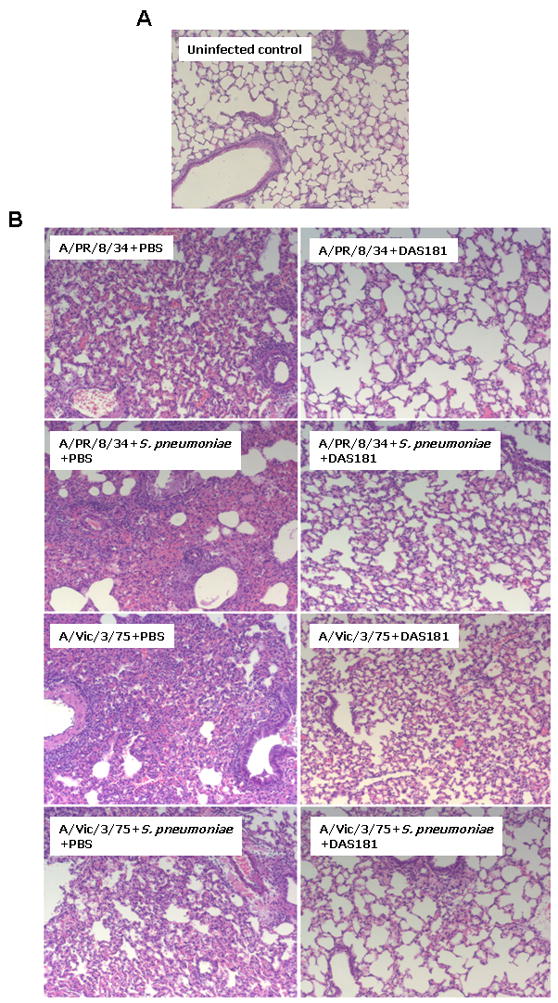
Lungs from non-infected control mice (A) and mice infected with either A/PR/8/34 or A/Victoria/3/75, with or without 104 cfu of S. pneumoniae (B), were harvested at 24 hours post bacterial challenge and processed for paraffin sections and stained with H&E for histology review. The photomicrographs shown here are representative of 3/3 mice from each respective group. (Magnification; ×100).
Effect of delayed DAS181 treatment and bacterial challenge
In a previous study the time of bacterial challenge subsequent to IFV infection was found to affect the rate of mortality in the mouse co-infection model, with the time point of bacterial challenge associated with the greatest mortality rate combined with the most rapid death at 7 days post viral challenge (23). Hence, in the next study, we selected this time frame to evaluate the effect of DAS181 on secondary bacterial infection. Mice were infected with either A/PR/8/34 or A/Victoria/3/75 on day 0. DAS181 treatment was initiated 48 hours post-infection and continued for 5 days until day 6. On day 7, we inoculated mice with S. pneumoniae, and followed the animals until day 21. All groups receiving DAS181 treatment showed increased survival and improved clinical signs compared to the PBS controls (Fig. 6A & B). In animals co-infected with bacteria and IFV A/PR/8/34 or A/Victoria/3/75, DAS181 significantly extended mean day to death to ≥21 days and 17±6.8 days, respectively, which are in contrast to their respective PBS treated control groups of 7.6±0.5 days and 8.5±3 days (p<0.001 and p<0.05, respectively). As expected, DAS181 treatment reduced viral titer by over 4 logs in all groups (Fig. 6C). Additionally, DAS181 treatment did not enhance S. pneumoniae colonization and growth in all animals (Fig. 6D).
Figure 6. Survival, body weight change, viral copy numbers and bacterial counts in mice infected with IFV and challenged with S. pneumoniae on day 7.
Mice (N=6–7 per group) were infected with 100 pfu of mouse-adapted A/PR/8/34 or 5,000 of mouse-adapted A/Victoria/3/75 and 48 hours later received intranasal treatment (q.d. ×5) with DAS181 (1 mg/kg). S. pneumoniae challenge was performed on day 7. At 24 hours post bacterial challenge, lungs from three mice per group were harvested and homogenized for analysis of bacteria counts and viral copy numbers. Graphpad Prism 4.2 was used to assess statistical differences in survival curves (A) by the Kaplan-Meier Log Rank test. (B) Body weights were recorded every two days, and differences in body weight loss were analyzed by Two-way ANOVA. Analysis of viral copy numbers (C) and bacterial counts (D) were performed by Student’s t-test. (E) Endogenous bacterial colonization was determined by counting the colonies not denoted as S. pneumoniae. These colonies were subsequently isolated and sent to IDEXX laboratories (Sacramento, CA) for identification. Citrobacter ssp titers are shown in the H1N1 infected animals and Klebsiella ssp titers are shown in H3N2 infected animals.
Interestingly, we also found that untreated animals were infected with other bacteria in addition to pneumococci. Upon analyzing the lungs of the IFV infected mice with or without the pneumococcal challenge, on day 8, we found that all of the mice of the PBS group were also infected with Citrobacter ssp (H1N1 infected animals) or Klebsiella ssp (H3N2 infected animals) (Fig. 6E), which was not observed at the earlier post IFV infection time points. These bacteria are known opportunistic pathogens in mice and humans (27,28). Strikingly, none of the DAS181 treated animals exhibited any secondary opportunistic bacterial infection. This observation suggests that DAS181 may prevent secondary infection by opportunistic bacterial pathogens.
Discussion
Our studies differ from the previous works on synergy between IFV and pneumococci in that we used a higher infectious dose of IFV than what was used before (14,15,23,29). However, we observed similar synergy between IFV and pneumococci as previously reported (14,15,23,29). Specifically, S. pneumoniae given to mice infected with IFV resulted in increased bacterial lung burden and increased mortality. While the previous reports attributed the increased severity of secondary bacterial infection to desialylation by IFV NA (14,15,23,29), our results contradict this hypothesis and indicate that desialylation per se does not increase susceptibility to secondary bacterial infection. Quite the contrary, desialylation by DAS181 seemed to protect animals by inhibiting IFV infection and subsequently reducing bacterial colonization. Because whole IFV virions were used in the previous reports (14,15,23,30), rather than a purified NA protein (a sialidase), the previous experimental system cannot distinguish the effects of IFV virions from that of the NA function alone. An IFV strain with higher NA activity, as used in the previous reports, is likely more virulent and thus causes more severe primary viral infection which may lead to more severe secondary bacterial infection. Prior to this report, there had not been any publication that directly addressed the effect of a purified sialidase on secondary bacterial infection.
A positive correlation between viral virulence and increased severity of secondary bacterial infection has been well known (31–33). IFV infection causes cellular and tissue damages, cytokine changes, and impaired immune function that all contribute to increased susceptibility to S. pneumoniae (11,26,34–37). Extensive airway epithelium denudation happens during severe influenza (38,39). It was reported that S. pneumoniae only adheres to the denuded basal cells and basement membrane but not to the intact tracheal epithelium (39). By inhibiting IFV infection as the primary cause of the epithelial damage, DAS181 treatment may protect the airway epithelium from inflammation and denudation which may be the mechanism to prevent secondary bacterial infection.
Consistent with the lack of effects by DAS181 on bacterial colonization in normal airway epithelium, we and others have observed that the subterminal polysaccharide structures exposed after sialidase treatment, Galβ1-4GlcNAc and Galβ1-3GalNAc, are abundantly present on the native human airway epithelium and normal lung tissue unexposed to a sialidase (24,40). Thus, sialidase treatment does not seem to introduce novel polysaccharide structures on the human airway surface.
In summary, results reported here indicate that DAS181 treatment does not increase S. pneumoniae colonization to either human lung epithelial cells or in the lungs of healthy mice. In mice co-infected with IFV and S. pneumoniae, DAS181 treatment inhibited IFV replication as well as secondary pneumococcal infection. It is noted that, however, the mouse influenza model in this study rapidly progressed to pneumonia which does not exactly mimics the common path of disease progression in human where influenza that is often associated with tracheobronchitis, rather than pneumonia. Ultimately, the effect of DAS181 on secondary bacterial infection in human will be elucidated by clinical trials.
Acknowledgments
This study has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200600015C, and grant U01AI070281.
Footnotes
Potential conflicts of interest. M.H., L.M.A., K.J., J.L.L and F.F. are shareholders in NexBio Inc. (San Diego, CA).
Reference List
- 1.HERS JF, MASUREL N, MULDER J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958 Nov 29;2(7057):1141–3. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay MI, Jr, Herrmann EC, Jr, Morrow GW, Jr, Brown AL., Jr Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA. 1970 Dec 7;214(10):1825–32. doi: 10.1001/jama.214.10.1825. [DOI] [PubMed] [Google Scholar]
- 3.LOURIA DB, BLUMENFELD HL, ELLIS JT, Kilbourne ED, ROGERS DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959 Jan;38(1 Part 2):213–65. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008 Oct 1;198(7):962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008 May;86(5):408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004 Aug;10(8):811–3. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006 Apr 6;354(14):1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 8.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007 Nov 1;196(9):1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 9.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006 Apr;50(4):1470–9. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ah-Tye C, Schwartz S, Huberman K, Carlin E, Moscona A. Virus-receptor interactions of human parainfluenza viruses types 1, 2 and 3. Microb Pathog. 1999 Nov;27(5):329–36. doi: 10.1006/mpat.1999.0313. [DOI] [PubMed] [Google Scholar]
- 11.Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 12.Tuomanen E. Molecular and cellular biology of pneumococcal infection. Curr Opin Microbiol. 1999 Feb;2(1):35–9. doi: 10.1016/s1369-5274(99)80006-x. [DOI] [PubMed] [Google Scholar]
- 13.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001 Jun;65(2):187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004 Jan;23(1 Suppl):S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 15.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005 Jul 15;192(2):249–57. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King SJ, Hippe KR, Weiser JN. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol. 2006 Feb;59(3):961–74. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- 17.Tong HH, Blue LE, James MA, DeMaria TF. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun. 2000 Feb;68(2):921–4. doi: 10.1128/iai.68.2.921-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manco S, Hernon F, Yesilkaya H, Paton JC, Andrew PW, Kadioglu A. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect Immun. 2006 Jul;74(7):4014–20. doi: 10.1128/IAI.01237-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis. 2004 Nov 1;190(9):1661–9. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- 20.Soong G, Muir A, Gomez MI, et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest. 2006 Aug;116(8):2297–305. doi: 10.1172/JCI27920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trappetti C, Kadioglu A, Carter M, et al. Sialic Acid: A Preventable Signal for Pneumococcal Biofilm Formation, Colonization, and Invasion of the Host. J Infect Dis. 2009 May 15;199(10):1497–505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 22.King QO, Lei B, Harmsen AG. Pneumococcal surface protein A contributes to secondary Streptococcus pneumoniae infection after influenza virus infection. J Infect Dis. 2009 Aug 15;200(4):537–45. doi: 10.1086/600871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003 Mar 15;187(6):1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls JM, Aschenbrenner LM, Paulson JC, et al. Comment on: concerns of using sialidase fusion protein as an experimental drug to combat seasonal and pandemic influenza. J Antimicrob Chemother. 2008 Aug;62(2):426–8. doi: 10.1093/jac/dkn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004 Aug 1;190(3):519–26. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 26.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004 Jun 15;172(12):7603–9. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 27.Bleich A, Kirsch P, Sahly H, et al. Klebsiella oxytoca: opportunistic infections in laboratory rodents. Lab Anim. 2008 Jul;42(3):369–75. doi: 10.1258/la.2007.06026e. [DOI] [PubMed] [Google Scholar]
- 28.Lipsky BA, Hook EW, III, Smith AA, Plorde JJ. Citrobacter infections in humans: experience at the Seattle Veterans Administration Medical Center and a review of the literature. Rev Infect Dis. 1980 Sep;2(5):746–60. doi: 10.1093/clinids/2.5.746. [DOI] [PubMed] [Google Scholar]
- 29.McCullers JA, Tuomanen EI. Molecular pathogenesis of pneumococcal pneumonia. Front Biosci. 2001 Aug 1;6:D877–D889. doi: 10.2741/mccullers. [DOI] [PubMed] [Google Scholar]
- 30.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002 Aug 1;186(3):341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 31.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000 Mar;181(3):831–7. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 32.Peltola VT, Boyd KL, McAuley JL, Rehg JE, McCullers JA. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun. 2006 May;74(5):2562–7. doi: 10.1128/IAI.74.5.2562-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giebink GS, Wright PF. Different virulence of influenza A virus strains and susceptibility to pneumococcal otitis media in chinchillas. Infect Immun. 1983 Sep;41(3):913–20. doi: 10.1128/iai.41.3.913-920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992 Oct;146(4):1067–83. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 35.Wilson R, Cole PJ. The effect of bacterial products on ciliary function. Am Rev Respir Dis. 1988 Dec;138(6 Pt 2):S49–S53. doi: 10.1164/ajrccm/138.6_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 36.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J Virol Methods. 2001 May;94(1–2):173–86. doi: 10.1016/s0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 37.van der Sluijs KF, Nijhuis M, Levels JH, et al. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006 Jan 15;193(2):214–22. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- 38.WALSH JJ, DIETLEIN LF, LOW FN, BURCH GE, MOGABGAB WJ. Bronchotracheal response in human influenza. Type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Arch Intern Med. 1961 Sep;108:376–88. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]
- 39.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986 Nov;134(5):1040–4. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 40.Chandrasekaran A, Srinivasan A, Raman R, et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol. 2008 Jan;26(1):107–13. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]