Abstract
Background
Alzheimer's disease (AD) and a host of other neurodegenerative central nervous system (CNS) proteinopathies are characterized by the accumulation of misfolded protein aggregates. Simplistically, these aggregates can be divided into smaller, soluble, oligomeric and larger, less-soluble or insoluble, fibrillar forms. Perhaps the major ongoing debate in the neurodegenerative disease field is whether the smaller oligomeric or larger fibrillar aggregates are the primary neurotoxin. Herein, we propose an integrative hypothesis that provides new insights into how a variety of misfolded protein aggregates can result in neurodegeneration.
Results
We introduce the concept that a wide range of highly stable misfolded protein aggregates in AD and other neurodegenerative proteinopathies are recognized as non-self and chronically activate the innate immune system. This pro-inflammatory state leads to physiological senescence of CNS cells. Once CNS cells undergo physiological senescence, they secrete a variety of pro-inflammatory molecules. Thus, the senescence of cells, which was initially triggered by inflammatory stimuli, becomes a self-reinforcing stimulus for further inflammation and senescence. Ultimately, senescent CNS cells become functionally impaired and eventually die, and this neurodegeneration leads to brain organ failure.
Conclusion
This integrative hypothesis, which we will refer to as the proteinopathy-induced senescent cell hypothesis of AD and other neurodegenerative diseases, links CNS proteinopathies to inflammation, physiological senescence, cellular dysfunction, and ultimately neurodegeneration. Future studies characterizing the senescent phenotype of CNS cells in AD and other neurodegenerative diseases will test the validity of this hypothesis. The implications of CNS senescence as a contributing factor to the neurodegenerative cascade and its implications for therapy are discussed.
Genetic, pathological, biochemical, animal and cell modeling studies provide strong support for the general hypothesis that accumulation of misfolded, aggregated proteins in the brain triggers a complex series of events that result in neuronal degeneration [1-4]. In Alzheimer's disease (AD) aggregation and accumulation of the amyloid β (Aβ) protein and microtubule associated protein tau (MAPT) have both been implicated as key pathogenic 'triggers' [5]. Aβ accumulates in senile plaques, cerebral vessels, and, to a more limited extent, within neurons [6]. Tau accumulates inside cells as neurofibrillary tangles and tau neurites [7]. In genetic forms of AD the data overwhelmingly support the 'Aβ aggregate/amyloid cascade' hypothesis, which posits that Aβ aggregation and accumulation precedes, and therefore drives, tau accumulation [3]. In 'sporadic' cases it is also possible that the two pathologies may arise, at least in part, through independent pathways [8]. Like familial AD, mutations in a number of genes result in accumulation of protein aggregates (for example, ABri, ADan, superoxide dismutase, α-synuclein, huntingtin, ataxins, and neuroserpin), triggering the pathological cascade that leads to many phenotypically distinct neurodegenerative diseases [1-3,9,10].
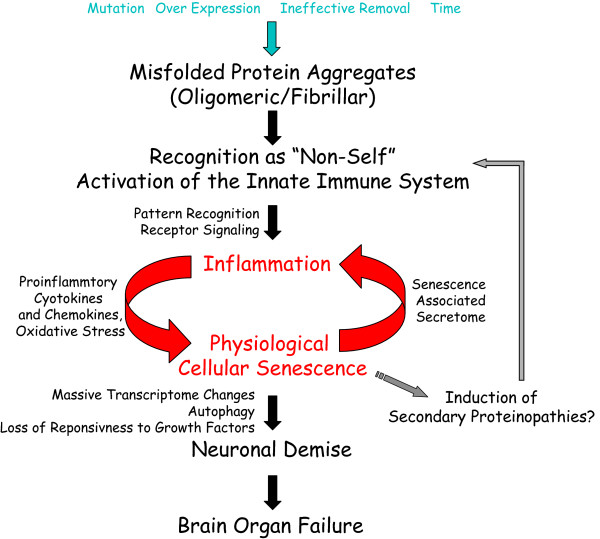
Herein, we will refer to the generic concept of misfolded protein aggregation and accumulation as a proteinopathy. Although there is reasonable consensus in the field regarding proteinopathies as 'triggers' of neurodegeneration, there is little consensus regarding the mechanisms that lead to neuronal demise. Using the Aβ and tau proteinopathies in AD as examples, we will present an integrated hypothesis of how central nervous system (CNS) proteinopathies cause neurodegeneration through a cascade initially involving innate immune activation, inflammation, induction of senescence, and subsequent neurodegeneration. We will refer to this integrated hypothesis as the proteinopathy-induced senescent cell hypothesis of neurodegeneration (Figure 1). Along with a detailed presentation of the hypothesis and current experimental data that support this hypothesis, we will outline the experimental steps needed to validate this hypothesis and explore its potential significance with respect to therapeutic development for AD and other neurodegenerative proteinopathies.
Figure 1.
Proteinopathy-induced neuronal senescence. A schematic of proteinopathy-induced neuronal senescence depicts protein misfolding and aggregation as a trigger for a self-reinforcing cycle of pro-inflammatory signals and senescence. As a critical mass of neurons acquire a physiologically senescent phenotype overt neurodegeneration and failures in the brain's cognitive and regulatory functions become clinically apparent.
Step 1: misfolding and aggregation of proteins into pathogen associated molecular patterns
Aβ, tau and other protein aggregates in neurodegenerative diseases are almost always found in an abnormal structural conformation compared to the non-aggregated protein [1-4]. Many aggregates show the characteristic features of amyloid and accumulate in an 'abnormal' fibrillar β-pleated sheet structure [11]. A common theme of genetic alterations that cause AD is that they increase the likelihood that Aβ will aggregate into amyloid [6]. Mutations in tau that cause frontal temporal dementia with parkinsonism linked to chromosome 17 (FTDP-17 MAPT) also alter tau in a way that increases its likelihood to aggregate into amyloid-like structures [12-14]. Furthermore, there is evidence that mutations in, or over-expression of, other proteins linked to neurodegeneration enhance the likelihood that they are assembled into misfolded aggregates, and many of these aggregates also have characteristic features of amyloid [1-4].
When a normal protein misfolds and aggregates, it no longer resembles a self protein; thus, it is subject to recognition by the immune system. Misfolded self protein aggregates resemble pathogen-associated molecular patterns (PAMPs) and thymus independent type 2 (TI-2) antigens [15-18]. PAMPs are a group of molecules that are capable of activating a wide array of innate immune defenses. They can be proteins, polysaccharides, or nucleotides and are characterized by a repetitive molecular motif that is recognized as non-self and can bind and activate evolutionarily conserved pattern recognition receptors (PRRs) that initiate innate immune signaling. Classic PAMPs are bacterial lipopolysaccharide, flagellin, peptidoglycan, some viruses and virus-like particles, and double-stranded RNA. TI-2 antigens are similar polymeric molecules that directly stimulate B cells to secrete IgM by crosslinking of plasma membrane immunoglobulin. Despite their chemical diversity, the unifying features of both PAMPs and TI-2 antigens are that they are large in size (typically greater then 100 kDa and often much larger), and have repetitive epitopes (and at least for TI-2 antigens require rigid presentation of the epitope with a two-dimensional spacing of 5 to 10 nm), poor in vivo degradability, and the ability to activate complement [18]. Notably, these features of PAMPS and TI-2 antigens are quite reminiscent of the features of amyloid deposits [11].
Misfolded protein aggregates can theoretically activate the adaptive immune system. However, the key step in activation of adaptive immunity, major histocompatibility complex (MHC) presentation of non-self peptides, is likely to limit such activation [19]. MHC binds small peptides that are typically cleaved from a larger protein [20]. Unless a small peptide derived from the protein aggregate retains an abnormal 'non-self' configuration following disaggregation, proteolytic cleavage, and binding to the MHC, it will not be a strong activator of the adaptive immune system [21]. Moreover, classic MHC molecules are typically expressed at low levels in the CNS and though there clearly is continuous surveillance of the CNS by T cells, the low levels of T cells and limited MHC expression are likely to limit adaptive immune responses to misfolded self proteins in the CNS. Thus, misfolded self protein aggregates are not likely to strongly activate the adaptive immune response. Instead, the proteinopathy will largely activate the innate immune system.
The recent description of proteins that can exist as functional amyloid-like structures within select organelles challenges, to some degree, the notion that all aggregated misfolded proteins are PAMPs. Examples of physiologically 'functional' mammalian amyloids are currently limited to amyloid formation by select peptide hormones in secretory granules of the pituitary, amyloid present in semen, and Pmel17 in melanocytes [22-24]. Notably, both the secretory granule amyloid and Pmel17 amyloid are contained within intracellular vesicles that most likely sequester them from interaction with PRRs and other forms of innate immune surveillance. Furthermore, because the peptide hormone amyloids must dissociate in order for them to be active, they are distinct from many pathological amyloids and PAMPs, which are highly stable structures.
Step 2: proteinopathy-mediated activation of innate immunity results in chronic inflammation
Amyloid or amyloid-like protein aggregates are highly resistant to degradation [11]. Perhaps the clearest illustration of this stability is seen in both in vivo imaging studies in Aβ protein precursor transgenic mice and cross-sectional pathology studies in inducible Aβ protein precursor transgenic mice [25-28]. These studies demonstrate that the amyloid deposits, once formed, are incredibly stable even in the absence of ongoing Aβ production. Though intracellular aggregates, such as those found in the polyglutamine diseases, can in certain circumstances be cleared in mice in which the transgene is turned off entirely, this situation is not replicated in the human disease and attempts to clear the aggregates may result in direct impairment of the protein quality control machinery [29-32]. Significantly, amyloid or amyloid-like protein aggregates catalyze the structural conversion of the normally folded protein into additional aggregates via a seeded nucleation-dependent process. Thus, following nucleation, the ongoing production of a 'normal' precursor drives additional amyloid formation [33,34]. In contrast to amyloid, the stability and 'seeding' or nucleating potential of other potentially pathogenic oligomeric structures formed by misfolded proteins has not been studied in detail [35,36].
We postulate that extracellular and intracellular protein aggregates act like PAMPs and result in chronic activation of the innate immune systems through PRRs. The concept that misfolded protein aggregates are PAMPs and activate innate immunity has been previously suggested by a number of groups and is supported by a plethora of experimental data [37-39]. Amyloid and amyloid like aggregates regardless of their peptide or protein subunit can be shown to bind and activate a whole array of PRRs, including Toll-like receptors, formyl peptide receptors, receptor for advanced glycation end products, scavenger receptors, complement and pentraxins [37-39]. Oligomeric assemblies of amyloidogenic proteins have not been studied as intensively with respect to PRR activation, but in the cases that they have they can be shown to elicit effects similar to amyloid fibrils (reviewed in [37]). Structurally, it is likely that oligomeric proteins resemble viruses or virus-like particles, which are known to function as PAMPs [40].
Most of the experiments that have established amyloid, amyloid like structures, and oligomers as PAMPs have involved direct application of these aggregates to cells in culture [37]. Such studies are complemented by histopathological studies that show co-localization of inflammatory cells and mediators with amyloid plaques and in vivo studies using multiphoton imaging that show the rapid mobilization of microglia to newly formed plaques [38,39,41-43]. These studies strongly support the concept that extracellular proteinopathies activate PRRs. Less well supported by direct experimental data is the notion that an intracellular protein aggregate acts like a PAMP, resulting in activation of PRRs and mobilization of the innate immune defenses. Nevertheless, a number of pathological features seen in intracellular proteinopathy-induced neurodegeneration suggest that these intracellular aggregates are activating the innate immune system. For example, binding to heat shock proteins, induction of autophagy and binding to intracellular PRRs can all activate the innate immune system [37,44-46].
The notion that intracellular proteinopathies activate innate immunity incorporates and extends some aspects of the danger theory of immune activation. This theory postulates that an intracellular stress or pathogen results in the cell generating a 'danger' signal that activates the immune system [47]. In this case we postulate that an intracellular protein aggregate causes the neuron or other CNS cell to send out 'danger signals' that activate the innate immune system. Such 'danger signaling' might explain the observation that inflammatory markers are often the earliest sign of pathology in experimental models of neurodegenerative proteinopathies [38,48-51]. Ultimately, intracellular or extracellular, stable protein aggregates acting as PAMPs will produce a chronic inflammatory condition.
A major ongoing debate in the AD and the larger neurodegenerative disease field is whether small soluble aggregates or larger, less soluble aggregates are the principle toxic species [52-55]. In the context of this hypothesis, both small soluble aggregates - oligomers - and larger aggregates - fibrils - will function as PAMPs. Depending on their concentration, location, and degradability, their ability to activate PRRs will likely vary. Significantly, the activation of PRRs by PAMPs elicits a response that is designed to result in clearance or sequestration and inactivation of the PAMP. In addition to a whole host of other variables, differential activation of the innate immune system by different protein aggregates and variable clearance of the aggregates following immune activation probably contribute to the imperfect correlations between the amounts and regional distribution of protein aggregates with clinical and neuropathological phenotypes [56]. Indeed, an aggregate that elicits the strongest innate immune response may be cleared more effectively. If this is the case, then it will always be challenging to link the aggregate to downstream pathology through cross-sectional analyses.
Step 3: chronic inflammation and senescence are mutually reinforcing states
Histopathological, biochemical and molecular studies unambiguously show that the AD brain is subject to a chronic inflammatory condition [38]. The widespread gliosis and increased levels of numerous inflammatory factors, including, but not-limited to, chemokines, cytokines, and acute phase reactants, in the absence of overt lymphocytic or mononuclear infiltrates is consistent with inflammation resulting from innate immune activation and not adaptive immune responses. In AD, the inflammatory changes are noted in the earliest stages of the disease process and have also been shown to be early events in some of the AD mouse models of amyloid and tau pathology. As noted above, in some cases the earliest pathology noted is microglial activation and increased levels of select cytokines [38,48-51]. Though often more focal in nature, inflammation is a hallmark of other CNS proteinopathies and is often seen as an early change in mouse models of these diseases [57].
Recent studies have revealed a remarkable connection between inflammatory mediators and replicative senescence [58-64]. These studies demonstrate that a hallmark of replicatively senescent cells is a massive increase in the secretion of multiple pro-inflammatory proteins, including IL-6, IL-8 (CXCL8), IL-1α and β and monocyte chemoattractant peptide-1 (MCP-1, CCL2) [60,63]. In certain cases it has been shown that these secreted proteins can act in an autocrine manner to further maintain the senescent state, drive senescence of neighboring cells in a paracrine fashion, and promote degenerative or proliferative changes in neighboring cells [58,60]. It has also been shown that key inflammatory pathways, including those mediated by IL-6 and CXCR2 ligands, are not only upregulated by senescence but may play a critical role in inducing and maintaining senescence [59,62-64]. Notably IL-6 and MCP-1 are markedly upregulated in AD, as are CXCR2 receptors [38,65-71]. Finally, it is well-established that oxidative stress, which almost invariably accompanies chronic inflammation, can also induce senescence [72-74]. Oxidative stress can also arise independently of inflammatory pathways in CNS proteinopathies [75]. Extracellular Aβ has been reported to directly cause oxidative stress through production of reactive oxygen species [76,77]. Oxidative stress arising from mitochondrial dysfunction has also been reported to be associated with numerous neurodegenerative proteinopathies [75]. Thus, senescence appears to be associated with an induction of a pro-inflammatory state, but can also result from an inflammatory state. Moreover, inflammatory mediators and oxidative stress can synergistically act to drive senescence.
Step 4: senescence and neurodegeneration
Senescence has largely been studied in the context of dividing cells, a phenomenon more specifically referred to as replicative senescence. Replicative senescence was first described by Hayflick, and the 'Hayflick limit' refers to the limited replicative capacity of primary human fibroblasts or other diploid cell lines to prolonged passaging in tissue culture [78]. Typically, such senescence results in cells with altered morphology (large, flattened cells with high cytoplasm to nucleus ratios), telomere shortening or telomerase malfunction, distinct senescence-associated hetrochromatic foci, and increased expression of a panel of senescence-associated biomarkers (for example, senescence-associated β-galactosidase activity, INK4A, IL-6) [58,60,79]. A functional definition is that a senescent cell has lost its replicative capacity and is no longer able to respond to growth factors. Though replicative senescence has been postulated to be a key driver of human aging, it is more likely that replicative senescence is simply one of many factors that contribute to the aging process.
To date there has been very little study of senescence of neurons or even glia cells. As neurons are terminally differentiated, it is immediately obvious that one of the critical hallmarks of replicative senescence, inability to divide, does not apply. Some would claim that because they are terminally differentiated, neurons cannot senesce [80]. However, we postulate that neurons do undergo physiological senescence and that this senescence is accelerated in AD and other CNS proteinopathies by inflammatory and oxidative stimuli. Moreover, we postulate that a senescent neuron will be defined functionally by its inability to respond appropriately to growth factors and its expression of senescence-associated proteins. In this scenario, other CNS cells, including glia, neuroglial stem cells, and endothelial and smooth muscle cells that form the cerebrovasculature, are also likely to undergo senescent changes in response to the chronic inflammatory environment. Senescent astrocytes might switch from a neuroprotective phenotype to one that is less suitable for supporting neuronal homeostasis. Senescence of microglia cells has been proposed as a mechanism for 'aging' microglia, less efficient scavenger cells with diminished phagocytic capacity and enhanced neurotoxic potential [81,82]. Put simply, we postulate that CNS cells will display a senescent phenotype that is physiologically similar to cells that have undergone replicative senescence, and be functionally impaired in a way that leads to neuronal dysfunction and degeneration. Ultimately, a growing number of senescent cells will lead to either widespread brain 'organ failure' as exhibited in AD, or more or less regional brain organ failure as seen in other neurodegenerative proteinopathies.
One of the key features of this hypothesis is that once triggered by the proteinopathy, senescent changes are likely to be both self-reinforcing and irreversible. In an autocrine fashion, inflammatory mediators secreted by the senescent neurons and glia would help to maintain the senescent state [60]. In a paracrine fashion, senescent cells induce additional inflammation and senescence of neighboring cells. Over long periods of time senescent neurons become increasingly dysfunctional and die due to a combination of diminished response to growth factors and possible pathophysiological effects of chronic exposure to an altered milieu of signaling factors as well as the direct signaling effects of the protein aggregates. Senescent changes can also affect neuronal stem cells, leading to diminished potential for renewal of neurons.
It is likely that the senescence response is not an 'all or none' phenomenon. There may be a graded continuum of responses to a proteinopathy-induced stress that depends both on the strength and acuteness of the stress as well as the preprogrammed response of the cell. Experimental data demonstrate that cells with high levels of anti-apoptotic proteins often undergo senescence whereas cells with lower levels of anti-apoptotic factors seem prone to undergo apoptosis [83-85]. Mature neurons, which are known to express high levels of anti-apoptotic proteins, may respond to potentially apoptotic stresses by senescing. At least in culture, a lower level of oxidative stress can drive senescence whereas a higher level can drive apoptosis. The notion that cellular 'stress', depending on the context, can result in two different endpoints, either apoptosis or senescence, may help to explain the disparate endpoints observed in various neurodegenerative proteinopathy models. In models where the cells are 'primed' to undergo apoptosis, a proteinopathy-driven stress will more likely drive apoptosis. In models where the proteinopathy is overwhelming, apoptosis may also be the primary endpoint. If the proteinopathy develops more insidiously, senescence may result.
Of course there is extensive neuronal loss in AD. So how would a senescent cell die? Senescent cells are stable for some period of time, and little information has been published on how they die. As noted above, in vitro studies suggest that senescence is an alternative pathway to apoptosis and that senescent cells are resistant to apoptosis. Of note, a recent study has shown that senescent keratinocytes die by autophagic cell death, a cell death pathway characterized by an increase in macroautophagic activity [86]. In many neurodegenerative diseases auto-phagic cell death has been implicated as an alternative to apoptotic or necrotic mechanisms [45,87].
A more speculative extension of this hypothesis in AD is that the senescent phenotype could be the key link between Aβ proteinopathy and secondary proteinopathies that are seen in the AD brain, including those involving tau, α-synuclein and TDP-43. At least for tau and α-synuclein there is experimental evidence that an Aβ proteinopathy can enhance, if not trigger, a tauopathy or synucleinopathy. Despite intense investigation, there is no consensus regarding the pathways that relay the signals between Aβ and tau, synuclein, or TDP-43. Although it is possible that we simply have not identified the single critical factor, it is perhaps more likely that Aβ proteinopathy induces a plethora of changes that drive the secondary proteinopathies. Given that senescence triggers gross changes in the transcriptome and secretome, perhaps senescent changes mediate the secondary proteinopathy?
Future studies: how do we prove that the AD brain is senescing?
Recent studies of the senescence-associated secretory phenotype (SASP), also termed the senescence-messaging secretome (SMS), have identified a number of secreted biomarkers associated with replicative senescence [58,60,63]. Depending on the cell type examined, the method of induction of senescence, and the methodology used to identify the secreted proteins, the secretome and transcriptome that defines the SASP/SMS can be variable. However, certain proteins are invariably identified in these studies. Many of the proteins consistently upregulated during senescence are inflammatory mediators, including IL-6, IL-8 and other chemokines and cytokines [58,60,63]. Though not a perfect correlation, there is extensive overlap between the biomarkers that comprise the core SASP/SMS phenotype and secreted biomarkers of AD (Table 1). This overlap provides evidence for a SASP/SMS in the AD brain, with many of these features seen in mouse models of AD. At least in AD, it has been challenging to define the nature of the 'immune-system dysregulation' based on pathway-type analyses; the overlap between the SAPS/SMS and biomarkers of AD suggest that the 'immune-system dysregulation' may, in fact, reflect senescence [71].
Table 1.
Senescence-associated secretory phenotype/senescence messaging secretome biomarkers in Alzheimer's disease
| Protein | Alteration(s) in ADa | Association with senescence |
|---|---|---|
| IL-6 | ↑ in B, C, P [65,71,120] | ↑ in oncogene-induced senescence (OIS); mediates the SASP in vitro; knockdown results in senescence bypass [59,63] |
| IL-8 (CXCL8) and other CXCR2 ligands/CXCR2 | ↑ IL-8 in B, C, P, focal ↑ CXCR2 in B in plaque-associated dystrophic neurites [66,68,69] | ↑ IL-8 and other CXCR2 ligands in multiple in vitro models of replicative senescence; CXCR2 signaling functionally implicated in replicative senescence [59,63,64] |
| MCP-1 | ↑ in B [65] | ↑ in vitro in multiple models of replicative senescence |
| IL1-α | ↓ in P [71], ∝ P [121,122], ↑ mRNA in B [123] | Implicated in endothelial cell senescence [124,125] |
| ICAM-1 | ↑ in B, P [71,126-128] | ↑ in vitro in replicative senescence [63] |
| IGFBP | ↑ IGFPB6 in P [71], ↑ IGFPB2 and 6 in C [129] | Various IGFBP ↑ in replicative senescence; IGFBP sufficient and required for replicative senescence in various models [130-133] |
| GM-CSF | ≅ to ↑ in C [134,135] | ↑ in vitro in replicative senescence [63] |
| Osteoprotegerin | ↑ in P [136] | ↑ in vitro in replicative senescence [63] |
| PAI-1 | CNS homolog neuroserpin ↑ in B [137] | ↑ in vitro in models of replicative senescence and critical for induction [138] |
| TGF-β | ↑ in B, C, P [38,139] | ↑ in vitro in multiple models of replicative senescence; implicated in inducing replicative senescence [140,141] |
| WNT2 | Wnt pathway implicated in pathogenic signaling cascades in AD. No rigorous biomarker studies. Aβ implicated as blocking Wnt signaling [142] | ↓ WNT2 in replicative senescence and OIS [143] |
| sPLA2/sPLA2R | ↑ group IV isoform of phospholipase A(2) in B [144] | ↑ sPLA2/sPLA2R in replicative senescence; sPLA2 (PLA2G2A) can induce senescence in vitro [145] |
| IGF-1 | Some reports indicate ↑ in AD brain [146] | Linked to life-span extension [58,60] |
| MMPs | Various MMPs ↑ in B and P [147] | MMP3 ↑ associated with replicative senescence [148] |
aB, brain; C, cerebrospinal fluid; P, plasma. Up and down arrows indicate increased and decreased levels, respectively. AD, Alzheimer's disease; CNS, central nervous system; GM-CSF, granulocyte-macrophage colony stimulating factor; ICAM, intracellular adhesion molecule; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; IL = interleukin; MCP, monocyte chemoattractant peptide; MMP, matrix metalloproteinase; OIS, oncogene-induced senescence; PAI, plasminogen activator inhibitor; sPLA2, soluble phospholipase A2; SASP, senescence-associated secretory phenotype; sPLA2R, soluble phospholipase A2 receptor; TGF, transforming growth factor.
Given the large number of inflammatory proteins that have been implicated in the SASP/SMS, one of the challenges in moving forward is to discriminate between 'classic' reactive neuroinflammation and senescence in the diseased brain. If one uses replicative senescence as a guide, the key observations that would distinguish a senescence from an inflammatory phenotype are: that inflammatory mediators are expressed by cells such as neurons that do not normally produce them; that neurons and other cells in the AD brain exhibit cytoplasmic and nuclear markers of senescence; and that the cells displaying these markers of senescence are both functionally impaired and exhibit a SASP/SMS phenotype. The links between senescence and inflammation could also be evaluated through a number of experimental paradigms. For example, does overexpression of inflammatory factors, such as IL-6 in the brain, drive senescent markers in CNS cells? Does forced expression of classic inducers of senescence, such as P53 or INK4A, in adult neurons drive senescence and inflammation?
Though clearly there is much work to be done to move this hypothesis forward, there is sufficient evidence in the literature to make the case for further study. In the AD brain neurons strongly stain for MCP-1 and IL-6, suggesting that these inflammatory mediators are being 'ectopically expressed', or at least dramatically upregulated, in cells that are not professional immune cells [65]. In addition, neurons stain and can be shown to actually express the mRNA for a number of other secreted inflammatory proteins [37,38]. Notably, some of the neuronal expression of inflammatory markers can be seen in mouse models of AD and other neurodegenerative disorders [48,88]. Cytoplasmic or nuclear protein markers associated with senescent cells are often cell-cycle proteins, tumor suppressors, or cell-cycle regulators [80]. In AD and other neurodegenerative diseases there is often marked upregulation of cyclins, p53 and related proteins, and cyclin-dependent kinase inhibitors that have also been implicated in the senescent phenotype (Table 2) [89-91]. Often these markers are upregulated in tangle-bearing neurons. Notably, there are no reports about the presence of a widely used 'biomarker' of senescence, senescence associated β-galactosidase activity, in AD or any other neurodegenerative condition [92].
Table 2.
Cytoplasmic and nuclear protein biomarkers of senescence in Alzheimer's disease
| Protein | Alteration(s) in AD | Association with senescence |
|---|---|---|
| p53 | ↑ in neurons, astrocytes [149,150] | Constitutively active p53 can induce senescence [151,152] |
| INK4A (p16) | ↑ in neurons with NFT [153,154] | Activated in senescence [155,156] |
| Senescence associated-β-galactosidasea | Not examined | Classic widely accepted biomarker of senescence [92] |
| Cylcins D, E | ↑ in neurons [157] | ↑ in endothelial cells and fibroblasts during senescence [158,159] |
aSenescence associated-β-galactosidase is β-galactosidase activity detected at pH 6.0. AD, Alzheimer's disease; NFT, neurofibrillary tangle.
Changes in telomere length, telomere activity, and the presence of distinct heterochromatic nuclear bodies (called senescence-associated heterochromatic foci) are also well-established markers of replicative senescence [80,93]. However, given the terminally differentiated state of neurons, it is unclear whether these markers would be expected to be seen in physiological senescence of neurons in the AD brain, or even in any of the CNS cells. For example, even senescence-associated heterochromatic foci are typically only seen in human cells and have been much more difficult to demonstrate in mouse cells [94,95]. Furthermore, mouse cells can undergo replicative senescence without shortened telomeres [80]. Thus, it is clear that the nuclear biomarkers of human replicative senescence may not apply to studies of CNS senescence.
Autophagy represents an additional potential link between senescence and neurodegeneration. Altered autophagy has been implicated in AD and many other neurodegenerative conditions [45,96]. Genetic removal of genes involved in autophagy results in neurodegeneration [97,98]. More generally, autophagy plays a key role in organismal aging and lifespan [99,100]. Genetic alterations that induce premature aging phenotypes are associated with autophagy induction. Autophagosomes accumulate in senescent fibroblasts [101]. In addition, it also has been shown that autophagy is an effector mechanism of replicative senescence [102]. It is activated during senescence, as are a subset of autophagy-related genes, and inhibition of autophagy delays oncogene-induced replicative senescence [103,104]. Finally, it has recently been postulated that autophagy is a key mechanism in immune responses to intracellular pathogens [105].
Alternative theories
The main working hypothesis in AD and other CNS proteinopathies has been that some species of the aggregated misfolded protein are directly neurotoxic [1-4,106]. There have been many variations on the theme of the presumptive neurotoxic protein aggregate. In primary neuronal culture systems it has been possible to reproducibly demonstrate that a variety of protein aggregates cause some form of 'neurotoxicity'. For example, Aβ aggregates ranging from oligomers (dimers, trimers, tetramers, dodecamers to 50-to 100-mers), soluble protofibrils, actively growing fibrils, to mature fibrils have been implicated as potential pathological entities in AD [3,107-114]. Despite this intense focus on finding the exact assembly that is the real 'neurotoxin', there is little, if any, consensus in the field regarding this issue, in large measure because it has been difficult to unequivocally demonstrate that some of the proposed misfolded neurotoxins exist in vivo. Given the considerable body of data showing that protein aggregates can be directly neurotoxic by causing calcium influx, altering synaptic plasticity, impairing axonal transport and mitochondrial function, or altering other homeostatic functions in the cell, we believe that direct neurotoxic effects of protein aggregates probably do play some role in AD and other neurodegenerative diseases. However, we would argue that a slow degenerative phenotype is hard to reconcile with a direct toxic mechanism and that if indeed there were a 'smoking gun' aggregate that was directly neurotoxic, that it would likely show a much better and more consistent correlation with disease or disease progression than do any of the current aggregates.
Organisms have developed many ways to adapt to stressful stimuli. In the mature nervous system a key adaptive mechanism is to try to keep largely irreplaceable neurons alive. One of the most remarkable examples of this is that pathogenic viruses can be cleared from neurons by activation of the innate and adaptive immune systems in a non-cytolytic fashion [115]. In the periphery this immune activation would typically result in significant collateral damage and killing of the infected cells. As noted previously, there is some evidence that, in response to stress, a cell can either undergo apoptosis or senescence [104]. Given that mature neurons have many mechanisms to protect them from apoptosis, we might speculate that the same stress that induces apoptosis in a primary embryonic neuronal culture may induce senescence in the intact mature CNS.
Therapeutic implications
Replicative senescence does not appear to be easily reversible [80]. In the few examples where replicative senescence has been reversed in culture, the reversal typically requires inactivation or downregulation of tumor suppressors [116,117]. Thus, from a therapeutic point of view, reversal of a replicatively senescent phenotype poses serious problems as it increases the likelihood for tumorigenesis. Indeed, it is generally thought that replicative senescence is a mechanism designed to suppress tumorigenesis. If cells in the AD or other neurodegenerative disease brain are physiologically senescent, it may be very challenging to reverse the senescent phenotype. Of course, an enhanced understanding of physiological neuronal or glial senescence may reveal distinct differences between replicative senescence and the physiological senescence of these specialized cells. Understanding whether such differences are present may reveal new therapeutic approaches to treat many neurodegenerative diseases.
Recognizing that proteinopathy-induced inflammation may drive senescence, and thereby induce neurodegeneration, reinforces therapeutic efforts designed to prevent the formation of or clear the proteinopathy and also potentially reveals new pathways that could be the focus of therapeutic efforts. In the former case, the rationale is obvious - prevent the proteinopathy and the downstream cascade is prevented. Of course, as discussed in recent reviews, this type of therapy targeting the trigger of the disease is likely to be much more effective as primary prevention and may have little therapeutic benefit once degeneration is entrenched [5,118]. If some aspects of the degenerative cascade downstream of the proteinopathy are self-reinforcing and difficult to reverse, then therapeutics aimed at the initiator of the cascade will almost certainly have limited benefit when administered once these downstream cascades have begun. In the latter case, the notion that the inflammatory and senescent phenotypes may be mutually reinforcing responses that are capable of inducing pathological changes in a paracrine fashion establishes a new framework for understanding the interplay between chronic neuroinflammation and neuronal dysfunction. Further elucidation of an inflammatory senescence network may reveal multiple new targets for intervention. In particular, novel anti-inflammatory approaches designed to reduce the paracrine effects of the SASP/SMS may limit the spread of a neurodegenerative process, and thereby limit the collateral damage caused by a proteinopathy.
Summary: age, aging, senescence, and neurodegeneration
The major risk factor for developing AD and other neurodegenerative diseases is age. Because of this association many in the field have proposed that aging contributes to the risk for developing AD and other neurodegenerative diseases. Often the semantic distinction between age and aging is not well-defined even by those who use the terms. We use the term aging to refer to distinct biological processes that are altered as an organism grows older, and age will simply be used to denote time. Though it remains possible, and even likely, that aging does contribute to the risk of developing AD or other neurodegenerative conditions, genetic studies indicate that aging effects can be overcome. Essentially, the same neurodegenerative disease can be driven in a relatively young person by a genetic alteration that accelerates the induction of the proteinopathy. For example, when the polyglutamine expansion is large enough, Huntington's disease can occur in children [119].
As alluded to previously, replicative senescence has been implicated as a key component of the aging process. In the context of a neurodegenerative cascade we would propose that senescence of CNS cells is a reinforcing pathway downstream of a proteinopathy that can, in an autocrine and paracrine fashion, create an environment that results in aging of the brain. Indeed, senescence appears to reinforce both chronic inflammation and oxidative stress, two factors that are thought to play a key role in the aging process. This might explain why genetically driven early onset forms of AD and other neurodegenerative diseases mimic late onset sporadic forms of the disease.
The proteinopathy-induced senescent cell hypothesis of AD and neurodegenerative disease that we describe here provides a novel integrative intellectual framework for future studies of pathological cascades in AD and other neurodegenerative diseases. Such studies may broaden our understanding of the phenotype of senescing cells and also identify novel therapeutic targets for the treatment or prevention of AD and other neurodegenerative disorders.
Abbreviations
Aβ: amyloid β; AD: Alzheimer's disease; CNS: central nervous system; IL: interleukin; MAPT: microtubule associated protein tau; MCP: monocyte chemoattractant peptide; MHC: major histocompatibility complex; PAMP: pathogen-associated molecular pattern; PRR: pattern recognition receptor; SASP: senescence-associated secretory phenotype; SMS: senescence-messaging secretome; TI-2: thymus independent type 2.
Competing interests
TEG is a co-editor in chief of this journal, for which he receives an honorarium. He serves on the SAB for Alzheimer's disease for Élan Pharmaceuticals, and has received sponsored research support from Lundbeck and Myriad Genetics. He is an inventor on several patents related to gamma-secretase modulators and anti-Aβ immune therapy. VMM has no conflicts.
Contributor Information
Todd E Golde, Email: Golde.Todd@mayo.edu.
Victor M Miller, Email: miller.victor@mayo.edu.
Acknowledgements
This work was supported by grants from the NIA (AG18454, AG29866, AG25531) and NINDS (NS39072), the CART fund, and the Mayo Foundation for Medical Research.
References
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenga S. Conformational mutations in neuroserpin and familial dementias. Lancet. 2002;360:1696. doi: 10.1016/S0140-6736(02)11626-6. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Révész T, Holton J, Rostagno A, Lashley T, Houlden H, Gibb G, Anderton B, Bek T, Bojsen-Møller M, Wood N, Vidal R, Braendgaard H, Plant G, Frangione B. Chromosome 13 dementia syndromes as models of neurodegeneration. Amyloid. 2001;8:277–284. doi: 10.3109/13506120108993826. [DOI] [PubMed] [Google Scholar]
- Glenner GG. Amyloid deposits and amyloidosis. N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Hutton M. Molecular genetics of chromosome 17 tauopathies. Ann N Y Acad Sci. 2000;920:63–73. doi: 10.1111/j.1749-6632.2000.tb06906.x. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem. 2001;276:48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- McDermott MF, Tschopp J. From inflammasomes to fevers, crystals and hypertension: how basic research explains inflammatory diseases. Trends Mol Med. 2007;13:381–388. doi: 10.1016/j.molmed.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–397. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- Merwe PA van der, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. doi: 10.1111/j.0105-2896.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, Eisenberg D, Rivier J, Sawchenko P, Vale W, Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Giménez-Gallego G, Sánchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Gonzales V, Savonenko AV, Wen JC, Jenkins NA, Copeland NG, Younkin LH, Lester HA, Younkin SG, Borchelt DR. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med. 2005;2:e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, Holtzman DM. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005;115:428–433. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada CM, Garcia-Alloza M, Betensky RA, Zhang-Nunes SX, Greenberg SM, Bacskai BJ, Frosch MP. Antibody-mediated clearance of amyloid-beta peptide from cerebral amyloid angiopathy revealed by quantitative in vivo imaging. J Neurosci. 2007;27:1973–1980. doi: 10.1523/JNEUROSCI.5426-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitinproteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Venkatraman P, Wetzel R, Tanaka M, Nukina N, Goldberg AL. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol Cell. 2004;14:95–104. doi: 10.1016/s1097-2765(04)00151-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–8861. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Lansbury PT Jr, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT Jr. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Bitan G, Fradinger EA, Spring SM, Teplow DB. Neurotoxic protein oligomers - what you see is not always what you get. Amyloid. 2005;12:88–95. doi: 10.1080/13506120500106958. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I. et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Innate immunity, local inflammation, and degenerative disease. Sci Aging Knowledge Environ. 2002;2002:re3. doi: 10.1126/sageke.2002.29.re3. [DOI] [PubMed] [Google Scholar]
- Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Rozemuller JM, van Muiswinkel FL. Inflammation and Alzheimer's disease: relationships between pathogenic mechanisms and clinical expression. Exp Neurol. 1998;154:89–98. doi: 10.1006/exnr.1998.6920. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS Jr, Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007;6:352–361. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr. How the immune system recognizes invaders. Sci Am. 1993;269:72–79. doi: 10.1038/scientificamerican0993-72. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Lee IY, Yoo H, Gehlenborg N, Cho JH, Petritis B, Baxter D, Pitstick R, Young R, Spicer D, Price ND, Hohmann JG, Dearmond SJ, Carlson GA, Hood LE. A systems approach to prion disease. Mol Syst Biol. 2009;5:252. doi: 10.1038/msb.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Möller T, Tabrizi SJ. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Sahara N, Maeda S, Takashima A. Tau oligomerization: a role for tau aggregation intermediates linked to neurodegeneration. Curr Alzheimer Res. 2008;5:591–598. doi: 10.2174/156720508786898442. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–230. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, Horst CM van der, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Boysen M, Sun CH, Wong BJ, Kang MK, Park NH, Desprez PY, Campisi J, Krtolica A. A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol Cancer Res. 2008;6:1085–1098. doi: 10.1158/1541-7786.MCR-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte Chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2008;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Hyman BT. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer's disease? J Neuroimmunol. 2002;122:55–64. doi: 10.1016/s0165-5728(01)00463-5. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Hyman BT. Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- Xia M, Qin S, McNamara M, Mackay C, Hyman BT. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer's disease. Am J Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corrà B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40:1277–1283. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- Bush AI, Tanzi RE. The galvanization of beta-amyloid in Alzheimer's disease. Proc Natl Acad Sci USA. 2002;99:7317–7319. doi: 10.1073/pnas.122249699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65:1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Campisi J, Dimri GP, Nehlin JO, Testori A, Yoshimoto K. Coming of age in culture. Exp Gerontol. 1996;31:7–12. doi: 10.1016/0531-5565(95)02024-1. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Nelyudova A, Aksenov N, Pospelov V, Pospelova T. By blocking apoptosis, Bcl-2 in p38-dependent manner promotes cell cycle arrest and accelerated senescence after DNA damage and serum withdrawal. Cell Cycle. 2007;6:2171–2177. doi: 10.4161/cc.6.17.4610. [DOI] [PubMed] [Google Scholar]
- Tombor B, Rundell K, Oltvai ZN. Bcl-2 promotes premature senescence induced by oncogenic Ras. Biochem Biophys Res Commun. 2003;303:800–807. doi: 10.1016/s0006-291x(03)00402-9. [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- Gosselin K, Deruy E, Martien S, Vercamer C, Bouali F, Dujardin T, Slomianny C, Houel-Renault L, Chelli F, De Launoit Y, Abbadie C. Senescent keratinocytes die by autophagic programmed cell death. Am J Pathol. 2009;174:423–435. doi: 10.2353/ajpath.2009.080332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- Owens T, Wekerle H, Antel J. Genetic models for CNS inflammation. Nat Med. 2001;7:161–166. doi: 10.1038/84603. [DOI] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer's disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberham U, Arendt T. The expression of cell cycle proteins in neurons and its relevance for Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2005;4:293–306. doi: 10.2174/1568007054038175. [DOI] [PubMed] [Google Scholar]
- Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Adams PD. Remodeling chromatin for senescence. Aging Cell. 2007;6:425–427. doi: 10.1111/j.1474-9726.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Adams PD. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397:84–93. doi: 10.1016/j.gene.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Vicencio JM, Kepp O, Joza N, Tajeddine N, Kroemer G. Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans. 2008;36:786–790. doi: 10.1042/BST0360786. [DOI] [PubMed] [Google Scholar]
- Gerland LM, Peyrol S, Lallemand C, Branche R, Magaud JP, Ffrench M. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp Gerontol. 2003;38:887–895. doi: 10.1016/s0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavaré S, Arakawa S, Shimizu S, Watt FM, Narita M. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med. 2008;8:78–91. doi: 10.2174/156652408783769616. [DOI] [PubMed] [Google Scholar]
- Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero S, Kroemer G. Senescence, apoptosis or autophagy? When a damaged cell must decide its path - a mini-review. Gerontology. 2008;54:92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26:523–528. doi: 10.1016/j.it.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Yankner BA. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Glabe CC. Amyloid accumulation and pathogensis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem. 2005;38:167–177. doi: 10.1007/0-387-23226-5_8. [DOI] [PubMed] [Google Scholar]
- Tanzi RE. The synaptic Abeta hypothesis of Alzheimer disease. Nat Neurosci. 2005;8:977–979. doi: 10.1038/nn0805-977. [DOI] [PubMed] [Google Scholar]
- Klein WL, Stine WB Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder GK, Griffin DE. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 2003;5:439–448. doi: 10.1016/s1286-4579(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac AM, Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J Biol Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- Golde TE. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol Neurodegener. 2009;4:8. doi: 10.1186/1750-1326-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FO. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Franco-Maside A, Alvarez XA. Interleukin-1 in Alzheimer's disease and multi-infarct dementia: neuropsychological correlations. Methods Find Exp Clin Pharmacol. 1991;13:703–708. [PubMed] [Google Scholar]
- Cacabelos R, Barquero M, Garcia P, Alvarez XA, Varela de Seijas E. Cerebrospinal fluid interleukin-1 beta (IL-1 beta) in Alzheimer's disease and neurological disorders. Methods Find Exp Clin Pharmacol. 1991;13:455–458. [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the lifespan of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Hsu MY, Sorger T, Herlyn M, Levine EM. Heparin/endothelial cell growth supplement regulates matrix gene expression and prolongs life span of vascular smooth muscle cells through modulation of interleukin-1. In Vitro Cell Dev Biol Anim. 1999;35:647–654. doi: 10.1007/s11626-999-0105-6. [DOI] [PubMed] [Google Scholar]
- Rentzos M, Michalopoulou M, Nikolaou C, Cambouri C, Rombos A, Dimitrakopoulos A, Kapaki E, Vassilopoulos D. Serum levels of soluble intercellular adhesion molecule-1 and soluble endothelial leukocyte adhesion molecule-1 in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2004;17:225–231. doi: 10.1177/0891988704269822. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Differential expression of intercellular adhesion molecule-1 (ICAM-1) in the A beta-containing lesions in brains of patients with dementia of the Alzheimer type. Acta Neuropathol. 1996;91:608–615. doi: 10.1007/s004010050474. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Westphal JR, Wesseling P, Ruiter DJ, de Waal RM. Accumulation of intercellular adhesion molecule-1 in senile plaques in brain tissue of patients with Alzheimer's disease. Am J Pathol. 1994;144:104–116. [PMC free article] [PubMed] [Google Scholar]
- Tham A, Nordberg A, Grissom FE, Carlsson-Skwirut C, Viitanen M, Sara VR. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect. 1993;5:165–176. doi: 10.1007/BF02257671. [DOI] [PubMed] [Google Scholar]
- Kim KS, Seu YB, Baek SH, Kim MJ, Kim KJ, Kim JH, Kim JR. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Kim MS, Seu YB, Chung HY, Kim JH, Kim JR. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim WY, Kim JH, Younes MN, El-Naggar AK, Myers JN, Kies M, Cohen P, Khuri F, Hong WK, Lee HY. Identification of insulin-like growth factor binding protein-3 as a farnesyl transferase inhibitor SCH66336-induced negative regulator of angiogenesis in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:653–661. doi: 10.1158/1078-0432.CCR-05-1725. [DOI] [PubMed] [Google Scholar]
- Sprenger CC, Vail ME, Evans K, Simurdak J, Plymate SR. Over-expression of insulin-like growth factor binding protein-related protein-1(IGFBP-rP1/mac25) in the M12 prostate cancer cell line alters tumor growth by a delay in G1 and cyclin A associated apoptosis. Oncogene. 2002;21:140–147. doi: 10.1038/sj.onc.1205021. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Wallin A, Regland B, Blennow K, Tarkowski A. Local and systemic GM-CSF increase in Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:166–174. doi: 10.1034/j.1600-0404.2001.103003166.x. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Peros E, Scioli GA, D'Angelo A, Olivieri C, Montagna L, Geroldi D. Plasma osteoprotegerin as a biochemical marker for vascular dementia and Alzheimer's disease. Int J Mol Med. 2004;13:849–853. [PubMed] [Google Scholar]
- Fabbro S, Seeds NW. Plasminogen activator activity is inhibited while neuroserpin is up-regulated in the Alzheimer disease brain. J Neurochem. 2009;109:303–315. doi: 10.1111/j.1471-4159.2009.05894.x. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer's disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- Tremain R, Marko M, Kinnimulki V, Ueno H, Bottinger E, Glick A. Defects in TGF-beta signaling overcome senescence of mouse keratinocytes expressing v-Ha-ras. Oncogene. 2000;19:1698–1709. doi: 10.1038/sj.onc.1203471. [DOI] [PubMed] [Google Scholar]
- Glick AB, Lee MM, Darwiche N, Kulkarni AB, Karlsson S, Yuspa SH. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 1994;8:2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer's disease: up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Ye X, Zerlanko B, Kennedy A, Banumathy G, Zhang R, Adams PD. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol Cell. 2007;27:183–196. doi: 10.1016/j.molcel.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cissé M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augert A, Payre C, de Launoit Y, Gil J, Lambeau G, Bernard D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10:271–277. doi: 10.1038/embor.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2008. [DOI] [PubMed]
- Baig S, Kehoe PG, Love S. MMP-2, -3 and -9 levels and activity are not related to Abeta load in the frontal cortex in Alzheimer's disease. Neuropathol Appl Neurobiol. 2008;34:205–215. doi: 10.1111/j.1365-2990.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. Faseb J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Kamoshima W, Matsuoka Y, Nomura Y, Taniguchi T. Changes of p53 in the brains of patients with Alzheimer's disease. Biochem Biophys Res Commun. 1997;232:418–421. doi: 10.1006/bbrc.1997.6301. [DOI] [PubMed] [Google Scholar]
- Itahana K, Dimri G, Campisi J. Regulation of cellular senescence by p53. Eur J Biochem. 2001;268:2784–2791. doi: 10.1046/j.1432-1327.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- Yaswen P, Campisi J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell. 2007;128:233–234. doi: 10.1016/j.cell.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease. Neuroreport. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- McShea A, Harris PL, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. Am J Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- Collins CJ, Sedivy JM. Involvement of the INK4a/Arf gene locus in senescence. Aging Cell. 2003;2:145–150. doi: 10.1046/j.1474-9728.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami-Kobayashi J, Mitsui Y. Cyclin D1 inhibits cell proliferation through binding to PCNA and cdk2. Exp Cell Res. 1999;246:338–347. doi: 10.1006/excr.1998.4306. [DOI] [PubMed] [Google Scholar]
- Lucibello FC, Sewing A, Brusselbach S, Burger C, Muller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993;105:123–133. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]