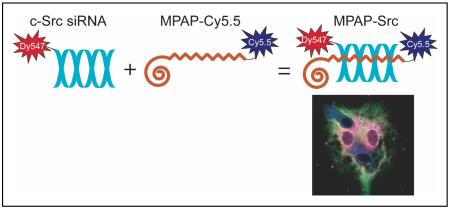
Synopsis
We report the generation and characterization of a peptide-based siRNA probe for ischemic stroke treatment. Complexing siRNA with myristoylated polyarginine peptides promoted probe uptake by primary and transformed cells and protection from serum nucleases. Application of siRNA probe directed against c-Src, a protein implicated in stroke pathology, led to a reduction of endogenous c-src mRNA in all cell types tested. Results suggest the value of peptide-siRNA probes as tools for dual imaging and therapeutic applications.
RNA interference (RNAi) is a sequence-specific gene silencing technique that has been applied to multiple pathological conditions. In this report, we describe the generation and in vitro characterization of an RNAi-based fluorescent probe for use as a therapeutic in the setting of ischemic stroke. Probe delivery to bEnd.3 brain endothelial cells and primary cortical neurons and astrocytes was promoted by incorporating small interfering RNA (siRNA) into complexes with fluorescently labeled myristoylated polyarginine peptides. The resulting probe was partially protected from serum nuclease degradation and was efficiently internalized by cells as confirmed by flow cytometry and confocal microscopy. In addition, application of siRNA probe directed against c-Src, a protein implicated in stroke pathology, led to statistically significant reduction of endogenous c-src mRNA levels in all cell types tested. Results demonstrate the proof-of-principle that functionalized peptide-siRNA probes can be used as potential tools for dual imaging and therapeutic applications.
INTRODUCTION
In the past decade, RNA interference (RNAi) technology has been rapidly integrated into basic biomedical research, emerging as a premier method for regulating gene expression in a variety of model systems. As RNAi-based therapies progress to clinical trials and are applied to increasingly complex human diseases, the need for more robust strategies for small RNA delivery and monitoring becomes critical (1). These considerations are particularly relevant in neurological pathologies, for which an intact blood brain barrier (BBB) opposes direct contact between diseased cells and most systemically administered agents. One approach for overcoming biological membranes exploits naturally-occurring membrane translocation peptides (2, 3) known as cell penetrating peptides (CPPs), cationic peptides that have been adapted to accommodate diverse molecular cargoes, including proteins (4), liposomes (5), and nanoparticles (6). Recently it was demonstrated that intravenously injected small interfering RNA (siRNA), when used in conjunction with a peptide sequence from rabies virus glycoprotein, is delivered to the brain and retains its gene silencing capabilities (7).
In the present study we utilized optically labeled poly-arginine peptides modified with a 14-carbon myristic acid moiety (MPAP) for siRNA delivery. It has been shown previously that myristoylation increases the affinity of the peptide for lipid bilayer membranes, enhancing peptide entry into cells by several-fold relative to Tat and unmodified poly-arginine cell penetrating peptides (8). This peptide was used by us to promote cellular delivery of nanoparticle imaging agents to solid tumors after intravenous injection(9, 10). Furthermore, MPAP is BBB permeable in vivo, where it was shown to localize to neurons, astrocytes and endothelial cells after intravascular administration (11). These properties make MPAP ideally suited for use with siRNA to form a dual optical imaging and therapeutic probe for neurological applications, provided that a method of complexation is developed that does not interfere with the bioactivity of siRNA molecules.
This work details the generation and in vitro testing of an MPAP and siRNA (MPAP-siRNA) imaging probe complex for the treatment of ischemic stroke. In the setting of ischemic stroke, disruption of blood flow to the brain induces a cascade of pathological cell signaling events, culminating in widespread death of neurons and supporting cells (12). We hypothesize that if siRNA directed against proteins implicated in stroke pathology were effectively delivered to primary neurons and astrocytes to intercede in this process, neuroprotection could be elicited. With this in mind, we developed dual MPAP-siRNA optical imaging probes directed against c-Src, a protein tyrosine kinase that becomes upregulated after stroke and is associated with increased tissue damage in cerebral ischemia models (13, 14). Probes were assembled by utilizing the natural affinity of cationic peptides for oligonucleotides, a process that was optimized to ensure maximum siRNA incorporation. MPAP-siRNA complexes were tested for stability and their internalization in primary cells and an endothelial cell line was monitored by flow cytometry and confocal microscopy. The probes were also evaluated for their cytotoxic effects and siRNA silencing efficiency. Results confirm that MPAP-siRNA complexes become internalized by cells and cause statistically significant reductions in endogenous c-src levels without significantly effecting cell viability, paving the way for the future studies of the dual imaging and therapeutic probe in vivo.
EXPERIMENTAL PROCEDURES
Myristoylated polyarginine peptide (MPAP), C14-βAla-(Arg)7-Cys(SH)-NH2, was synthesized as described previously (11) and conjugated to Cy5.5 fluorescent dye (Amersham, Piscataway, NJ) to form MPAP-Cy5.5. To promote probe complex formation, MPAP-Cy5.5 was mixed through gentle aspiration with a solution of siRNA conjugated to Dy547 (siRNA-Dy547), a fluorescent tag with absorbance/emission max of 548/562nm. The resulting probe mixture was incubated for 20 minutes at room temperature to induce complete complexation of the two components.
To optimize stoichiometry of MPAP-Cy5.5 and siRNA-Dy547 complexes, we performed polyacrylamide gel electrophoresis. Various dilutions of MPAP-Cy5.5 solution were added to siRNA-Dy547 (20 pmol) to obtain 1:10, 1:5, 1:2.5, 1:1, 2.5:1, 5:1 and 10:1 siRNA-to-peptide molar ratios. Samples were run on polyacrylamide gels and stained with ethidium bromide for visualization of uncomplexed siRNA.
Solutions of free siRNA-Dy547 or complexes of siRNA-Dy547 and MPAP-Cy5.5, hereafter termed MPAP-Src, were incubated in mouse serum to evaluate the protective effect of MPAP-Cy5.5 on nuclease digestion of siRNA. MPAP-Cy5.5 and c-Src siRNA were combined at a 1:5 siRNA-to-peptide molar ratio to form MPAP-Src. MPAP-Src was incubated in fresh mouse serum for 0, 0.5, 1, 2, 4, or 8 hours. The amount of siRNA spared from nuclease digestion was determined by gel electrophoresis, SYBR Gold (Molecular Probes, Invitrogen, Eugene OR) staining, and quantification using ImageJ software (NIH).
Cortical neuron cultures were generated from embryonic day 18 C57BL/6 mouse fetuses (see Supporting Information). Cell culture of cortical astrocytes was performed using a slight modification of the procedure for neurons, with cortices dissected from C57BL/6 mouse pups, postnatal day 1-2 (see Supporting Information). A brain endothelial cell line, bEnd.3, was obtained from American Type Culture Collection (Manassas, VA) and maintained according to the vendor’s instructions (see Supporting Information). To promote probe internalization by cells, MPAP-Src solutions were added to primary astrocytes, primary neurons, or bEnd.3 cells plated in 12-well plates in serum-free media and incubated for 24 hours. To determine the cytotoxicity of probe incubation, cells exposed to MPAP-Src for 48 hours or untreated cells were processed using MTT and CASP-3-C (Sigma, St. Louis, MO) caspase-3 assays (see Supporting Information).
The extent of probe internalization by cells was investigated using FACS analysis and confocal microscopy (see Supporting Information). Confocal microscopy was also used in conjunction with LysoSensor Green staining of living cells to evaluate the mechanism of MPAP-Src uptake. To assess the effect of MPAP-Src internalization, total RNA was purified from cells treated with MPAP-Src or scrambled control siRNA probes (MPAP-ctrl) and subjected to real-time quantitative RT-PCR (TaqMan) to determine relative levels of c-src mRNA.
RESULTS AND DISCUSSION
MPAP has proven effective as a membrane translocation moiety when covalently linked to optical probes and iron oxide nanoparticles (9, 10). In this report we wished to extend the use of optically labeled MPAP further, by taking advantage of the potential for its poly-arginine residues to electrostatically interact with negatively-charged siRNA molecules. To promote the formation of siRNA dual optical imaging probes, c-Src siRNA-Dy547 and MPAP-Cy5.5 were mixed in several molar ratios. The efficiency of MPAP binding to siRNA was determined by performing gel electrophoresis on the resulting MPAP-Src solutions to assess the proportion of free siRNA in each mixture. MPAP-Src complexes remained in the loading well during gel electrophoresis, while uncomplexed siRNA readily entered the gel and stained with ethidium bromide (Figure 1). In solutions containing a 1:5 siRNA-to-MPAP molar ratio or higher peptide content, the vast majority of siRNA was associated with MPAP. Full incorporation of siRNA into probes was deemed important to maximize siRNA internalization and silencing potential. Thus, the siRNA-to-peptide ratio at which no discernible siRNA remained unassociated with MPAP-Cy5.5, 1:5, was used for subsequent experiments.
Figure 1.

Gel mobility shift assay. MPAP-Cy5.5 was incubated with c-Src siRNA at several molar ratios to form MPAP-Src probes. At the 1:5 siRNA-to-MPAP ratio, most siRNA was incorporated into complexes.
Ultimate utility of MPAP-Src in vivo requires protection of siRNA from nucleases in the bloodstream. Generally, siRNAs are chemically modified to extend their serum half-life relative to naked siRNA, which becomes degraded and inactivated within minutes of blood exposure (15, 16). To investigate the stability of the siRNA incorporated into MPAP-Src, siRNA complexes and naked siRNA-Dy547 were exposed to serum for up to 8 hours. Following removal of serum proteins and MPAP with proteinase K (1mg/ml), intact siRNA was analyzed by gel electrophoresis and quantified (Figure 2). It was observed that the optical label Dy547 conjugated to c-Src siRNA partially protects it from degradation by nucleases, with just over 25% of the total siRNA retained after 8 hours. The presence of MPAP appears to confer an additional measure of protection, such that the amount of free siRNA after 8 hours of MPAP-Src incubation in serum was close to 55%. These results indicate that MPAP complexation has a stabilizing effect on c-Src siRNA.
Figure 2.

Serum Stability. Stability of MPAP-Src (open squares) in serum relative to Dy547-labeled c-Src siRNA alone (closed squares) was investigated by incubating probes in 75% mouse serum for 0-8 hours. MPAP complexation to siRNA confers partial protection from serum nucleases, yielding a higher percentage of intact siRNA after incubation in serum than siRNA alone (mean±s.d.) relative to undigested controls (*).
Internalization of MPAP-Src probes was evaluated in vitro in a variety of cell types implicated in ischemic stroke pathology, including primary cortical neurons, astrocytes and the bEnd.3 brain endothelial cell line. Cells incubated for 24 hours with MPAP-Src or MPAP associated with scrambled control siRNA (MPAP-ctrl) were subjected to flow cytometry and monitored for Cy5.5 and Dy547 fluorescence. Figure 3 shows significant signal from both siRNA and MPAP relative to untreated controls in all cell types. Internalization of MPAP-Src and MPAP-ctrl occurred in similar patterns, indicating that MPAP association with c-Src siRNA is not sequence dependent. Furthermore, flow cytometry scatter plots (data not shown) showed that roughly 46% of bEnd3 cells, 77% of neurons and 78% of astrocytes with MPAP uptake also contained c-Src siRNA, suggesting that siRNA did not dissociate from MPAP but formed a stable complex in solution.
Figure 3.

Flow cytometry. Astrocytes, neurons and bEnd.3 cells were subjected to MPAP-Src or MPAP-ctrl incubation for 24 hours, followed by flow cytometry. All cell types were positive for the presence of both MPAP-Cy5.5 and siRNA-Dy547.
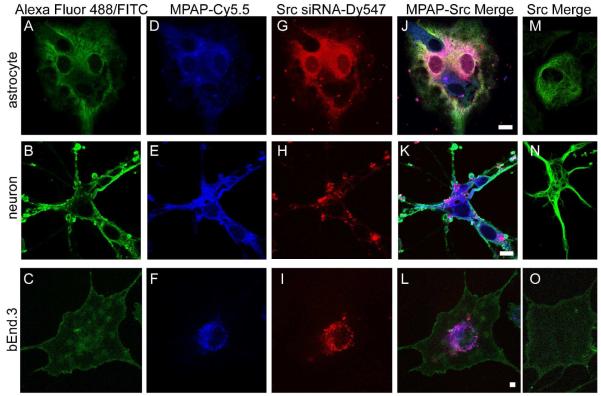
To confirm that MPAP-Src probe was internalized by cells, and not passively associated with the cell membrane, confocal microscopy was performed. Neurons, astrocytes and bEnd.3 cells stained with cell-specific antibodies (Figure 4A-C) were positive for the presence of both MPAP (Figure 4D-F) and c-Src siRNA (Figure 4G-I). In general, c-Src siRNA colocalized with MPAP (Figure 4J-L), though, for neurons in particular, there was evidence that MPAP and siRNA become dissociated to some degree when taken up by cells (Figure 4K). The presence of MPAP was crucial for internalization, as evident by the complete absence of c-Src siRNA uptake relative to MPAP-Src when cells were incubated with equivalent quantities of siRNA alone (Figure 4M-O).
Figure 4.
Confocal microscopy of astrocytes, neurons and bEnd.3 stained with GFAP (A), beta-III tubulin (B), and CD31 (C), respectively. Following incubation with MPAP-Src probe for 24 hours and cell-specific staining, cells were visualized by confocal microscopy. MPAP (D-F) and c-SRC siRNA (G-I) were taken up by cells following MPAP-Src incubation, whereas incubation with 100 pmoles of c-Src siRNA alone resulted in no significant siRNA uptake (M-O). Scale bar = 10 μm.
The mechanism of cellular internalization was investigated in unfixed astrocytes, bEnd.3 cells and neurons using Lysosensor Green staining that is specific for endosomal vesicles (Figure 5D-F). As in fixed cells, MPAP-Src substantially entered cells (Figure 5M-O). A significant portion of c-Src and MPAP signal appeared to originate from vesicles in all cell types, suggesting that a dominant mechanism of MPAP uptake is endocytosis. These findings prompt concerns that MPAP-Src becomes sequestered in vesicles where it would be unable access RNAi machinery to achieve successful c-Src silencing. To address this, expression levels of endogenous c-src mRNA were evaluated by RT-PCR following MPAP-Src uptake. Total c-src expression after MPAP-Src treatment was compared to expression after treatment with MPAP-ctrl, a control used to differentiate siRNA-induced c-src inhibition from off-target effects potentially caused by the MPAP method of siRNA delivery. Endogenous c-src levels were reduced to a statistically significant degree in all cell types subjected to MPAP-Src treatment for 48 hours relative to controls (Figure 6). For astrocytes and bEnd.3 cells, c-src expression decreased to approximately 66% of controls, while in neurons the decrease was to 75%. Similar reductions in c-src levels were achieved following 24-hour MPAP-Src treatment in astrocytes and bEnd.3 cells (data not shown). Thus, despite the observation that MPAP-Src appears to traffic to some extent in endosomal vesicles, the endosomal escape of siRNA and its separation from MPAP is sufficient to mediate a significant degree of endogenous gene inhibition. To determine if MPAP-Src mediated siRNA delivery was cytotoxic, MTT and caspase-3 assays were performed (Figure 7). MPAP-Src treatment resulted in no discernable increase in cytotoxicity in either astrocytes or bEnd.3 cells relative to untreated controls as assessed by MTT and caspase-3 analysis. Likewise, MTT analysis showed no relative decrease in viability in neurons. Although caspase-3 levels in neurons appeared elevated in neurons, suggesting the presence of apoptosis following 48 hours of MPAP incubation, the effect was not statistically significant. These data indicate that MPAP-Src treatment induced partial endogenous gene silencing without the degree of cytotoxic effects that are often observed in other peptide-based delivery systems (17).
Figure 5.

Cells were visualized using confocal microscopy following Lysosensor Green staining of unfixed astrocytes, bEnd.3 cells and neurons (D-F) to determine the mechanism of cellular uptake of MPAP-Src. MPAP (G-I) and c-Src siRNA (M-O) generally co-localize with endosomal vesicles. Scale bar = 10 μm.
Figure 6.

Quantitative RT-PCR analysis of endogenous c-src mRNA expression levels (mean ±s.d., n = 7) in astrocytes, neurons and bEnd.3 cells treated with MPAP-ctrl (black bars) or MPAP-Src (gray bars) for 48 hours. After administration of MPAP-Src, there was a significant reduction in c-src levels in all cell types tested.
Figure 7.

A. The percent survival (mean±s.d.) of cells treated with MPAP-Src or staurosporine (gray and white bars, respectively) relative to untreated controls (black bars) was obtained by MTT analysis. MPAP-Src treatment resulted in no significant increase in cytotoxicity in any of the cell types. B. Caspase-3 activity (mean±s.d.) in cells treated with MPAP-Src (gray bars) presented relative to activity in untreated cells (black bars). Though slight increases in caspase-3 signal were apparent, particularly in neurons, these were not statistically significant in any of the cells tested.
This report details the characterization and in vitro testing of a fluorescent siRNA imaging probe for application to ischemic stroke. MPAP, an amphipathic membrane translocation peptide derivative capable of crossing the blood-brain barrier, was shown to form stable complexes with siRNA and mediate cell entry in primary neurons and astrocytes and an endothelial cell line without significant cytotoxicity. The internalized probe was biologically active, reducing endogenous levels of c-src mRNA that codes for a protein associated with detrimental outcome following ischemic stroke.
Previous studies have shown successful non-covalent complexation of siRNA to cell penetrating peptides for delivery to model and therapeutic targets (7, 18-20). Here we demonstrate the proof-of-principle that simple complexation techniques can be employed to produce a dual optical imaging and therapeutic probe. We intend to investigate the probe as a method of monitoring siRNA delivery and treatment of cerebral ischemia in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
This publication was made possible by grant T32 EB001680 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Footnotes
Supporting Information Available: Detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–33. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- (3).Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–50. [PubMed] [Google Scholar]
- (4).Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- (5).Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–7. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–4. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- (7).Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- (8).Pham W, Kircher MF, Weissleder R, Tung CH. Enhancing membrane permeability by fatty acylation of oligoarginine peptides. Chembiochem. 2004;5:1148–51. doi: 10.1002/cbic.200400063. [DOI] [PubMed] [Google Scholar]
- (9).Kumar M, Medarova Z, Pantazopoulos P, G D, A M. Novel membrane permeable contrast agent for brain tumor detection by MRI. Magn Reson Imaging. 2009 doi: 10.1002/mrm.22216. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- (11).Pham W, Zhao BQ, Lo EH, Medarova Z, Rosen B, Moore A. Crossing the blood-brain barrier: a potential application of myristoylated polyarginine for in vivo neuroimaging. Neuroimage. 2005;28:287–92. doi: 10.1016/j.neuroimage.2005.06.007. [DOI] [PubMed] [Google Scholar]
- (12).Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- (13).Korematsu K, Goto S, Nagahiro S, Ushio Y. Microglial response to transient focal cerebral ischemia: an immunocytochemical study on the rat cerebral cortex using anti-phosphotyrosine antibody. J Cereb Blood Flow Metab. 1994;14:825–30. doi: 10.1038/jcbfm.1994.103. [DOI] [PubMed] [Google Scholar]
- (14).Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–7. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- (15).Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–75. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- (16).Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- (17).Saar K, Lindgren M, Hansen M, Eiriksdottir E, Jiang Y, Rosenthal-Aizman K, Sassian M, Langel U. Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem. 2005;345:55–65. doi: 10.1016/j.ab.2005.07.033. [DOI] [PubMed] [Google Scholar]
- (18).Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31:2717–24. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, Kim SW. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther. 2006;14:343–50. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- (20).Lundberg P, El-Andaloussi S, Sutlu T, Johansson H, Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. Faseb J. 2007;21:2664–71. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.