Abstract
Closely related strains of Escherichia coli have been shown to cause extraintestinal infections in unrelated persons. This study tests whether a food reservoir may exist for these E. coli. Isolates from 3 sources over the same time period (2005–2007) and geographic area were compared. The sources comprised prospectively collected E. coli isolates from women with urinary tract infection (UTI) (n = 353); retail meat (n = 417); and restaurant/ready-to-eat foods (n = 74). E. coli were evaluated for antimicrobial drug susceptibility and O:H serotype and compared by using 4 different genotyping methods. We identified 17 clonal groups that contained E. coli isolates (n = 72) from >1 source. E. coli from retail chicken (O25:H4-ST131 and O114:H4-ST117) and honeydew melon (O2:H7-ST95) were indistinguishable from or closely related to E. coli from human UTIs. This study provides strong support for the role of food reservoirs or foodborne transmission in the dissemination of E. coli causing common community-acquired UTIs.
Keywords: Escherichia coli, molecular epidemiology, urinary tract infections, extraintestinal infections, antimicrobial resistance, retail meat, foodborne transmission, food reservoir, bacteria, research
Foodborne transmission of extraintestinal E. coli is common.
Extraintestinal infections caused by Escherichia coli cause serious illness and death. Every year, 6–8 million cases of uncomplicated urinary tract infections (UTI) occur in the United States and 130–175 million cases occur globally; >80% are associated with E. coli (1,2). The urinary tract is the most common source for E. coli causing bloodstream infections, which cause 40,000 deaths from sepsis each year in the United States (1). Uncomplicated UTIs alone are responsible for an estimated $1–$2 billion of direct healthcare costs in the United States annually (1,2). Antimicrobial drug resistance among extraintestinal E. coli is further adding to the cost of treating these infections (3). Drug-resistant infections often require more complicated treatment regimens and result in more treatment failures.
The immediate reservoir of E. coli that causes extraintestinal infections is the intestinal tract of the person. Although extraintestinal infections caused by E. coli are not usually associated with outbreaks, mounting evidence shows that extraintestinal E. coli may be responsible for community-wide epidemics. For instance, in 2001, we reported the discovery of E. coli O11/O77/O17/O73:K52:H18-ST69. This clonal group caused 11% of all E. coli UTIs and 49% of all trimethoprim/sulfamethoxazole-resistant E. coli UTIs in 1 California community over a 4-month period (4). It caused antimicrobial drug–resistant UTIs in Michigan, Minnesota, and Colorado (5), and pyelonephritis in several states (6). Other outbreaks of UTIs caused by E. coli have been described, including a large E. coli O15:K52:H1 outbreak in South London (7), clusters of cases in Copenhagen, Denmark, caused by E. coli O78:H10 (8), and cases in Calgary, Alberta, Canada, caused by an extended-spectrum β-lactamase-producing E. coli (9).
Identification of these outbreak strains has suggested that environmental sources, possibly contaminated meat and other foods, may play a role in the local spread of closely related E. coli strains. If there is a food animal reservoir for extraintestinal E. coli, then the use of antimicrobial agents in food animal production may select for antimicrobial drug–resistant forms of extraintestinal E. coli (10,11). Links between antimicrobial resistance and specific strains of extraintestinal E. coli in animal food products, specifically chicken meat, and human infections have been observed (12–16). In a previous study, we noted an increase in antimicrobial drug–resistant UTIs among women who report frequent chicken and pork consumption (17).
Evidence showing that food can be a reservoir for extraintestinal E. coli includes 1) community-based outbreaks of extraintestinal infections caused by epidemic strains of E. coli causing uncomplicated UTIs (4,18) and other severe infections (6,19,20); 2) the determination that these epidemic strains share antimicrobial drug susceptibility patterns and genotypes with isolates from retail meat (12–15); and 3) the epidemiologic association between retail meat consumption and intestinal acquisition of antimicrobial drug–resistant E. coli causing UTIs (17). On the basis of these observations, we hypothesize that retail chicken is the main reservoir for E. coli causing human extraintestinal infections.
Methods
Study Design
E. coli isolates from human clinical samples, restaurant/ready-to-eat foods, and retail meat were systematically sampled over the same period. Human clinical isolates and restaurant/ready-to-eat isolates were obtained from Montréal, Québec, Canada. Retail meat isolates from Québec and Ontario were included because women with infections were primarily from these regions. We hoped to maximize the probability that matching genotypes between E. coli from these 3 sources could be identified. E. coli isolates from each source were cultured and processed separately to prevent cross-contamination. The study protocol was approved by the McGill University Institutional Review Board (A01-M04-05A).
Sampling of E. coli Causing Human UTIs
E. coli isolates from women with UTIs in Montréal from June 1, 2005, to May 30, 2007, were included. Women 18–45 years of age with a suspected UTI were enrolled. UTI was defined as the presence >2 relevant symptoms including dysuria, increased urinary frequency or urgency, pyuria, and hematuria and >102 colony-forming units of E. coli per milliliter of clean-catch urine (21). A total of 1,395 consecutive UTI samples were obtained. Details about specimen culture and bacterial identification of E. coli are provided in Manges et al. (18). One E. coli isolate from each urine culture was arbitrarily selected for further analysis. If a woman had had recurrent UTIs, only the isolate from the first infection was included. The study sample (n = 353) of E. coli isolates was assembled in the following manner. All cephalothin-resistant E. coli (n = 19) were included. Isolates known to be members of a clonal group (n = 46) found to be closely related to or indistinguishable from other E. coli causing UTI in unrelated women were included (4,18,22) because we hypothesized that these E. coli would be more likely to be associated with food sources. A random sample of E. coli isolates resistant to >1 antimicrobial agents was assembled (n = 172). We chose to oversample resistant E. coli, as antimicrobial resistance has been associated with possible outbreaks of extraintestinal E. coli infections. A random sample of fully susceptible E. coli isolates (n = 116) was selected.
Sampling of E. coli from Retail Meat
A total of 417 E. coli isolates from fresh, raw retail chicken, beef, and pork products were selected from the collection of the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), which monitors antimicrobial resistance in bacteria from meat obtained from grocery and other retail stores in several provinces in Canada (23). Isolates collected by the CIPARS in Montréal, areas of Québec outside Montréal, and parts of Ontario from January 1, 2005, to July 31, 2007, were included as follows. All CIPARS isolates from Montréal were included because all cases of UTI occurred in Montréal (n = 197). All CIPARS nalidixic acid–resistant E. coli from all regions of Canada were included (n = 24); these isolates have been associated with reduced susceptibility to fluoroquinolones. Randomly selected susceptible and resistant isolates from outside Montréal, including other regions of Québec and Ontario, were selected to better represent the possible sources of retail meat exposure for the UTI cases. The overall sampling fraction for retail chicken meat-associated isolates was ≈60%, given that our primary hypothesis focused on retail chicken meat. The sampling fraction for retail beef was 20% and for retail pork 20%. A strong association between extraintestinal E. coli clonal groups and antimicrobial resistance has been reported (4,7,9,18). Our targeted sampling fraction for antimicrobial resistance was 60% for each retail meat category; however, only 25% of retail beef isolates were resistant.
Sampling of E. coli from Restaurant/Ready-to-Eat Food Sources
We included all 74 E. coli isolates from restaurant and ready-to-eat food sources for Montréal collected from January 1, 2005, to December 31, 2007, by the Division de l’Inspection des Aliments (24,25). These isolates were recovered from a range of prepared and ready-to-eat foods, including meat, fruit, vegetables, and other items. Isolates were collected as part of routine surveillance activities and from complaint-related inspections of restaurants and establishments offering ready-to-eat foods.
Antimicrobial Drug Susceptibility
We determined the minimum inhibitory concentration values for 15 antimicrobial agents for all E. coli isolates by the broth microdilution method (26), using the Sensititre Automated Microbiology System (Trek Diagnostic Systems Ltd., Cleveland, OH, USA). National Antimicrobial Resistance Monitoring System (NARMS) susceptibility panel CMV1AGNF was used for E. coli testing. Human clinical and restaurant/ready-to-eat isolates were also evaluated for resistance to cephalothin and nitrofurantoin by a standard disk diffusion method (27). Isolates were defined as resistant, intermediate, or susceptible according to Clinical and Laboratory Standards Institute and NARMS guidelines (23). Isolates exhibiting intermediate resistance were interpreted as susceptible.
Multilocus Variable Number Tandem Repeat Analysis
We performed multilocus variable number tandem repeat analysis (MLVA) on all isolates using capillary electrophoresis methods as described previously in Manges et al (28). Essentially, 8 loci were amplified in separate PCRs by using fluorescent primers. Raw fragment lengths for each locus were binned manually using a minimum threshold of ± 3 bp to distinguish alleles. E. coli CFT073, K12, and O157:H7 were used as positive controls. The set of 8 alleles for each isolate was defined as the MLVA profile.
Enterobacterial Repetitive Intergenic Consensus Sequence 2 PCR Fingerprinting
E. coli isolates exhibiting indistinguishable MLVA profiles were compared by enterobacterial repetitive intergenic consensus sequence 2 PCR (ERIC2 PCR) fingerprinting (29). Isolates with fingerprints that were indistinguishable on visual inspection were grouped and selected for further typing.
Clonal Group Definition
A clonal group was defined as >2 E. coli isolates exhibiting indistinguishable MLVA and ERIC2 PCR patterns. We focused only on groups identified by MLVA and ERIC2 PCR that contained members from >1 source. Groups containing isolates from retail meat and restaurant/ready-to-eat food sources were included to determine whether related extraintestinal E. coli from retail meat isolates could be identified in prepared food. These groups were given a designation that included the serogroup and multilocus sequence type (MLST), as in serogroup O25:H4 and ST131 (O25:H4-ST131). Selected isolates representing each clonal group were chosen and evaluated by pulsed-field gel electrophoresis (PFGE), serotyping, MLST, and phylogenetic typing to confirm the identities of these clonal groups and to define their within-group variability.
Pulsed-Field Gel Electrophoresis
The standard Centers for Disease Control and Prevention protocol for molecular subtyping of E. coli O157:H7 by PFGE was used (30). PFGE of XbaI- and NotI-digested DNA was performed on selected isolates belonging to each clonal group. Isolates exhibiting identical PFGE patterns were considered genetically indistinguishable, those exhibiting 1–3 band differences were considered closely related, and those exhibiting 4–6 band differences were considered possibly related (31).
Serotyping
The Public Health Agency of Canada Laboratory for Foodborne Zoonoses performed O- and H-serotyping using established protocols. Isolates that did not react with O antiserum were classified as nontypeable (ONT), and those that were nonmotile were denoted NM.
MLST and Phylotyping
MLST on selected E. coli isolates was performed as previously described (32). Gene amplification and sequencing were performed by using the primers specified at the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli). Allelic profile and sequence type determinations were assigned according to this website’s scheme. Determination of the major E. coli phylogenetic groups (A, B1, B2, and D) was performed by multiplex PCR (33).
Statistical Analyses
Proportions and 95% confidence intervals for proportions were estimated. Differences in proportions were assessed by χ2 tests; statistical significance was defined as a p value <0.05. All analyses were conducted using Stata version 9.0 (StataCorp LP, College Station, TX, USA).
Results
Final Sample Assembly
We analyzed 844 E. coli isolates obtained from human UTIs (n = 353), retail meat (n = 417), and restaurant/ready-to-eat foods (n = 74). Table 1 contains details regarding the year of isolation, geographic location, and specific meat or food source.
Table 1. Sources of 844 Escherichia coli isolates collected and analyzed in Canada, by year and location, 2005–2007*.
| Source | Total no. (%) isolates | Year, no. (%) isolates |
Location, no. (%) isolates |
|||||
|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | Quebec | Ontario | Other† | |||
| Clinical | ||||||||
| UTI |
353 (42) |
103 (29) |
175 (50) |
75 (21) |
|
353 (100) |
0 |
0 |
| Retail meat | ||||||||
| All | 417 (49) | 178 (43) | 158 (38) | 81(19) | 264 (63) | 139 (33) | 14 (3) | |
| Chicken | 253 (61) | 107 (42) | 101 (40) | 45 (18) | 141 (56) | 99 (39) | 13 (5) | |
| Beef | 82 (20) | 37 (45) | 26 (32) | 19 (23) | 81 (99) | 1 (1) | 0 | |
| Pork |
82 (20) |
34 (41) |
31 (38) |
17 (21) |
|
42 (51) |
39 (48) |
1 (1) |
| Restaurant/ready-to-eat foods | ||||||||
| All | 74 (9) | 19 (26) | 33 (45) | 22 (30) | 74 (100) | 0 | 0 | |
| Chicken | 21 (28) | 7 (33) | 6 (29) | 8 (38) | 21 (100) | 0 | 0 | |
| Beef | 13 (18) | 3 (23) | 6 (46) | 4 (31) | 13 (100) | 0 | 0 | |
| Pork | 5 (7) | 0 | 4 (80) | 1 (20) | 5 (100) | 0 | 0 | |
| Fish/seafood | 6 (8) | 2 (33) | 2 (33) | 2 (33) | 6 (100) | 0 | 0 | |
| Other meat‡ | 9 (12) | 1 (11) | 7 (78) | 1 (11) | 9 (100) | 0 | 0 | |
| Other food§ |
20 (27) |
6 (30) |
8 (40) |
6 (30) |
|
20 (100) |
0 |
0 |
| Total | 844 (100) | 300 (36) | 366 (43) | 178 (21) | 691 (82) | 139 (16) | 14 (2) | |
*UTI, urinary tract infection. †British Columbia (n = 4) and Saskatchewan (n = 10). ‡Bison, lamb, duck, and snail. §Fruits (honeydew melon), vegetables, cheese, rice, couscous, and pasta.
Clonal Group Identification and Characterization
Seventeen clonal groups were identified (containing a total of 72 isolates). Eleven groups contained isolates from human infections and retail meat sources; 5 groups contained isolates from retail meat and restaurant/ready-to-eat food sources; and 1 group contained isolates from restaurant/ready-to-eat food and human infections. Fifty-seven representative isolates were selected for evaluation by PFGE, MLST, serotyping, and phylotyping (Table 2).
Table 2. Characteristics of Escherichia coli clonal groups identified within isolates from 3 types of samples, Canada, 2005–2007*†.
| Group and strain | Type of sample | Isolate source | Location‡ | Year | Genotype | MLST ST | Serotype | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MLVA | ERIC2 | XbaI PFGE | |||||||||
| 1 | |||||||||||
| EC01DT06-1737-01 | Retail meat | Chicken | Montreal | 2006 | 1.033 | 33.01 | 33A.0 | 131 | O25:H4 | ||
| MSHS 161 | Clinical | Human | Montreal | 2005 | 1.033 | 33.01 | 33A.0 | 131 | O25:H4 | ||
| MSHS 1134A | Clinical | Human | Montreal | 2007 | 1.033 | 33.01 | 33A.1 | 131 | O25:H4 | ||
| 2 | |||||||||||
| 68616.01 | RTE | Honeydew | Montreal | 2005 | 1.018 | 18.01 | 18A.0 | 95 | O2:H7 | ||
| MSHS 100 | Clinical | Human | Montreal | 2005 | 1.018 | 18.01 | 18A.0 | 95 | O2:H7 | ||
| MSHS 186 | Clinical | Human | Montreal | 2005 | 1.018 | 18.01 | 18A.0 | 95 | O2:H7 | ||
| MSHS 811 | Clinical | Human | Montreal | 2006 | 1.018 | 18.01 | 18A.0 | 95 | O2:H7 | ||
| MSHS 1229 | Clinical | Human | Montreal | 2007 | 1.018 | 18.01 | 18A.1 | 95 | O2:H7 | ||
| MSHS 95 | Clinical | Human | Montreal | 2005 | 1.018 | 18.01 | 18A.2 | 95 | O2:H7 | ||
| MSHS 1062 | Clinical | Human | Montreal | 2007 | 1.018 | 18.01 | 18A.2 | 95 | O2:NM | ||
| MSHS 782 | Clinical | Human | Montreal | 2006 | 1.018 | 18.01 | 18A.4 | 95 | O2:H7 | ||
| MSHS 819 | Clinical | Human | Montreal | 2006 | 1.018 | 18.01 | 18A.4 | 95 | O2:H7 | ||
| 3 | |||||||||||
| EC01DT05-0789-01 | Retail meat | Chicken | Ontario | 2005 | 1.023 | 23.01 | 23A.0 | 117 | O114:H4 | ||
| MSHS 1014A | Clinical | Human | Montreal | 2007 | 1.023 | 23.01 | 23A.5 | 117 | O114:H4 | ||
| EC01DT05-0224-01 | Retail meat | Chicken | Ontario | 2005 | 1.023 | 23.01 | 23B | 117 | ONT:NM | ||
| EC01DT06-1887-01 | Retail meat | Chicken | Montreal | 2006 | 1.023 | 23.01 | 23C | 117 | O143:H4 | ||
| EC01DT07-0956-01 | Retail meat | Chicken | Other | 2007 | 1.023 | 23.01 | 23D | 117 | O53:H4 | ||
| EC01DT05-1700-01 | Retail meat | Chicken | Quebec | 2005 | 1.023 | 23.01 | NT | 117 | O160:H4 | ||
| EC01DT07-1050-01 | Retail meat | Chicken | Ontario | 2007 | 1.023 | 23.01 | NT | 117 | O45:H4 | ||
| EC01DT07-1090-01 | Retail meat | Chicken | Montreal | 2007 | 1.023 | 23.01 | NT | 117 | O24:H4 | ||
| MSHS 133 | Clinical | Human | Montreal | 2005 | 1.023 | 23.01 | NT | 117 | O24:NM | ||
| 4 | |||||||||||
| EC01DT06-0649-01 | Retail meat | Pork | Montreal | 2006 | 1.116 | 116.01 | 116A | 69 | O17/73/106:H18 | ||
| MSHS 719 | Clinical | Human | Montreal | 2006 | 1.116 | 116.01 | 116C | 69 | O44:H18 | ||
| MSHS 956 | Clinical | Human | Montreal | 2007 | 1.116 | 116.01 | 116D | 69 | ONT:H18 | ||
| 5 | |||||||||||
| EC01DT05-1012-01 | Retail meat | Pork | Ontario | 2005 | 1.102 | 102.01 | 102A | 493 | O4:H5 | ||
| MSHS 769 | Clinical | Human | Montreal | 2006 | 1.102 | 102.01 | 102B | 493 | O4:H5 | ||
| 6 | |||||||||||
| EC01DT06-1265-01 | Retail meat | Beef | Montreal | 2006 | 2.107 | 107.01 | 107A | 401 | O36:NM | ||
| 76083.08 | RTE | Chicken | Montreal | 2007 | 2.107 | 107.01 | 107B | 401 | O36:NM | ||
| 7 | |||||||||||
| EC01DT06-0274-01 | Retail meat | Chicken | Quebec | 2006 | 2.097 | 97.01 | 97A | 295 | O172:H16 | ||
| 79287 | RTE | Chicken | Montreal | 2007 | 2.097 | 97.01 | 97B | 295 | O172:H16 | ||
*MLST, multilocus sequence typing; MLVA, multilocus variable number tandem repeat analysis; ERIC2, enterobacterial repetitive intergenic consensus sequence 2; PFGE, pulsed-field gel electrophoresis; ST, sequence type; RTE, restaurant/ready-to-eat foods; NT, nontypeable; ONT, serogroup nontypeable; NM, non-motile; UNK, unknown. An expanded version of Table 2 containing all isolates is available online at www.cdc.gov/EID/content/16/1/zzz-T2.htm. †All isolates in groups 1, 2, and 5 were phylotype B2; all isolates in groups 3, 4, and 8 were phylotype D; all isolates in groups 6, 9, 10, and 11, as well as isolate MSHS 689 in group 17, were phylotype A; all isolates in groups 7, 12, 13, 14, 15, and 16, as well as isolate EC01DT05-0469-01 from group 17, were phylotype B1. ‡Other locations were Saskatchewan or British Columbia.
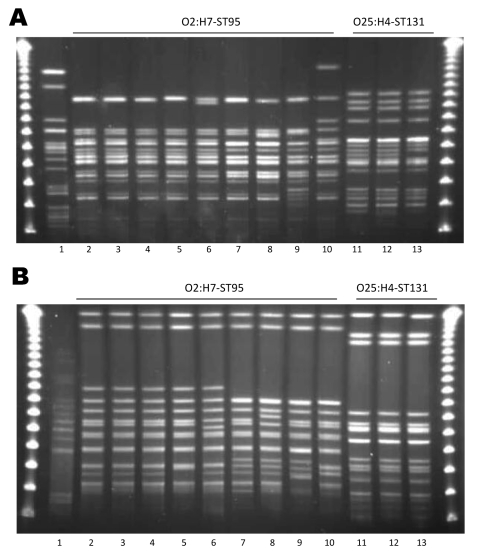
On the basis of PFGE patterns, we identified 2 clonal groups (group 1 and group 2) that contained genetically indistinguishable isolates and 1 clonal group (group 3) that contained closely related isolates from food sources and human UTIs. Group 1 contained E. coli characterized as O25:H4-ST131, which was identified in 1 sample of retail chicken meat and in 2 cases of human infection. The XbaI PFGE patterns of the human isolate (MSHS 161) and the retail chicken isolate (EC01DT06-1737-01) were indistinguishable, and the second human isolate (MSHS 1134A) differed by 1 band from the other 2 patterns (Figure 1, panel A). The NotI PFGE patterns of the 2 human isolates, which were indistinguishable, differed from the retail chicken isolate by a single band (Figure 1, panel B). The retail meat isolate from this group was susceptible to all antimicrobial agents tested, while 1 of the 2 isolates from human infections was resistant to cephalothin and the second was resistant to ampicillin, streptomycin, sulfisoxazole, and tetracycline.
Figure 1.
Pulsed-field gel electrophoresis patterns for Escherichia coli O2:H7-ST95 and E. coli O25:H4-ST131. A) XbaI; B) NotI. Lane 1 is the positive control E. coli O11:H18-ST69 (SEQ102); lane 2 is an E. coli O2:H7-ST95 isolate from a restaurant sample of honeydew melon (68616.01); lanes 3–10 are isolates from human urinary tract infection cases (UTIs; lane 3, MSHS 100; lane 4, MSHS 186; lane 5, MSHS 811; lane 6, MSHS 1229; lane 7, MSHS 95; lane 8, MSHS 1062; lane 9, MSHS 782; lane 10, MSHS 819); lane 11 is an E. coli O25:H4-ST131 isolate from a retail chicken sample (EC01DT06-1737-01); and lanes 12 and 13 are E. coli isolates from human UTIs (lane 12, MSHS 161; lane 13, MSHS 1134A). Outer lanes are pulsed-field molecular weight markers.
Group 2 contained E. coli characterized as O2:H7-ST95; one isolate was from a restaurant/ready-to-eat food source (a honeydew melon) and 8 isolates were from cases of human infection. The XbaI PFGE patterns were indistinguishable for 3 of the human infection isolates (MSHS 100, 186, and 811) and the restaurant/ready-to-eat food isolate (68616.01); the other 5 O2:H7-ST95 isolates differed by 1 band (MSHS 1229), two bands (MSHS 95 and MSHS 1062), and 4 bands (MSHS 782 and MSHS 819) from the food source isolate, respectively (Figure 1, panel A ). The NotI PFGE patterns for MSHS 100 and MSHS 186 were indistinguishable from the restaurant/ready-to-eat isolate, and the other human infection isolates differed by 1 to 7 bands (Figure 1, panel B). The E. coli isolate from the food source was fully susceptible, as were most isolates from the human infections, except for 2 (one was resistant to ampicillin, and the second to ampicillin, sulfisoxazole, and trimethoprim/sulfamethoxazole).
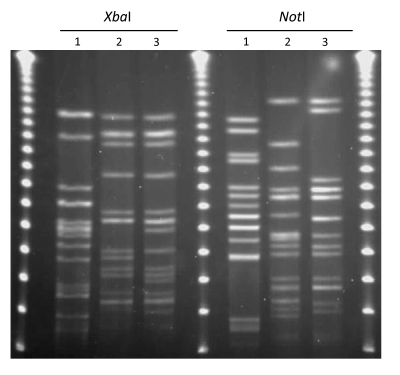
Group 3 contained E. coli characterized as O114:H4-ST117; one isolate was from retail chicken meat and the second was from a human UTI. The XbaI PFGE patterns of the human infection isolate (MSHS 1014A) and retail meat isolate (EC01DT05-0789-01) differed by 5 bands (Figure 2). The NotI PFGE patterns differed by >6 bands (Figure 2). Both isolates were fully susceptible. In addition to shared PFGE patterns, these 3 groups of E. coli shared the same MLSTs, serotypes, and phylotypes.
Figure 2.
XbaI and NotI pulsed-field gel electrophoresis patterns for Escherichia coli O114:H4-ST117 (lanes 2 and 3). Lane 1 is the positive control E. coli O11:H18-ST69 (SEQ102), lane 2 is an E. coli O25:H4-ST131 isolate from a retail chicken sample (EC01DT06-1737-01), and lane 3 is an E. coli isolate from a human urinary tract infection case (MSHS 1014A). Outer and center lanes are pulsed-field molecular weight markers.
The clonal group characterized as E. coli O17/O73/O77:H18-ST69, also known as clonal group A (4), was identified in human and retail meat samples, although closely related PFGE patterns were not observed (group 4, Table 2). Three other groups (groups 5–7, Table 2), characterized as E. coli O4:H5-ST493, O36:NM-ST401, and O172:H16-ST295, exhibited shared MLSTs, serotypes, and phylotypes, but the PFGE patterns were not related.
Discussion
We report the identification of E. coli isolates from retail chicken and other food sources that are indistinguishable from or closely related to isolates from human UTIs. Our a priori hypothesis, based on results from previous studies, suggests that retail meat, specifically retail chicken meat, could be a reservoir for E. coli causing human extraintestinal infections. This study provides strong support for this hypothesis on the basis of genetic similarities between food and human clinical isolates.
Johnson et al. have proposed that antimicrobial drug–resistant E. coli from human feces (and human bloodstream infections) tend to be more similar to antimicrobial-resistant and -susceptible E. coli from retail poultry meat sources (14,15). These observations indicate that the selection of resistant E. coli is more likely to occur in the animal food reservoir than in humans. In this study, we observed that genetically related E. coli from food sources and human infections tended to be susceptible, suggesting that both resistant and susceptible isolates causing UTIs in women may be transmitted through the food supply. Our study also identified members of the O2:H7-ST95 group, previously associated with extraintestinal disease in both humans and avian hosts (34). The O2:H7-ST95 food source isolate from this study was from a honeydew melon. Potential origins of this E. coli contamination could include human or food animal sources.
The E. coli O25:H4-ST131 clonal group, also identified in this study, has been associated with extended spectrum β-lactamase production and fluoroquinolone resistance and has been found across Europe and in Canada (18,35–37). The E. coli O25:H4-ST131 isolates identified in this study are susceptible; however, because this clonal group may be found in a food animal reservoir and transmitted by food, amplification and transmission of these highly resistant organisms could be possible. Extended spectrum β-lactamase-producing E. coli have not yet been identified by CIPARS (23,38,39).
This study was ecologic in design and presents several limitations. Epidemiologic information on the UTI cases was not available. Information on travel, history of antimicrobial drug use, dietary information, and other factors would have been useful to describe the study population and to assess the significance of other possible transmission routes that might explain our results. The study also oversampled retail chicken meat and consequently undersampled isolates from retail pork and beef. It is possible that closely related clonal groups could be identified that contain isolates from both human infections and pork or beef samples. Because of insufficient power in our sampling strategy we could not exclude the existence of these groups; additional sampling of isolates from retail pork and beef are underway to address this question. Despite oversampling isolates from retail chicken meat, we observed that 82% (a greater fraction than the 60% sampling fraction) of E. coli belonging to the 17 clonal groups were associated with retail chicken meat. We also oversampled antimicrobial drug-resistant isolates; however, most (53%) isolates that belonged to a clonal group were fully susceptible. Even though the size and scope of this study was limited, we were able to detect several instances of groups containing closely related isolates from human and food sources. It is therefore probable that a food reservoir exists and that foodborne transmission of extraintestinal E. coli is common.
The identification of 2 clonal groups containing isolates from retail chicken meat and human infections supports our a priori hypothesis. We cannot exclude the possibility that food source isolates were present because of human contamination during food production, processing or handling, even though it is very unlikely. Subsequent research will help determine whether these E. coli occur in a food animal reservoir or whether transfer of these E. coli results from contamination during food processing or preparation and reflects human-to-human transmission by food.
This study demonstrates that some E. coli from retail chicken meat and other food sources are closely related to E. coli causing human UTIs. Since a food animal reservoir apparently exists for E. coli that cause urinary tract and other extraintestinal infections, this further reinforces the need for responsible antimicrobial drug stewardship in veterinary medicine and food animal production as well as in human medicine.
Acknowledgments
We thank members of the surveillance team of the Canadian Integrated Program for Antimicrobial Resistance Surveillance (Brent Avery); the Division de l’Inspection des Aliments, Ville de Montréal (Myrto Mantzavrakos and Annie Laviolette); and Christiane Lacombe, the Student Health Services Clinical Technician.
This study was supported by the Public Health Agency of Canada and by the Canadian Institutes of Health Research, CGM84898 (C.V.).
Biography
Ms Vincent is a graduate student in the Department of Microbiology and Immunology at McGill University in Montréal, Québec. Her research interests include bacteriology and molecular epidemiology and she is currently investigating the possible foodborne transmission of E. coli causing urinary tract infections.
Footnotes
Suggested citation for this article: Vincent C, Boerlin P, Daignault D, Dozois CM, Dutil L, Galanakis C, et al. Food reservoir for Escherichia coli causing urinary tract infections. Emerg Infect Dis [serial on the Internet]. 2010 Jan [date cited]. Available from http://www.cdc.gov/EID/content/16/1/88.htm
References
- 1.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–56. 10.1016/S1286-4579(03)00049-2 [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. 10.1016/S0002-9343(02)01054-9 [DOI] [PubMed] [Google Scholar]
- 3.Alam MF, Cohen D, Butler C, Dunstan F, Roberts Z, Hillier S, et al. The additional costs of antibiotics and re-consultations for antibiotic-resistant Escherichia coli urinary tract infections managed in general practice. Int J Antimicrob Agents. 2009;33:255–7. 10.1016/j.ijantimicag.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 4.Manges AR, Johnson JR, Foxman B, O’Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med. 2001;345:1007–13. 10.1056/NEJMoa011265 [DOI] [PubMed] [Google Scholar]
- 5.Burman WJ, Breese PE, Murray BE, Singh KV, Batal HA, MacKenzie TD, et al. Conventional and molecular epidemiology of trimethoprim-sulfamethoxazole resistance among urinary Escherichia coli isolates. Am J Med. 2003;115:358–64. 10.1016/S0002-9343(03)00372-3 [DOI] [PubMed] [Google Scholar]
- 6.Johnson JR, Manges AR, O’Bryan TT, Riley LW. A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis. Lancet. 2002;359:2249–51. 10.1016/S0140-6736(02)09264-4 [DOI] [PubMed] [Google Scholar]
- 7.Phillips I, Eykyn S, King A, Gransden WR, Rowe B, Frost JA, et al. Epidemic multiresistant Escherichia coli infection in West Lambeth Health District. Lancet. 1988;331:1038–41. 10.1016/S0140-6736(88)91853-3 [DOI] [PubMed] [Google Scholar]
- 8.Olesen B, Kolmos HJ, Orskov F, Orskov I. Cluster of multiresistant Escherichia coli O78:H10 in Greater Copenhagen. Scand J Infect Dis. 1994;26:406–10. 10.3109/00365549409008613 [DOI] [PubMed] [Google Scholar]
- 9.Pitout JDD, Gregson DB, Church DL, Elsayed S, Laupland KB. Community-wide outbreaks of clonally related CTX-M-14 beta-lactamase-producing Escherichia coli strains in the Calgary Health Region. J Clin Microbiol. 2005;43:2844–9. 10.1128/JCM.43.6.2844-2849.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones TF, Schaffner W. New perspectives on the persistent scourge of foodborne disease. J Infect Dis. 2005;191:1029–31. 10.1086/428509 [DOI] [PubMed] [Google Scholar]
- 11.Stamm WE. An epidemic of urinary tract infections. N Engl J Med. 2001;345:1055–7. 10.1056/NEJM200110043451409 [DOI] [PubMed] [Google Scholar]
- 12.Johnson JR, Kuskowski MA, Smith K, O’Bryan TT, Tatini S. Antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in retail foods. J Infect Dis. 2005;191:1040–9. 10.1086/428451 [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Delavari P, O’Bryan TT, Smith KE, Tatini S. Contamination of retail foods, particularly turkey, from community markets (Minnesota, 1999–2000) with antimicrobial-resistant and extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2005;2:38–49. 10.1089/fpd.2005.2.38 [DOI] [PubMed] [Google Scholar]
- 14.Johnson JR, Kuskowski MA, Menard M, Gajewski A, Xercavins M, Garau J. Similarity between human and chicken Escherichia coli isolates in relation to ciprofloxacin resistance status. J Infect Dis. 2006;194:71–8. 10.1086/504921 [DOI] [PubMed] [Google Scholar]
- 15.Johnson JR, Sannes MR, Croy C, Johnston B, Clabots C, Kuskowski MA, et al. Antimicrobial drug–resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg Infect Dis. 2007;13:838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder CM, White DG, Ge B, Zhang Y, McDermott PF, Ayers S, et al. Isolation of antimicrobial-resistant Escherichia coli from retail meats purchased in Greater Washington, DC, USA. Int J Food Microbiol. 2003;85:197–202. 10.1016/S0168-1605(02)00508-1 [DOI] [PubMed] [Google Scholar]
- 17.Manges AR, Smith SP, Lau BJ, Nuval CJ, Eisenberg JN, Dietrich PS, et al. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathog Dis. 2007;4:419–31. 10.1089/fpd.2007.0026 [DOI] [PubMed] [Google Scholar]
- 18.Manges AR, Tabor H, Tellis P, Vincent C, Tellier PP. Endemic and epidemic lineages of Escherichia coli that cause urinary tract infections. Emerg Infect Dis. 2008;14:1575–83. 10.3201/eid1410.080102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manges AR, Dietrich PS, Riley LW. Multidrug-resistant Escherichia coli clonal groups causing community-acquired pyelonephritis. Clin Infect Dis. 2004;38:329–34. 10.1086/380640 [DOI] [PubMed] [Google Scholar]
- 20.Manges AR, Perdreau-Remington F, Solberg O, Riley LW. Multidrug-resistant Escherichia coli clonal groups causing community-acquired bloodstream infections. J Infect. 2006;53:25–9. 10.1016/j.jinf.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 21.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–81. 10.1016/S0891-5520(05)70373-1 [DOI] [PubMed] [Google Scholar]
- 22.Manges AR, Natarajan P, Solberg OD, Dietrich PS, Riley LW. The changing prevalence of drug-resistant Escherichia coli clonal groups in a community: evidence for community outbreaks of urinary tract infections. Epidemiol Infect. 2006;134:425–31. 10.1017/S0950268805005005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), 2006. Guelph (Ontario, Canada): Public Health Agency of Canada; 2009. [Google Scholar]
- 24.Government of Canada. Enumeration of E. coli and coliforms in food products and food ingredients using 3M Petrifilm E. coli count plates. 2001. Report No.: MFHPB–34.
- 25.International Organization for Standardization. Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli —Part 2: Colony-count technique at 44 degrees C using 5-bromo-4-chloro-3-indoyl beta-D-glucuronide. 2001. Report No.: ISO 16649–2.
- 26.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 7th ed. CLSI M07–A7. Wayne (PA): The Institute; 2006. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. NCCLS M2–A7. Wayne (PA): The Committee; 2000. [Google Scholar]
- 28.Manges AR, Tellis PA, Vincent C, Lifeso K, Geneau G, Reid-Smith RJ, et al. Multi-locus variable number tandem repeat analysis for Escherichia coli causing extraintestinal infections. J Microbiol Methods. 2009;79:211–3. 10.1016/j.mimet.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 29.Johnson JR, O’Bryan TT. Improved repetitive-element PCR fingerprinting for resolving pathogenic and nonpathogenic phylogenetic groups within Escherichia coli. Clin Diagn Lab Immunol. 2000;7:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender JB, Hedberg CW, Besser JM, Boxrud DJ, MacDonald KL, Osterholm MT. Surveillance for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med. 1997;337:388–94. 10.1056/NEJM199708073370604 [DOI] [PubMed] [Google Scholar]
- 31.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60:1136–51. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–8. 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson TJ, Wannemuehler Y, Johnson SJ, Stell AL, Doetkrott C, Johnson JR, et al. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol. 2008;74:7043–50. 10.1128/AEM.01395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cagnacci S, Gualco L, Debbia E, Schito GC, Marchese A. European emergence of ciprofloxacin-resistant Escherichia coli clonal groups O25:H4-ST 131 and O15:K52:H1 causing community-acquired uncomplicated cystitis. J Clin Microbiol. 2008;46:2605–12. 10.1128/JCM.00640-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JR, Menard M, Johnston B, Kuskowski MA, Nichol K, Zhanel GG. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada (2002–2004). Antimicrob Agents Chemother. 2009;53:2733–9. 10.1128/AAC.00297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Canica MM, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008;61:273–81. 10.1093/jac/dkm464 [DOI] [PubMed] [Google Scholar]
- 38.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), 2005. Guelph (Ontario, Canada): Public Health Agency of Canada; 2007. [Google Scholar]
- 39.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2007. —preliminary results. Guelph (Ontario, Canada): Public Health Agency of Canada; 2008. [Google Scholar]