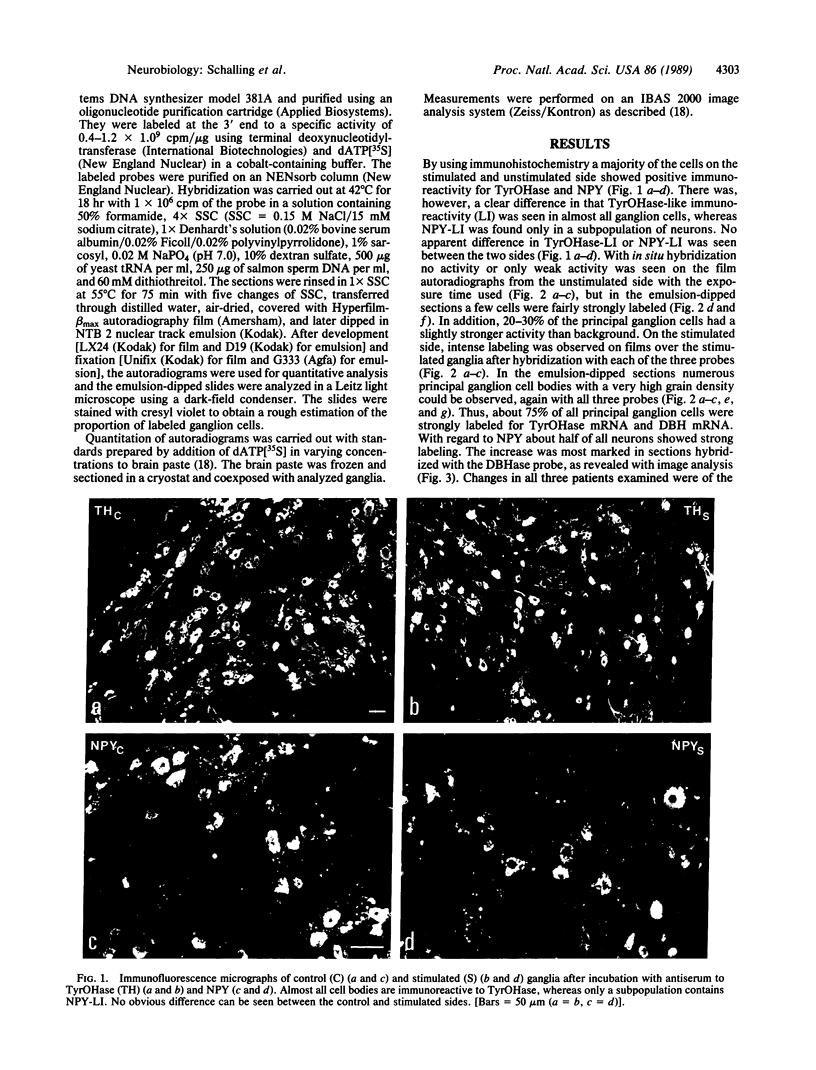
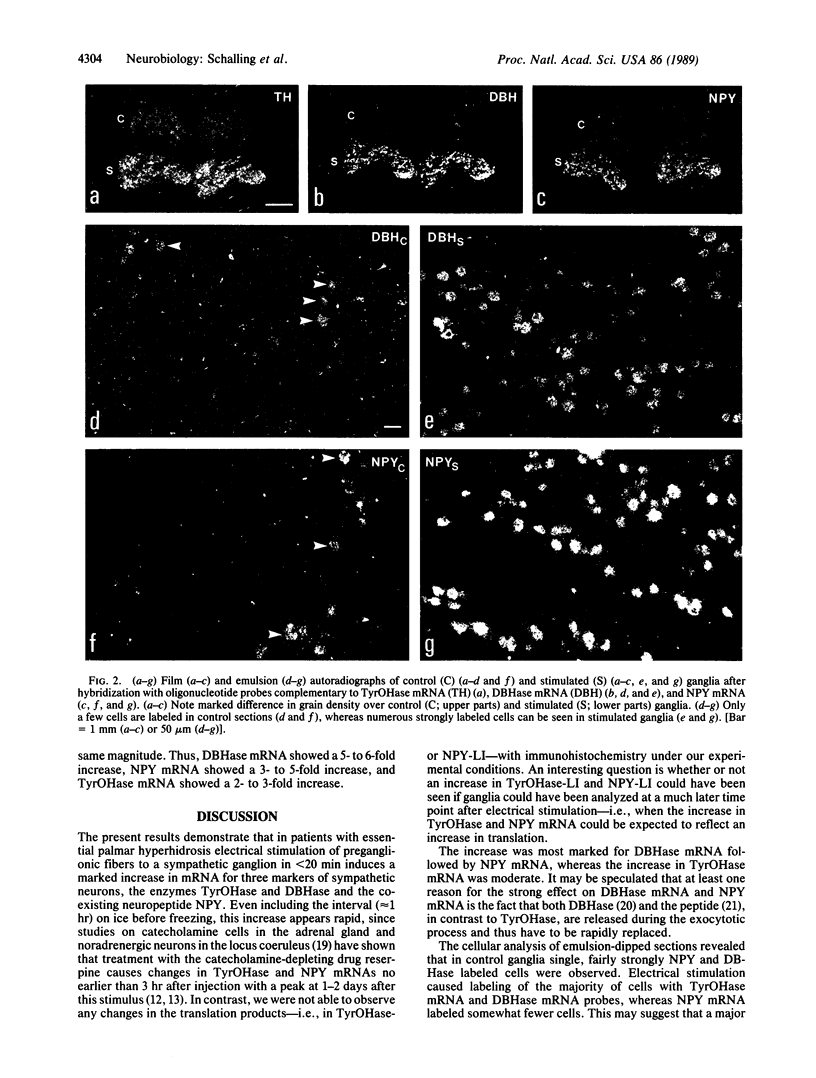
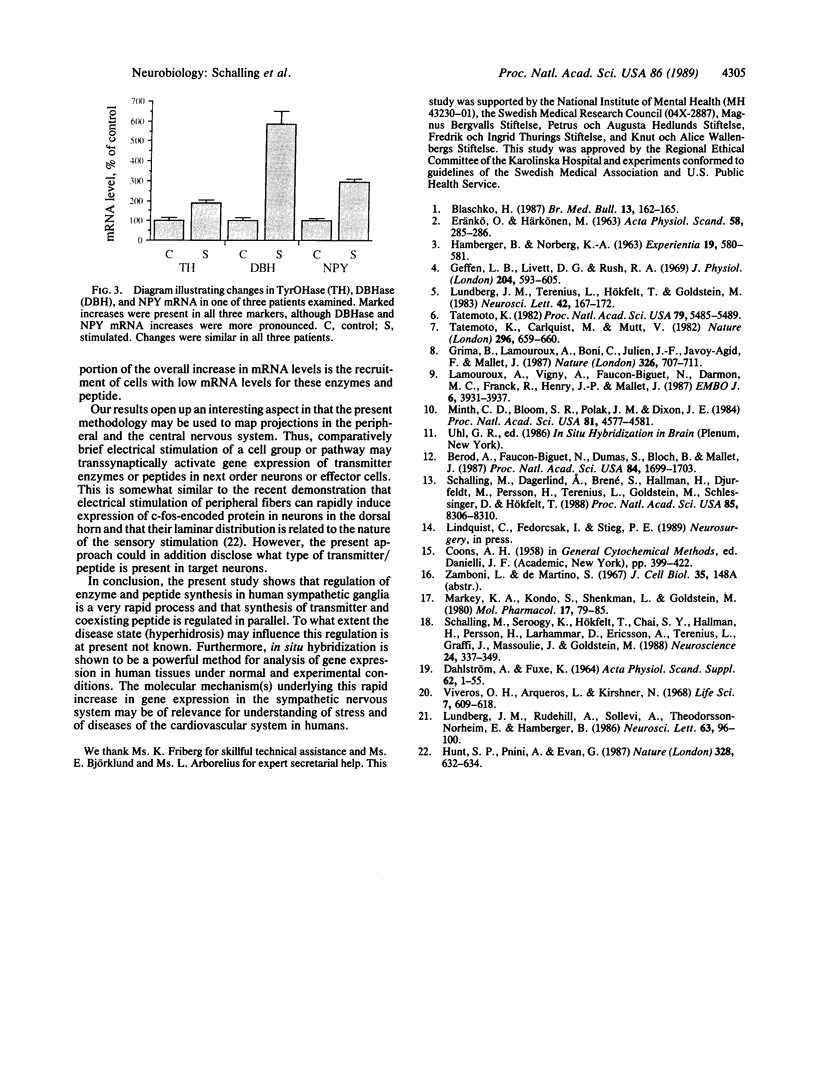
Abstract
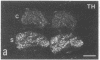
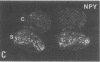
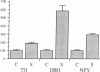
Thoracic ganglia in humans were studied after electrical, preganglionic stimulation using in situ hybridization with synthetic oligonucleotide probes against the catecholamine-synthesizing enzymes tyrosine hydroxylase (EC 1.14.16.2) and dopamine beta-hydroxylase (EC 1.14.17.1) and neuropeptide tyrosine. Immunohistochemical analysis was also performed. Following short peroperative stimulation a severalfold increase in all three mRNAs was found in principal ganglion cells, whereas no definite changes could be detected in enzyme or peptide levels with immunohistochemistry. The results suggest a very rapid and sensitive regulation of genes involved in signal transmission in the sympathetic nervous system of humans. Moreover, they indicate that electrical stimulation of neurons and/or pathways combined with in situ hybridization may be used as a method to define neuronal projections by visualizing increases in mRNAs for transmitter enzymes and/or peptide in target cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLASCHKO H. Formation of catechol amines in the animal body. Br Med Bull. 1957 Sep;13(3):162–165. doi: 10.1093/oxfordjournals.bmb.a069606. [DOI] [PubMed] [Google Scholar]
- Berod A., Biguet N. F., Dumas S., Bloch B., Mallet J. Modulation of tyrosine hydroxylase gene expression in the central nervous system visualized by in situ hybridization. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1699–1703. doi: 10.1073/pnas.84.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G., Rush R. A. Immunohistochemical localizatio of protein components of catecholamine storage vesicles. J Physiol. 1969 Oct;204(3):593–605. doi: 10.1113/jphysiol.1969.sp008934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- HAMBERGER B., NORBERG K. A. MONOAMINES IN SYMPATHETIC GANGLIA STUDIED WITH FLUORESCENCE MICROSCOPY. Experientia. 1963 Nov 15;19:580–581. doi: 10.1007/BF02150999. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Lamouroux A., Vigny A., Faucon Biguet N., Darmon M. C., Franck R., Henry J. P., Mallet J. The primary structure of human dopamine-beta-hydroxylase: insights into the relationship between the soluble and the membrane-bound forms of the enzyme. EMBO J. 1987 Dec 20;6(13):3931–3937. doi: 10.1002/j.1460-2075.1987.tb02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Rudehill A., Sollevi A., Theodorsson-Norheim E., Hamberger B. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: differential release of noradrenaline and neuropeptide Y from pig spleen. Neurosci Lett. 1986 Jan 2;63(1):96–100. doi: 10.1016/0304-3940(86)90020-0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Terenius L., Hökfelt T., Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983 Dec 2;42(2):167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- Minth C. D., Bloom S. R., Polak J. M., Dixon J. E. Cloning, characterization, and DNA sequence of a human cDNA encoding neuropeptide tyrosine. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4577–4581. doi: 10.1073/pnas.81.14.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brené S., Hallman H., Djurfeldt M., Persson H., Terenius L., Goldstein M., Schlesinger D., Hökfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and neuropeptide tyrosine in the rat and bovine adrenal gland: effects of reserpine. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Seroogy K., Hökfelt T., Chai S. Y., Hallman H., Persson H., Larhammar D., Ericsson A., Terenius L., Graffi J. Neuropeptide tyrosine in the rat adrenal gland--immunohistochemical and in situ hybridization studies. Neuroscience. 1988 Jan;24(1):337–349. doi: 10.1016/0306-4522(88)90335-1. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Carlquist M., Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982 Apr 15;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]