Abstract
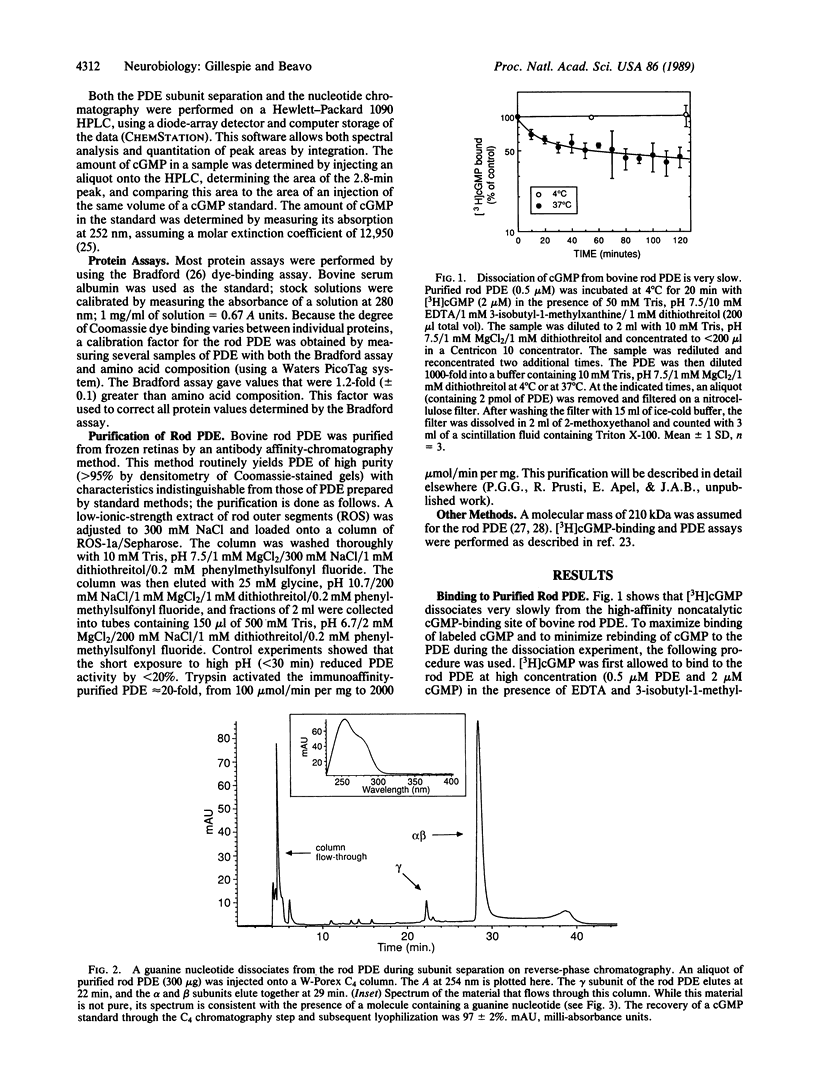
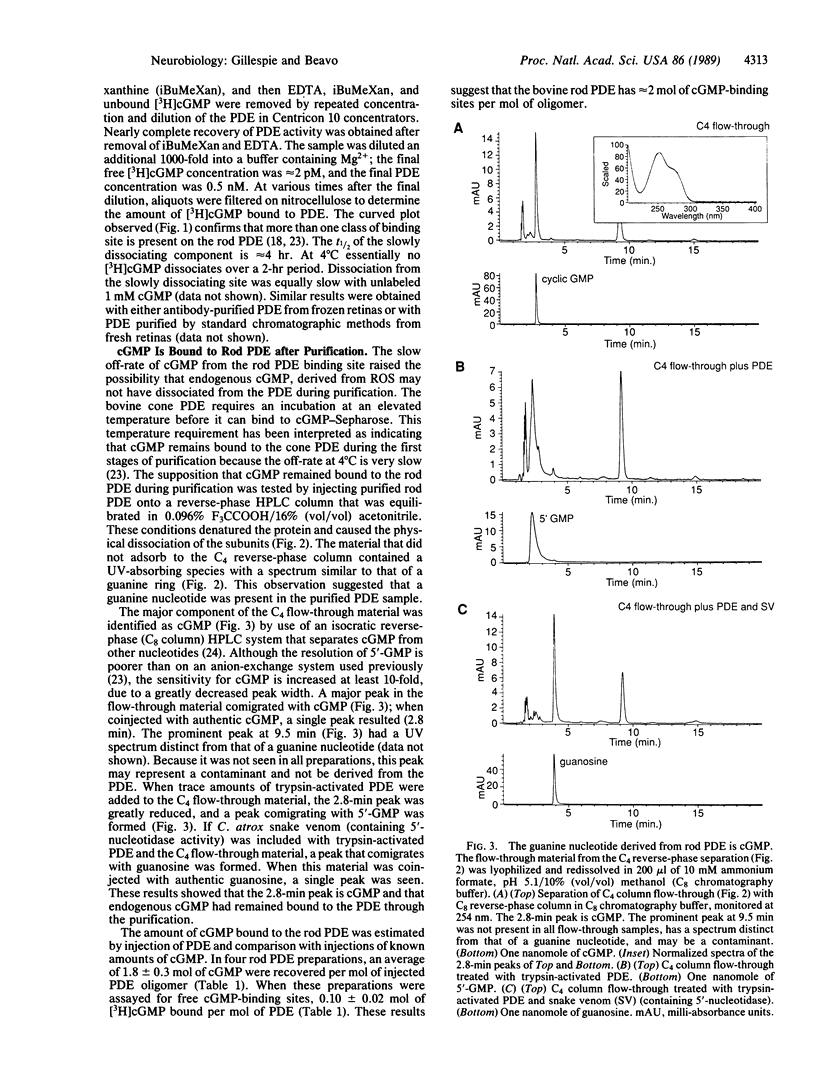
Although the total concentration of cGMP in rod outer segments is thought to be substantially greater than the free concentration, no quantitatively relevant site for the bound cGMP has been described in mammalian photoreceptors. We have found that preparations of purified bovine rod photoreceptor cyclic nucleotide phosphodiesterase (PDE) contain 1.8 +/- 0.3 mol of tightly bound cGMP per mol of PDE. When subunits of the purified PDE were separated by reverse-phase HPLC in 0.1% trifluoroacetic acid and acetonitrile, a peak of material having spectral properties characteristic of a guanine ring was seen. This material was identified as cGMP by comigration with authentic cGMP on HPLC, conversion to 5-GMP by trypsin-activated rod PDE, and conversion to guanosine by a combination of trypsin-activated PDE and 5'-nucleotidase-containing snake venom. When incubated with 1 microM [3H]cGMP, only 0.1 mol of [3H]cGMP bound per mol of purified PDE, presumably because nearly all binding sites were occupied by tightly bound endogenous cGMP carried through the purification. Scatchard plots of [3H]cGMP binding have indicated that two classes of binding sites are present on the rod PDE. The off-rate of cGMP from the slowly dissociating site is extremely slow; it has a t1/2 of approximately 4 hr at 37 degrees C. At lower temperatures, very little cGMP dissociates; the amount of [3H]cGMP bound to rod PDE after 2 hr at 4 degrees C was essentially the same as at the beginning of the incubation. The observation that stoichiometric amounts of cGMP are tightly bound to PDE accounts for the inability to purify the bovine rod PDE on cGMP affinity columns or to demonstrate stoichiometric high-affinity binding sites with [3H]cGMP. More significantly, the tightly bound cGMP may resolve the apparent discrepancy between the free and total cGMP concentrations of photoreceptor outer segments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Walseth T. F., Heyman R. A., Barad M., Graeff R. M., Goldberg N. D. Light-induced increases in cGMP metabolic flux correspond with electrical responses of photoreceptors. J Biol Chem. 1986 Oct 5;261(28):13034–13042. [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Baylor D. A. Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):34–49. [PubMed] [Google Scholar]
- Blazynski C., Cohen A. I. Rapid declines in cyclic GMP of rod outer segments of intact frog photoreceptors after illumination. J Biol Chem. 1986 Oct 25;261(30):14142–14147. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985 Feb 14;313(6003):585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Cote R. H., Biernbaum M. S., Nicol G. D., Bownds M. D. Light-induced decreases in cGMP concentration precede changes in membrane permeability in frog rod photoreceptors. J Biol Chem. 1984 Aug 10;259(15):9635–9641. [PubMed] [Google Scholar]
- Cote R. H., Nicol G. D., Burke S. A., Bownds M. D. Changes in cGMP concentration correlate with some, but not all, aspects of the light-regulated conductance of frog rod photoreceptors. J Biol Chem. 1986 Oct 5;261(28):12965–12975. [PMC free article] [PubMed] [Google Scholar]
- Dawis S. M., Graeff R. M., Heyman R. A., Walseth T. F., Goldberg N. D. Regulation of cyclic GMP metabolism in toad photoreceptors. Definition of the metabolic events subserving photoexcited and attenuated states. J Biol Chem. 1988 Jun 25;263(18):8771–8785. [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Beavo J. A. Characterization of a bovine cone photoreceptor phosphodiesterase purified by cyclic GMP-sepharose chromatography. J Biol Chem. 1988 Jun 15;263(17):8133–8141. [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Hamm H. E., Bownds M. D. Protein complement of rod outer segments of frog retina. Biochemistry. 1986 Aug 12;25(16):4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- Kilbride P., Ebrey T. G. Light-initiated changes of cyclic guanosine monophosphate levels in the frog retina measured with quick-freezing techniques. J Gen Physiol. 1979 Sep;74(3):415–426. doi: 10.1085/jgp.74.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Kondo H., Miller W. H. Rod light adaptation may be mediated by acceleration of the phosphodiesterase-guanylate cyclase cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1322–1326. doi: 10.1073/pnas.85.4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Kumarev V. P., Gubanov V. V., Khramtsov N. V., Akhmedov N. B., Zagranichny V. E., Muradov K. G. Cyclic GMP phosphodiesterase from cattle retina. Amino acid sequence of the gamma-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1986 Aug 18;204(2):288–292. doi: 10.1016/0014-5793(86)80830-4. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Cobbs W. H. Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 1986;26(10):1613–1643. doi: 10.1016/0042-6989(86)90051-9. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Harkness J., Parkes J. H., Gonzalez-Oliva C., Liebman P. A. Kinetic studies suggest that light-activated cyclic GMP phosphodiesterase is a complex with G-protein subunits. Biochemistry. 1986 Feb 11;25(3):651–656. doi: 10.1021/bi00351a021. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds D., Green S. H., Morrisey J. L., Shedlovsky A. Guanosine 3',5'-cyclic monophosphate and the in vitro physiology of frog photoreceptor membranes. J Gen Physiol. 1977 May;69(5):667–679. doi: 10.1085/jgp.69.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Bartucca F., Ting A., Bitensky M. W. Reciprocal effects of an inhibitory factor on catalytic activity and noncatalytic cGMP binding sites of rod phosphodiesterase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3702–3706. doi: 10.1073/pnas.79.12.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Sen I., Bitensky M. W., Casnellie J. E., Greengard P. Cyclic GMP-specific, high affinity, noncatalytic binding sites on light-activated phosphodiesterase. J Biol Chem. 1980 Dec 10;255(23):11619–11624. [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]



