Abstract
Rationale: Clinical testing of oxygen-conserving devices is not mandated before marketing. Consequently, little is known about individual or comparative therapeutic effectiveness.
Objectives: To relate oxygen delivery from prototypical instruments to physiological performance.
Methods: Thirteen subjects with obstructive lung disease performed progressive treadmill exercise while inhaling either room air, 2 L O2/min, or bolus oxygen from four commercially available conserving devices at regulator settings of 2, 5, and continuous. The devices were studied blindly in random order after first being tested to determine performance characteristics. Pulse oximetry, oxygen delivery, and nasal and oral ventilations were monitored at rest and with exertion.
Measurements and Main Results: At a setting of 2 at rest, all conservers maintained saturation greater than 90%, but there were significant differences in oxygenation between systems. Only one equaled 2 L O2/min. With exertion, saturation decreased with all conservers but not with 2 L O2/min. One device did not perform any better than room air. Two systems provided less oxygen than predicted, one more, and in one the expected and actual amounts were equal only at rest. Breath-by-breath performance was highly variable, with irregular activation and inconsistent oxygen bolus size delivery. Increasing oxygen pulse volume to the point of eradicating conservation with the continuous setting did not eliminate all disparities.
Conclusions: The mechanical and clinical performances of current oxygen conservers are highly variable and in some instances actually contribute to limitations in exercise ability. Seemingly equivalent technical features do not guarantee equivalent therapeutic functionality.
Keywords: oxygen, conservers, physiology, engineering, chronic obstructive pulmonary disease
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Clinical testing of O2 conservers is not mandated before marketing. Consequently, little is known about individual or comparative physiologic effectiveness.
What This Study Adds to the Field
The present study demonstrates that the mechanical and clinical performances of the current systems are highly variable both within and between devices and fall short of technical expectations. Such inconsistencies interfere with oxygenation during exertion and can actually contribute to limitations in patient exercise ability.
Persistent hypoxemia is a frequently encountered clinical problem that has devastating consequence if left unattended. By far, the most common cause is chronic obstructive pulmonary disease (COPD), and long-term supplemental oxygen (O2) is the consensus treatment for reducing morbidity and mortality (1, 2). Quality of life and patient acceptance are greatly enhanced when ambulatory O2 is made part of the regimen. To this end, O2 conservation systems that are light enough to carry comfortably during physical activity yet provide sufficient O2 to allow unfettered outdoor mobility for reasonable periods have been developed (3). The quintessential components of these instruments are small reservoirs that supply metered amounts of O2 only during inspiration to extend tank life (4).
To date, a large number of conservers have appeared on the market incorporating different technological features aimed at optimizing O2 bolus sizes and delivery efficiency (5). Because these devices represent variations on a basic theme, they have been assumed to be functionally identical by regulatory agencies. As a result, they have received new device exemption waivers (510K) from the Food and Drug Administration obviating the need for clinical testing. However, based on hints about device performance in the literature, this assumption may or may not be correct. For example, it is commonly held that demand and pulse instruments are equivalent to each other and to continuous flow, but this belief is rooted in limited clinical and technical assessments (6–14). A detailed review of such studies reveals inconsistencies between and within designs in the ability to maintain oxygenation, particularly during periods of physiologic stress (6, 7, 11, 13, 14). Thus far, the mechanisms for such phenomena and their potential therapeutics relevance have remained unexplained.
In the present study, we postulated that the simple demonstration of engineering equivalency ex vivo may be insufficient to guarantee similar performance in vivo, and important clinical differences in efficacy between the various designs could be present and go undetected unless specifically sought. It is possible that O2 bolus sizes may not be large enough during exertion to meet metabolic demands, the completeness of reservoir filling could be frequency limited, or there may be poor synchronization between triggering and respiratory activity. Alternatively, all three events could coexist. The presence of any one can suffice to lead to arterial desaturation with unnecessary limitation of patient activities that could be misinterpreted as disease related, rather than device malfunction. To provide data on these possibilities, we compared the clinical performance and equivalency of prototypical conserving devices to each other, room air breathing, and standard O2 supplementation during progressive exercise in subjects with COPD. Our observations form the basis of this report. Some of our findings have been previously reported in the form of an abstract (15).
METHODS
We investigated four O2 conserving systems in a randomized double-blinded prospective fashion in a group of ambulatory adults with COPD. Our subjects were identified by chart review and recruited sequentially during outpatient visits to the pulmonary clinics. The admission criteria were the presence of persistent airway obstruction with hypoxemia that required therapy with supplemental O2 at flows of 2 L/min (2LO2). Hypoxemia was defined as an arterial O2 saturation less than 90% at rest on pulse oximetry (SpO2) or during the performance of a 6-minute walk (16). No attempt was made to include or exclude individuals with a predetermined level of severity. All subjects were clinically stable at the time of enrollment and remained on their routine medications during testing.
Spirometry was measured using consensus recommendations (17). Maximum forced exhalations were performed in triplicate with a waterless spirometer. The curves with the largest FEV1 and FVC were analyzed (17). The data were expressed in both absolute terms and as a percentage of predicted normal (18).
To focus our efforts on the state of the industry, we surveyed manufacturers, durable medical equipment vendors, and home health care organizations to determine the most commonly used conservers on the market at the time of study. From this, four prototypical production systems were purchased from commercial sources. The devices chosen were characteristic of two general categories: electronically triggered, single-cannula demand systems and pneumatically triggered single-cannula pulse instruments. The systems differed in engineering features and were representative of the most popular models. Each was bench tested using standard techniques to determine performance characteristics (11). Oxygen bolus volume was measured throughout the range of regulator settings at a simulated respiratory frequency of 20. The latter was chosen to imitate a typical exercise rate. Five sets of measurements were obtained and mean values ± 1 SEM computed. The “2,” “5,” and “continuous” settings on the regulators were chosen for study herein to cover the ranges commonly used therapeutically. After the measurements were completed, the devices were coded D1 through D4 and then clinically evaluated. Only the person setting up the experimental procedure on a given day knew which device was being assessed. The O2 tanks supplying the conservers were freshly filled before each experiment. Once attached to the testing circuit, the conserver was covered so that neither the individuals performing the protocols nor the patients could determine its identity. The unblinded technician then monitored patient safety and was not involved in data collection. The blind was maintained through study completion and data analysis. None of the medical personnel knew the engineering features of the conservers.
To establish whether breathing patterns played a role in system performance and to document that the devices activated appropriately with inspiration, the participants were instructed to inhale sequentially through their noses and then their mouths with the first use of the conservers while O2 delivery was recorded.
Exercise challenges were performed by having the subjects walk on a treadmill to tolerance using a modified Naughton protocol (20). Treadmill speed, grade, and duration of exertion were programmed to increment so that work levels increased in predefined stages. The metabolic equivalent task units (METs) per stage were obtained from published tables (21). The SpO2 was monitored with a Nellcor N-595 sensor (Pleasanton, CA) worn on the finger. Exercise was continued until SpO2 decreased to less than 90% or the subjects wished to stop.
Oral and nasal airflow were independently measured by having the participants wear separate low dead-space masks over their mouths and noses that contained a pneumotachograph (Hans Rudolph 4719 Series; Kansas City, MO) and a hot wire anemometer (TSI 4000 series; Shoreview, MN), respectively. The mask assemblies were suspended from a support rod over the subjects' heads. This spatial configuration of flow meters allowed an optimal fit of the masks and provided maximum comfort and ease of use for the volunteers. The two flows were electronically integrated to produce orifice-specific tidal volumes, respiratory frequencies, and minute ventilations (V·e).
The subjects wore standard-length nasal cannulas during testing. The flow of O2 into the tubing was sensed with a hot wire anemometer (TSI 4000 series; Shoreview, MN) in series with the outflow ports of the conserving systems. The O2 flow signal was used as a marker of triggering. It was also integrated per device activation and summed to provide the quantity of O2 actually delivered over the periods of observation.
The frequency responses of the experimental wave forms were matched electronically and displayed continuously on a time-based monitor. They were also digitized and stored in a personal computer for subsequent analysis. Heart rate was recorded continuously and blood pressure intermittently.
To determine the volume of O2 (VO2) potentially supplied to the subjects during each experimental condition, we first computed the amount present in a standard tidal breath of 500 ml of room air (RA) by multiplying by 21%. The measured pulse volumes for D1 through D4 at regulator settings of 2 and 5 were then added to the RA value and conserver-specific values for FiO2 were calculated for each setting. These values were then multiplied by 500 ml to ascertain the actual VO2 contained in a standardized breath provided by each instrument. In the continuous mode, O2 flow was measured for each device and found to lie between 1 and 3 L/min. To compute VO2, FiO2 values of 24, 28, and 32% were used for flows of 1, 2, and 3 L/min, respectively (22). Intermediate values were obtained by linear interpolation.
To compare conserver performances, the O2 pulse volumes measured for each conserver at the index settings in the ex vivo experiments were multiplied by the respiratory frequencies found at rest and during exercise in the clinical trials to predict the volume of O2 that should have been dispensed. These values were then contrasted with the actual amounts delivered.
The experimental protocol was performed in six sections during which the two control and four conserver experiments were undertaken. In all, the subjects completed a total of 14 exercise tasks over a 3- to 4-week period. To try to simulate real-world activity in a sedentary group of people and avoid training effects, day-to-day testing was scheduled solely according to subject availability and not placed on a rigorous timetable. The number of trials performed on a given visit varied depending upon the subjects' wishes and stamina. Before the first test on a given day, and in between trials, the subjects used their prescribed O2 devices and settings while they sat comfortably in a chair. During this time their gas exchange and vital signs were monitored. When the measurements were stable for 10 to 15 minutes, the exercise trial commenced as described below. If they had performed a previous period of work, the measurements had to have returned to their preexercise baseline and remained constant as above before undertaking another experiment. If a participant did not wish to be reexercised, they were rescheduled.
Each challenge followed the same general pattern. At the start of the test, resting measurements were obtained with the patient standing quietly on the treadmill for 1 minute. Exercise was then progressively increased to tolerance, during which time the various indices were constantly recorded. The last completed Naughton stage was used for analysis. Resting and final exercise data were compared. The first two trials in the test sequence consisted of RA breathing followed by 2LO2 from a wall supply through a standard flowmeter. During the RA experiment, the nasal cannulae were not attached to an O2 source. The RA and 2LO2 control trials were performed on the same day. With this information in hand, the identical procedures were repeated with the four conserving systems at 2, 5, and continuous settings. The devices were studied in random order on separate occasions. The different regulator settings for a given device were also randomly examined.
The primary endpoints were the work stages completed, SpO2, V·e, O2 delivered, and the synchronization of conserver activation with respiratory frequency. The breathing patterns were secondary variables. Statistical comparisons were performed with one- and two-factor analyses of variance, paired t tests, and regression analysis. To limit cumulative Type 1 statistical errors from multiple comparisons, post hoc testing was performed with Tukey comparison of means. Ninety-five percent confidence intervals (CI) were computed. Two-tailed P values of 0.05 or less were considered significant.
The institutional review board for human investigations approved the study protocol and written informed consent was obtained from each subject.
RESULTS
Subjects
Thirteen individuals with COPD (5 men and 8 women) with a mean age of 61.0 ± 1.7 years served as our subjects (Table 1). All had severe airway obstruction and were O2 dependent. Their FEV1 averaged 0.93 ± 0.11 L (31.2 ± 2.5% of predicted) (mean ± 1 SEM). The mean FVC was 2.59 L (69.0 ± 3.2% of predicted). The FEV1/FVC ratio was 36.5 ± 3.0%. The arterial saturation was 90.5 ± 1.0% on RA. Nine used auxiliary O2 at rest and four with exercise. On average they participated in 3.3 ± 0.2 exercise challenges per visit (range 1–6) with 8.1 ± 1.0 days (range 2–14 d) between testing periods.
TABLE 1.
SUBJECT CHARACTERISTICS
| N | 13 |
| Age, yr | 61.0 ± 1.7 |
| Sex, M/F | 5/8 |
| Race, W/AA | 10/3 |
| FEV1, L | 0.93 ± 0.11 |
| FEV1, % predicted | 31.2 ± 2.5 |
| FVC, L | 2.59 ± 0.20 |
| FVC, % predicted | 69.0 ± 3.2 |
| FEV1/FVC, % | 36.5 ± 3.0 |
| SpO2, % | 90.5 ± 1.02 |
| O2 at rest, n/% | 9/69 |
| O2 exertion only, n/% | 4/31 |
Definition of abbreviations: AA = African American; F = female; M = male; N = number of subjects; SpO2 = arterial oxygen saturation as measured by pulse oximetry; W = white.
The last two rows indicate the number and percentage of subjects using supplemental O2 at rest and with exertion.
Engineering Features of Conservers
The technical features of the conserving devices are displayed in Table 2. A variety of combinations of performance categories, inspiratory trigger sensitivities, and O2 bolus sizes were represented. Three systems were pulse types and pneumatically activated. One was a demand style and was electronically triggered. The O2 boluses delivered at regulator settings of 2 varied from 3.2 ± 0.2 to 33.2 ± 1.1 ml, whereas those at a setting of 5 ranged between 7.1 ± 0.5 and 83.4 ± 1.6 ml. At the continuous settings, the instruments provided 100% O2 at flows from 1.60 (D3) to 2.30 (D2) L/min (D1 = 1.80, D4 = 1.96 L/min) resulting in VO2 of 132 to 146 ml in a 500-ml breath.
TABLE 2.
DEVICE CHARACTERISTICS
| Device | Performance Category | Actuation Style | Trigger ΔP (cm H2O) | O2 Bolus Delivered (ml) Setting 2 | Setting 5 | Continuous* |
|---|---|---|---|---|---|---|
| D1 | Pulse | Pneumatic | 0.3 | 23.4 ± 1 | 43.4 ± 2.0 | 136 |
| D2 | Pulse | Pneumatic | 0.4 | 3.2 ± 0.2 | 7.1 ± 0.5 | 146 |
| D3 | Pulse | Pneumatic | 0.3 | 22.8 ± 0.7 | 35.4 ± 1.6 | 132 |
| D4 | Demand | Electronic | 0.3 | 33.2 ± 1.1 | 83.4 ± 1.6 | 136 |
Definition of abbreviations: D = device; ΔP = change in pressure.
The performance category indicates whether O2 is delivered only early in inspiration (pulse) or throughout (demand). The activation style describes the mechanism by which the flow from the conserver is initiated. Trigger pressure refers to the magnitude of the pressure drop necessary to start flow. The O2 bolus is the volume of gas delivered during activation. These data are means ± 1 SEM.
In the continuous setting, O2 boluses were not generated. Here, O2 was provided at a constant flow varying from 1.60 to 2.40 L/min. The data in this column represent the volume of O2 in ml that would be contained in a 500-ml tidal breath. (See text for details of calculation).
The resulting FiO2 values and expected volumes of O2 supplied per 500 ml air for each trial are shown in Table 3. With the RA and 2LO2 controls, the FiO2 values were 21.0 and 28.0%, respectively, and the VO2 values were 105 and 140 ml. With the conservers, using the mean O2 bolus delivery presented in Table 2 and rounding to the highest whole number, the FiO2 ranged from 21.6 to 27.6% at a setting of 2 resulting in VO2 between 108 and 138 ml. The rank order of the quantity of O2 provided from most to least was: 2LO2 ≥ D4 > D1 = D3 > D2 ≥ RA. At a setting of 5, VO2 increased 17.1% on average and the pattern changed to: D4 > D1 > D3 = 2LO2 > D2 > RA. At the continuous settings, the mean FiO2 and VO2 values tended to be intermediate between the 2 and 5 settings. The exception was D2, where settings 2 and 5 were similar and continuous was significantly larger. The pattern was now D2 > 2LO2 > D1 = D4 > D3 > RA.
TABLE 3.
OXYGEN DELIVERED
| Control Values | ||||||
|---|---|---|---|---|---|---|
| Trial | FiO2 (%) | VO2 (ml/500 ml) | ||||
| RA | 21.0 | 105 | ||||
| 2LO2 | 28.0 | 140 | ||||
| Conserver Values | ||||||
| Setting 2 |
Setting 5 |
Continuous |
||||
| Device |
FiO2 |
VO2 |
FiO2 |
VO2 |
FiO2 |
VO2 |
| D1 | 25.6 | 128 | 29.6 | 148 | 27.2 | 136 |
| D2 | 21.6 | 108 | 22.4 | 112 | 29.2 | 146 |
| D3 | 25.6 | 128 | 28.0 | 140 | 26.8 | 132 |
| D4 | 27.6 | 138 | 37.6 | 188 | 27.2 | 136 |
| Mean | 25.1 | 126 | 29.4 | 147 | 27.6 | 137 |
Definition of abbreviations: D = device; RA = room air; VO2 = ml of O2 present in a 500-ml breath of air at the stated FiO2; 2LO2 = O2 at a flow of 2 L/min.
Trial indicates resting and exercise protocols while breathing with the various gas delivery systems. Settings 2, 5, and continuous refer to the regulator settings examined.
Clinical Performance of Conservers
Impact of breathing patterns.
All of the conservers initiated O2 delivery with inspiration both through the nose and mouth and the subjects' patterns of breathing did not play a role in device activation or performance. Three (23%) inhaled primarily through their nose, two (13%) only through the mouth, and the remainder (62%) used both orifices.
Individual example of exercise performance and conserver activity.
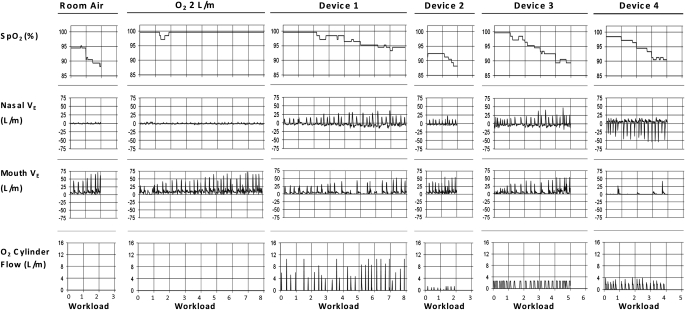
Figure 1 provides a representative example of the physiologic events and conserver performance seen during each experiment. These data were obtained at a regulator setting of 2. There was no O2 flow from the conservers during the RA and 2LO2 trials. Oxygen saturation decreased with exertion with RA and all of the conservers, but not with 2LO2. Note that the work loads completed varied considerably as a function of the device used and the sustainable SpO2. With RA and D2, only stage 2 was reached and SpO2 fell below 90%. With 2LO2 and D1, the work level quadrupled over that with RA. With D3 and D4, the amount achieved was 2 to 2.5 times RA and SpO2 hovered between 90 and 91%. This subject showed a mixed breathing pattern. She inhaled predominately through her mouth with RA and 2LO2 and her nose with D4. In the other experiments, she used both orifices. Note the inconsistent activations of the various conservers with inspiration.
Figure 1.
Representative example of the findings in an individual subject during each trial. The labeling at the top of each column denotes the separate trials; that at the bottom indicates the Naughton stage achieved. The nasal and mouth airflow signals are unidirectional because only inspiration is displayed. In the Device 4 experiment, the polarity was reversed. Note the decreases in percent arterial saturation as measured by pulse oximetry (SpO2) with exertion with each conserver, the fluctuating breathing pattern, and the inconsistent conserver actuations and bolus sizes. Nasal V·e = the minute ventilation with nose breathing in L/min. Mouth V·e = the minute ventilation with mouth breathing in L/min; O2 cylinder flow = the flow rate of O2 from each conserver in L/min.
Oxygen saturation, work loads, and conserver function.
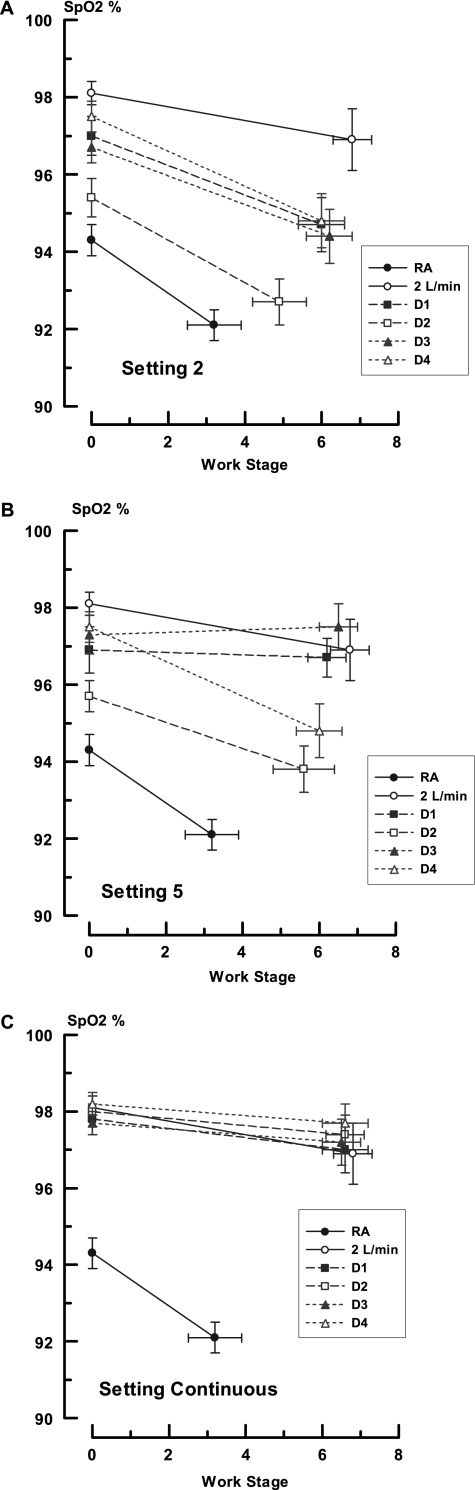
Group values for SpO2 during the trials with RA, 2LO2, and each of the devices at each setting are contained in Figure 2. Factorial analysis demonstrated that there were significant differences between conserver systems and settings (P < 0.001). At a regulator setting of 2, resting SpO2 varied from a low of 94.3 ± 0.4% on RA to a high of 98.1 ± 0.3% on 2LO2, with all of the conservers falling in between these extremes (CI, Δ 2LO2–RA, 5.2–2.4; P < 0.001). The rank order was 2LO2 = D4 = D1 > D3 > D2 = RA and generally followed the quantities of O2 provided by each system as shown in Table 3. Among the conservers, D1, D3, and D4 were equivalent (CI, D1–D4, 0.83–1.9; P = 0.84; CI, D1–D3, 1.7–1.1; P = 0.98; CI, D3–D4, 0.51–2.26; P = 0.44) and all three provided higher SpO2 values than RA (CI, D1–RA, 4.1–1.3; P < 0.001; CI, D3–RA, 3.7, 1.0; P = 0.001; CI, D4–RA, 4.6–1.9; P < 0.001). Devices D1 and D4 were significantly better than D2 (CI, D1–D2, 3.0–0.2; P = 0.02; CI, D4–D2, 0.76–3.5; P = 0.0004), but D3 was not (CI, D3–D2, 0.2–2.6; P = 0.09). Of note, the SpO2 with conserver D2 was statistically equivalent to RA (CI, D2–RA, 2.5–0.29; P = 0.20).
Figure 2.
Changes in pulse oximetry during rest and exercise in all experiments. The ordinate displays arterial saturation as measured by pulse oximetry in percent (SpO2) and the abscissa displays Naughton work stages. The insert indicates the studies performed with room air (RA), O2 at flows of 2 L/min (2LO2), and devices 1 through 4 (D1–D4). The data points are mean values and the brackets indicate 1 SEM. The zero values represent resting observations. A, B, and C depict the experiments performed at regulator settings of 2, 5, and continuous, respectively.
With exertion, SpO2 decreased significantly in all (P < 0.001) but the 2LO2 trial (P = 0.18 rest-to-exercise comparisons). The highest SpO2 was with 2LO2 (96.9 ± 0.8%) and the lowest with RA (91.8 ± 0.4%). As with the resting data, the values found with the conserving devices ranged between these boundaries (CI, 2LO2–RA, 7.2–2.5; P < 0.001) The rank order remained unchanged (2LO2 = D4 = D1 > D3 > D2 = RA). Conservers D1 and D4 were similar to 2LO2 (CI, D1–2LO2, 4.5–0.08; P = 0.06; CI, D4–2LO2, 4.4–0.2; P = 0.09). Devices D2 and D3 were less effective (CI, D2–2LO2, 6.6–2.0; P < 0.001; CI, D3–2LO2; 4.9–0.25; P = 0.02). The SpO2 values with D1, D3, and D4 were statistically similar (CI, D1–D3; 2.6–2.0; P = 0.99; CI, D1–D4, 2.21–2.4; P = 1.00; CI, D3–D4, 1.8–2.8; P = 0.99) and were all better than RA (CI, D1–RA, 4.9–0.3; P = 0.01; CI, D3–RA, 4.6–0.01; P = 0.03; CI, D4–RA, 5.0–0.5; P = 0.001). As in the resting experiments, D2 was no better than inhaling ambient air (CI, D2–RA, 2.9–1.7; P = 0.98). While breathing RA, the subjects were able to accomplish very little in the way of exercise (average Naughton stage = 3.2 ± 0.7; 1.5 ± 0.6 metabolic equivalents [METS]). The work level achieved increased significantly with 2LO2 (Naughton stage = 6. 8 ± 0.5; 4.6 ± 0.8 METS; P = 0.006). All of the conserving devices fell between these extremes and there were no statistical differences between systems (P = 0.27). Device 2 performed least well (final work stage = 4.9 ± 0.7).
At a regulator setting of 5 (Figure 2B), the mean resting SpO2 levels with the conservers varied from 95.4 ± 0.4% (D2) to 97.5 ± 0.5% (D4) (P < 0.001). All of the values were equivalent to 2LO2 (98.1 ± 0.03%) (CI, D1–2LO2, 2.7–0.3; P = 0.17; CI, D3–2LO2, 2.4–0.6; P = 0.50; CI, D4–2LO2, 1.1–1.9; P = 0.97) save for D2, which was smaller (CI, D2–2LO2; 3.9–0.9; P = 0.0002). Despite increased bolus sizes and VO2 (Tables 2 and 3), the resting SpO2 for D1 (96.9 ± 0.6%) and D2 (95.7 ± 0.4%) were not appreciably different from setting 2 (P = 0.70 and P = 0.37, respectively). The levels with D3 (97.3 ± 0.5%) and D4 (98.5 ± 0.4%), however, did improve significantly (P = 0.03 and P = 0.008, respectively). With exercise, the previously seen drop with exertion was blunted for the conservers as a whole, but there were still significant differences between performances (P < 0.001). Devices D1, D3, and D4 behaved like 2LO2 (CI, D1–2LO2, 2.3–1.8; P = 0.99; CI, D3–2LO2, 1.4–2.7; P = 0.95; CI, D4–2LO2, 1.0–3.2; P = 0.62). Conserver D2 was significantly less effective than any other form of O2 delivery (CI, D2–2LO2, 3.2–1.1; P = 0.0005; CI, D2–D4, 2.2–6.4; P < 0.0001). The new rank order was 2LO2 = D4 = D1 = D3 > D2 > RA. The expected response based on the theoretical considerations in Table 3 was D4 > D1 > D3 = 2LO2 > D2 > RA.
As O2 delivery increased during respiration, so did the work accomplished. With the 5 regulator setting, the maximum Naughton stage among devices now ranged from 5.6 ± 0.8 (3.6 ± 0.8 METS) with D2 to 6.5 ± 0.5 (4.3 ± 0.7 METS) with D3 (P = 0.78).
At a continuous setting (Figure 2C), the physiologic differences between delivery systems disappeared and all SpO2 values were equivalent to each other. The resting SpO2 varied from 97.7 ± 0.3% (D3) to 98.2 ± 0.3% (D4) (P = 0.78). Saturation did not change with exertion and the final values grouped between 96.7 ± 0.8% (2LO2) and 97.4 ± 0.5% (D2) (P = 0.90). The pattern was D4 = D2 = D3 = D1 = 2LO2 > RA. The expected response based on simple O2 availability was D2 > 2LO2 > D1 = D4 ≥ D3 > RA (Tables 2 and 3). The terminal work stages ranged between 6.8 ± 0.5 (4.3 ± 0.8 METS) (2LO2) and 6.5 ± 0.5 (4.5 ± 0.5 METS) (D3) (P = 0.99).
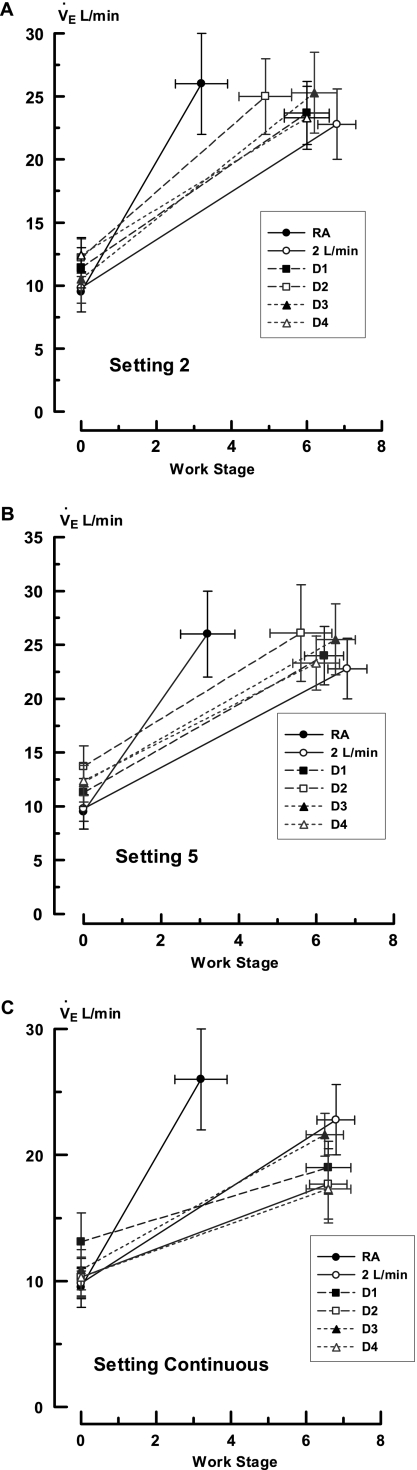
The changes in V·e are presented in Figure 3. Here too, factorial analysis demonstrated significant differences between conserver systems and settings (P < 0.001). At a regulator setting of 2, the resting V·e ranged from 9.8 ± 1.2 L/min with 2LO2 to 12.4 ± 1.3 L/min with D4 (Figure 3A). There were no significant differences between the different protocols (P = 0.60). With exertion, V·e approximately doubled and the final values with each challenge remained statistically identical (range 22.8 ± 2.8 L/min with 2LO2 to 26.0 ± 4.0 L/min with RA; P = 0.95); however, they occurred at different workloads. To account for this phenomenon, the data were recalculated as a V·e/work ratio (Table 4). Using this format, there were significant differences in V·e between trials with a conserver setting of 2, but not at 5 or continuous. The effect of a regulator setting of 2 was due to the relatively high V·e/work ratio in the RA trial. There were no significant differences among conservers or between them and 2LO2 (P = 0.99 for all comparisons). The performance distribution at this regulator position was 2LO2 = D4 = D3 = D1 = D2 > RA and varied from that of SpO2 at the same settings. The performance distribution from the best to worst V·e/work ratio was 2LO2 > D3 ≥ D4 ≥ D1 > D2 > RA (P < 0.001). This distribution varied from that of SpO2 at the same settings. Of note, the V·e/work values for D2 were significantly different between the various regulator settings. Those for D1, D3, and D4 were not.
Figure 3.
Minute ventilations during all experiments. The ordinate displays minute ventilation (V·e) in L/min and the abscissa displays Naughton work stages. The insert indicates the studies performed with room air (RA), O2 at flows of 2 L/min (2LO2), and devices 1 through 4 (D1–D4). The data points are mean values and the brackets indicate 1 SEM. The zero values represent resting observations. A, B, and C depict the experiments performed at regulator settings of 2, 5, and continuous, respectively.
TABLE 4.
VENTILATION–WORKLOAD RATIOS DURING EXERTION
| Setting | RA | 2LO2 | D1 | D2 | D3 | D4 | P Value |
|---|---|---|---|---|---|---|---|
| 2 | 12.6 ± 3.4 | 3.5 ± 0.4 | 4.5 ± 0.8 | 5.4 ± 0.9 | 4.3 ± 0.7 | 4.4 ± 0.8 | <0.001 |
| 5 | — | 3.5 ± 0.4 | 4.1 ± 0.5 | 5.4 ± 0.9 | 4.2 ± 0.7 | 3.7 ± 0.8 | 0.31 |
| Continuous | — | 3.5 ± 0.4 | 3.0 ± 0.4 | 2.7 ± 0.5 | 3.5 ± 0.6 | 2.8 ± 0.5 | 0.55 |
| P value | — | 1.0 | 0.18 | 0.03 | 0.63 | 0.26 | — |
Definition of abbreviations: D = device; RA = room air; 2LO2 = O2 at a flow of 2 L/min.
The P values at the end of the rows and columns represent between- and within-group comparisons, respectively. The RA and 2LO2 trials are compared with the conserver setting of 2 data. The 2LO2 trials are compared with the regulator setting of 2 conserver data. The lower the ratio, the less the ventilatory requirements for a given work load. The data are mean values ± 1 SEM.
Regulator settings of 5 did not change resting V·e but lessened the variance between devices. As a result, the V·e/work ratio differences disappeared (P = 0.31; Figure 3B). Delivering O2 continuously caused the V·e to decrease approximately 20%. The reduction was significant for all devices (P < 0.02) but there were no differences between them (P = 0.55).
Predicted versus actual O2 delivery.
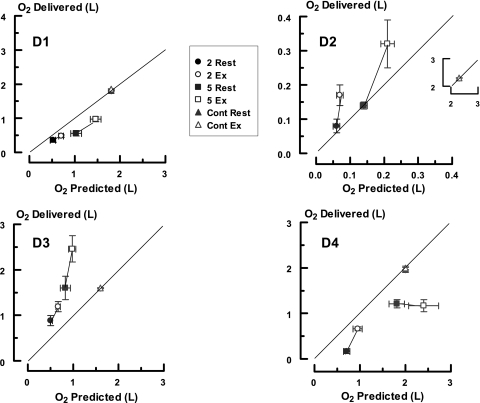
A comparison of the expected and delivered quantities of O2 for each instrument in each trial based on ex vivo pulse volume and in vivo integrated O2 flow is shown in Figure 4. There was no consistent pattern and each conserver displayed unique characteristics that deviated considerably from expected performance. By paired comparisons D1 always provided less O2 than predicted at regulator settings of 2 and 5 (P < 0.001 for both). At a continuous setting, it delivered 1.8 L/min O2 at rest and exertion. Device 2 behaved as expected during resting breathing at both settings but overshot during exercise (P < 0.001 for both). It delivered 2.3 L/min O2 when on a continuous setting. Device 3 constantly surpassed expectations at the 2 and 5 settings (P < 0.001) but greatly underperformed when on continuous. Here, O2 flow was 1.6 L/min. This value was actually 67% less than what was delivered with exercise at a regulator setting of 5. Device 4 constantly underachieved with rest and exercise at the 2 and 5 settings (P < 0.001 for both) but met performance specifications at rest and exercise when set on continuous (1.96 L/min O2). In addition, there were significant differences between O2 delivery at rest and during exercise for settings 2 and 5 for D1, D2, and D3 (P < 0.01 for all), but not D4 (P = 0.27 setting 2; P = 0.70 setting 5).
Figure 4.
Comparison of the predicted and delivered quantities of O2 provided by each conserver. The abscissa displays the predicted values derived from ex vivo testing and the ordinate the amounts actually provided during in vivo use. The data points are mean values and the brackets 1 SEM. The solid lines are the lines of identity. The solid symbols indicate data obtained at rest; open symbols depict exercise. The prefixes 2, 5, and cont refer to the 2, 5, and continuous regulator settings studied. The notations D1, D2, D3, and D4 indicate the various conservers. The insert in the D2 graph presents the findings with the continuous setting.
Mechanical performance of conservers.
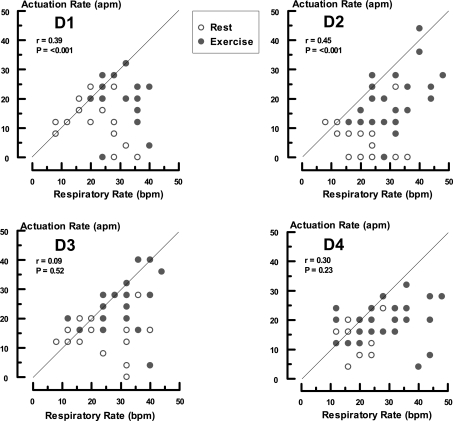
The reasons for the above phenomena lie in the mechanical characteristics of the conservers (Figures 5 and 6). All of the units demonstrated suboptimal activation with breathing. There was modest to poor synchronization between respiratory frequency and device triggering with correlation coefficients ranging from 0.45 (D2) to 0.09 (D3) (Figure 6). The average number of breaths/activation was 1.3 for D1, 1.9 for D2, 1.5 for D3, and 1.3 for D4. Conservers 1 and 2 had more favorable triggering ratios during exercise (D1 rest = 1.5, exercise = 1.2; D2 rest = 2.9, exercise = 1.6 breaths/activation). Device 4 was better during quiet breathing (D4 rest = 1.2, exercise = 1.7 breaths/activation) and with D3, the state of physical activity made no difference (D3 rest = 1.5, exercise = 1.5 breaths/activation).
Figure 5.
Comparison of conserver firing and respiratory frequency for each instrument. The ordinates indicate conserver actuation per minute (apm). The abscissa indicates breaths per min (bpm). The data points are individual values obtained at the 2 and 5 regulator settings. The notations D1 through D4 represent each device. The solid lines are the lines of identity.
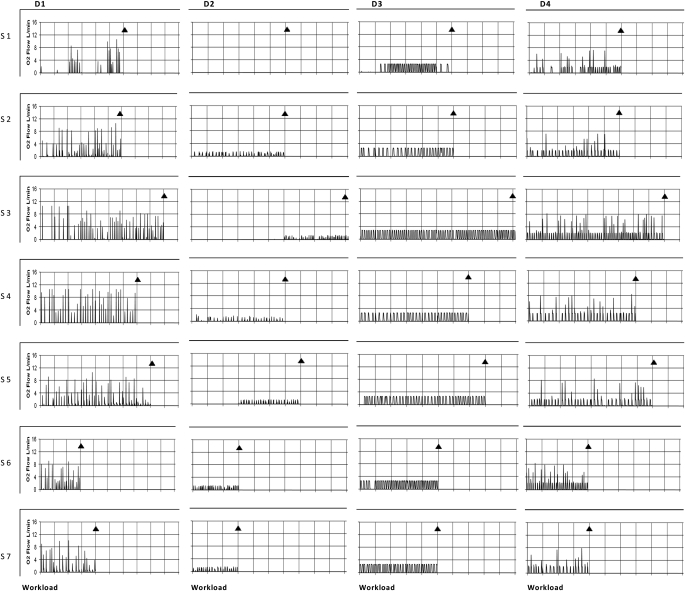
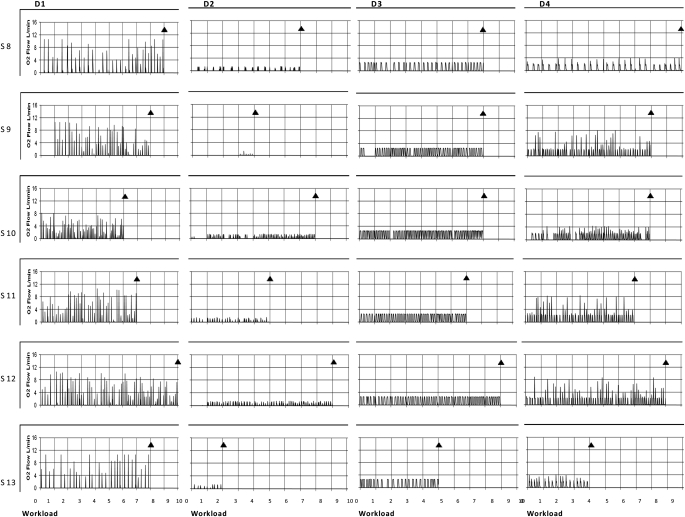
Figure 6.
The patterns of oxygen cylinder flow associated with each conserver at a regulator setting of 2. These data represent individual O2 flow profiles at rest and during exercise in each subject for each device. D1 to D4 indicates device number. S1 to S13 indicates subject number. The ordinates are O2 flow in L/min and the abscissas are the Naughton work stages completed. The zero time point indicates resting values. The termination of exercise is marked by diamonds in each graph. The prominent features are the sporadic pattern of firing and irregular flow profiles. Note that Device 2 went for long periods without activating.
The complete O2 flow profiles and triggering patterns are provided in Figure 6. All systems demonstrated sporadic firing frequencies. This was most marked in D2 where O2 delivery did not occur for prolonged periods. In addition, when the systems did activate, O2 rates were highly inconsistent and unpredictable. In three of the systems (D1, D2, and D4), flow changed 2.0 to 2.5 times on a breath–by-breath basis. With D3, flow was stable. A regulator setting of 5 increased the magnitude of the respective flow signals but had no major influence on behavior (data not shown). Only the continuous settings eliminated the performance disparities, but it destroyed the conserving activities. Delivery performance irregularities were constant within a prototype and independent of the patient using them.
DISCUSSION
The findings of the present study demonstrate that as a group the current classes of O2 conservers contain inherent technical limitations that result in highly inconsistent performances that vary with regulator setting and physical activity. None of the devices examined are functionally identical as assumed by regulatory agencies. Rather, there are major operational irregularities both between and within systems that materially compromise clinical usefulness. As a result, some devices work better at rest than with exercise and some work poorly with both. One instrument was no better than RA. Although each device activated during nose and mouth breathing, none consistently performed according to engineering expectations. There was poor synchronization between triggering and inspiration, erratic O2 flows, and major discrepancies between the predicted and actual amounts of O2 delivered. Two conservers provided less O2 than anticipated, one more, and in one only the resting values were in agreement with specifications. Such behavior was device rather than subject dependent and was similar from patient to patient. Raising O2 pulse volume by increasing the regulator settings had no major impact on instrument performance. Even abolishing conservation by using the continuous setting did not eradicate all disparities.
The physiologic impact of these abnormalities is a significant reduction in oxygenation and exercise capability. At a regulator setting of 2, only a single device provided a level of SpO2 similar to the 2LO2 standard at rest. With exercise, two devices met this criterion, but unlike 2LO2, none of the instruments could maintain the preactivity SpO2 values.
An important question to ask is whether such issues have any clinical relevance. Our data suggest the answer to be an unqualified “yes.” The primary purpose of O2 conservers is to allow people with hypoxemia to function freely outside of their usual care environment with minimal symptoms. Our findings show that instrument design is a major unrecognized impediment to achieving this goal. Simplistically stated, exercise tolerance derives from the quantity of O2 provided to working muscles. This, in turn, is determined by the FiO2, and the increases in V·e and cardiac output that occur with exertion (23). Pulmonary diseases diminish O2 availability by altering ventilation–perfusion relationships and interfering with the normal cardiopulmonary homeostatic mechanisms that increase O2 acquisition and delivery. When this occurs, arterial O2 saturation and content decrease, resulting in less physical activity (23). In such circumstances, with all else being equal, simply increasing FiO2 raises systemic O2 availability; hence, more work can be accomplished with fewer symptoms. Figures 3 and 4 clearly demonstrate that the achievable exercise levels were related to the conserver used and were independent of the underlying gas exchange impairments, severity of airway obstruction, or fitness level of the patients. From a practical standpoint, such phenomena readily translate into patients voluntarily limiting what they do physically to avoid unpleasant side effects. From here, it is a small step to the mistaken impression by patients and physicians alike that such behavior is disease related rather than conserver related. Possibilities such as these are likely part of the reason some have suggested that patients should undergo pulse oximetry titration with their prescribed devices to determine performance capabilities (24). Although this is certainly a worthwhile suggestion, given that there are at least 18 different systems on the market (5), it is a cumbersome task. A more functional approach would be to develop uniform performance standards rather than requiring patients to adapt to the limits of their machines.
To our knowledge, our study is the most detailed examination of general and comparative conserver behavior undertaken to date. Most (8–23), but not all (14) early studies concluded that both demand and pulse O2 delivery systems are equivalent to continuous flow; however, these efforts were either performed at rest or at low levels of exercise and included no, or minimal, assessments of the cardiopulmonary costs (8–14, 23). Thus, device performance was stressed neither technically nor physiologically. Nor was it completely evaluated. As shown in the present study such approaches materially underestimate operational limitations. Nonetheless even in the trials purporting to show equivalency a review of individual patient data demonstrates an unexplained inability of both types of conservers to uniformly maintain SpO2 (11, 13, 14).
Examination of conserver performance during more strenuous activity and/or comparison of different designs makes this thread more evident. Although there is no constant pattern of physiologic response, there is no consistent evaluation of integrated clinical engineering function. Roberts and colleagues (7) reported that demand O2 delivery devices were inferior to continuous flow in most measured variables during a 6-minute walk. In contrast, Garrod and associates (25) concluded that pulsed-dose oxygen conservers and continuous O2 were equally effective based on a lack of statistical differences in median values. In this study, however, the mean decrease in SpO2 with exertion was 35% greater with the conserver and more patients had major desaturation. Braun and colleagues (6) in their comparison of demand systems noted significant differences between instruments in maintaining SpO2 during activity. They also pointed out the presence of severe desaturation in some patients with each device when using prescribed O2 flows. Similar distinctions between devices were observed by Langenhof and Fichter (26). Fuhrman and associates (27) also found major disparities in the performance of the four devices they studied. In opposition, Strickland and colleagues (28) examined four systems and concluded that there were no significant differences in SpO2 or exercise performance between them. It is critical to appreciate that in both the Fuhrman and colleagues (27) and Strickland and colleagues (28) studies all of the conservers were associated with exercise desaturation so that the average SpO2 was less than 84%. From our data, it seems reasonable to speculate that the unexplained differences between patients and devices in the above trials might derive from the technical limitations of the instruments tested. Engineering specification were accepted at face value and not tested, and it was assumed that O2 bolus sizes were constant and delivered consistently with respiration.
We do not believe that our findings were influenced by our patient population, instrument selection, or study design. Our subjects' symptoms, degree of airway obstruction, exercise tolerance, and O2 needs are typical of those with COPD in the literature (6–14, 25–30) Admittedly, our subjects' O2 requirements are modest, but we would argue that if even these cannot be met, it is unlikely that more pressing needs can be accommodated. In an attempt to deal with real-world issues, conserver selection was based on popularity in the marketplace and types of technical features. Our instruments were all unmodified standard models that were purchased from commercial sources. No attempt was made to pick extreme examples. They were evaluated ex vivo with repetitive measures using published techniques (19) and studied clinically with a random double-blinded protocol using well-tested consensus endpoints (23). The O2 pulse volumes observed are similar to other investigations (5), as are the findings that the conserver regulator settings have no standard relationship to O2 liter flow (31). Here too, systematic invalidating errors seem unlikely.
We understand that although we are drawing general conclusions, because we did not test all of the instruments on the market, our results may not have bearing on specific models. This we believe to be the major limitation of our study. Nonetheless, the conservers we tested were among the most commonly used clinically and were representative of the various classes available, leading us to believe that our findings are likely widely applicable. At a minimum, they point out the pitfalls in assuming unquestioned equality. Conformation of our suppositions awaits systematic evaluation of the technical–clinical interactions present in existing devices. Our data strongly suggest that both of these elements will need to be evaluated in future instruments to ensure optimum performance.
Supported in part by General Clinical Research Center Grant M01 RR00080 from the National Center for Research Resources, United States Public Health Services, and a Grant in Aid from Invacare Corporation.
Originally Published in Press as DOI: 10.1164/rccm.200910-1638OC on February 4, 2010
Conflict of Interest Statement: A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.D. received $1,001–$5,000 from Invacare Corporation in consultancy fees for sleep therapy instrumentation development. E.R.M. received $1,001–$5,000 from Merck in lecture fees (CME), and more than $100,001 from GlaxoSmithKline in industry-sponsored grants for B2AR Genetics.
References
- 1.Nocturnal Oxygen Therapy Trial Group. Continuous nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed] [Google Scholar]
- 2.Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981;1:681–686. [PubMed] [Google Scholar]
- 3.Doherty DE, Petty TL, for the Writing and Organizing Committees. Recommendations of the 6th long-term oxygen therapy consensus conference. Respir Care 2006;51:519–525. [PubMed] [Google Scholar]
- 4.McCoy RW. Oxygen-conserving techniques and devices. Respir Care 2000;45:95–103. [PubMed] [Google Scholar]
- 5.Bliss PL, McCoy RW, Adams AB. Characteristics of demand oxygen delivery systems: maximum output and setting recommendations. Respir Care 2004;49:160–165. [PubMed] [Google Scholar]
- 6.Braun SR, Spratt G, Scott GC, Ellersieck M. Comparison of six oxygen delivery systems for COPD patients at rest and during exercise. Chest 1992;102:694–698. [DOI] [PubMed] [Google Scholar]
- 7.Roberts CM, Bell J, Wedzicha JA. Comparison of the efficacy of a demand oxygen delivery system with continuous low flow oxygen in subjects with stable COPD and severe oxygen desaturation on walking. Thorax 1996;51:831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mecikalski M, Shigeoka JW. A demand valve conserves oxygen in subjects with chronic obstructive pulmonary disease. Chest 1984;86:667–670. [DOI] [PubMed] [Google Scholar]
- 9.Tiep BL, Nicotra B, Carter R, Phillips R, Otsap B. Low-concentration oxygen therapy via a demand oxygen delivery system. Chest 1985;87:636–638. [DOI] [PubMed] [Google Scholar]
- 10.Rinow ME, Saltzman AR. Effectiveness of a new oxygen demand valve in chronic hypoxemia. Chest 1986;90:204–207. [DOI] [PubMed] [Google Scholar]
- 11.Tiep BL, Carter R, Nicotra B, Berry J, Phillips RE, Otsap B. Demand oxygen delivery during exercise. Chest 1987;91:15–20. [DOI] [PubMed] [Google Scholar]
- 12.Bower JS, Brook CJ, Zimmer K, Davis D. Performance of a demand oxygen saver system during rest, exercise, and sleep in hypoxemic patients. Chest 1988;94:77–80. [DOI] [PubMed] [Google Scholar]
- 13.Carter R, Tashkin D, Djahed B, Hathaway E, Nicotra MB, Tiep BL. Demand oxygen delivery for patients with restrictive lung disease. Chest 1989;96:1307–1311. [DOI] [PubMed] [Google Scholar]
- 14.Senn S, Wanger J, Fernandez E, Cherniack RM. Efficacy of a pulsed oxygen delivery device during exercise in patients with chronic respiratory disease. Chest 1989;96:467–472. [DOI] [PubMed] [Google Scholar]
- 15.Palwai A, Skowronski M, Coreno A, Drummond C, McFadden ER Jr. Comparison of O2 conserving devices for patients with hypoxemia [abstract]. Am J Respir Crit Care Med 2007;175:A372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society. ATS statement; guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, et al. Standardization of spirometry. Series “ATS/ERS task force: standardization of lung function testing.” Number 2. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 19.Bliss PL, McCoy RW, Adams AB. A bench study comparison of demand oxygen delivery systems and continuous flow oxygen. Respir Care 1999;44:925–931. [Google Scholar]
- 20.Naughton J, Sevellus G, Balke B. Physiologic responses of normal and pathologic subjects to a modified work capacity test. J Sports Med 2001;31:963. [PubMed] [Google Scholar]
- 21.Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990;13:555–565. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro BA, Harrison RA, Walton JR. Clinical application of blood gases, 3rd ed. Chicago: Year Book Medical Publishers; 1994. pp. 169–179.
- 23.Wasserman IK, Hansen JE, Darryl YS, Sue DY, Casaburi R, Whipp DJ. Principles of exercise testing and interpretation. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 1999.
- 24.Gallegos LC, Shigeoka JW. Novel oxygen-concentrator-based equipment: take a test drive first! Respir Care 2006;51:25–28. [PubMed] [Google Scholar]
- 25.Garrod R, Gestall JC, Paul E, Wedzicha JA. Evaluation of pulse dose oxygen delivery during exercise in patients with severe chronic obstructive pulmonary disease. Thorax 1999;54:242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langenhof S, Fichter J. Comparison of two demand oxygen delivery devices for administration of oxygen in COPD. Chest 2005;128:2082–2087. [DOI] [PubMed] [Google Scholar]
- 27.Fuhrman C, Chouaid C, Herigault R, Housset B, Adnot S. Comparison of four demand oxygen delivery systems at rest and during exercise for chronic obstructive pulmonary disease. Respir Med 2004;98:938–944. [DOI] [PubMed] [Google Scholar]
- 28.Strickland SL, Hogan MT, Hogan RG, Sohal HS, McKenzie WN, Petroski GF. A randomized multi-arm repeated-measures prospective study of several modalities of protable oxygen delivery during assessment of functional exercise capacity. Respir Care 2009;54:344–349. [PubMed] [Google Scholar]
- 29.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2008. Accessed June 11, 2009. Available from http://www.goldcopd.com/
- 30.Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 31.Knight A. No LPM equivalent for pulse dose. Letters. Respir Manage 2009;June:9.