Abstract
Rationale: The clinical pathology describing infants with chronic lung disease of infancy (CLDI) has been limited and obtained primarily from infants with severe lung disease, who either died or required lung biopsy. As lung tissue from clinically stable outpatients is not available, physiological measurements offer the potential to increase our understanding of the pulmonary pathophysiology of this disease.
Objectives: We hypothesized that if premature birth and the development of CLDI result in disruption of alveolar development, then infants and toddlers with CLDI would have a lower pulmonary diffusing capacity relative to their alveolar volume compared with full-term control subjects.
Methods: We measured pulmonary diffusing capacity and alveolar volume, using a single breath-hold maneuver at elevated lung volume. Subjects with chronic lung disease of infancy (23–29 wk of gestation; n = 39) were compared with full-term control subjects (n = 61) at corrected ages of 11.6 (4.8–17.0) and 13.6 (3.2–33) months, respectively.
Measurements and Main Results: Alveolar volume and pulmonary diffusing capacity increased with increasing body length for both groups. After adjusting for body length, subjects with CLDI had significantly lower pulmonary diffusing capacity (2.88 vs. 3.23 ml/min/mm Hg; P = 0.0004), but no difference in volume (545 vs. 555 ml; P = 0.58).
Conclusions: Infants and toddlers with CLDI have decreased pulmonary diffusing capacity, but normal alveolar volume. These physiological findings are consistent with the morphometric data obtained from subjects with severe lung disease, which suggests an impairment of alveolar development after very premature birth.
Keywords: pulmonary diffusing capacity, alveolar volume, lung parenchyma, bronchopulmonary dysplasia
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The pulmonary pathology obtained from infants with chronic lung disease of infancy (CLDI) has been limited and we know little about the development of the lung parenchyma of premature infants.
What This Study Adds to the Field
Our study demonstrates that clinically stable infants and toddlers with chronic lung disease of infancy after very premature birth have reduced pulmonary diffusing capacity, but normal alveolar volume. These physiological findings are consistent with morphometric data suggesting an impairment of alveolar development.
Treatment of premature infants with artificial surfactant, as well as improved respiratory and nutritional support, has lowered the limits of viability of extremely preterm infants to less than 25 weeks of gestation (1). Very premature birth now occurs in the saccular stage of parenchymal development, which may have an important impact on subsequent growth and development of the lung parenchyma. Improved respiratory management of very premature infants may have resulted in less severe airway disease than seen in the original description of bronchopulmonary dysplasia (BPD) (2); however, there appears to be a new parenchymal pathology referred to as “new” BPD or chronic lung disease of infancy (CLDI) (3). Autopsied lungs from infants who were born between 24 and 30 weeks of gestation and received mechanical ventilation ranging from days to weeks have enlarged alveoli, reduced alveolar number, decreased internal surface area, and marked simplification of acinar structure (4–7). These pathologic findings are believed to be the sequelae of very premature birth, as well as lung injury secondary to the ventilator and oxygen support provided to sustain these very premature infants. The clinical pathology describing infants with CLDI has been limited and obtained primarily from infants with severe lung disease, who either died or required lung biopsy. Therefore, we know little about the development of the lung parenchyma of premature infants with chronic lung disease of infancy, particularly those who leave the hospital and are clinically stable outpatients. As lung tissue from these clinically stable outpatients is not readily available, physiological measurements offer the potential to increase our understanding of the pulmonary pathophysiology of CLDI. We hypothesized that if premature birth and the development of CLDI results in disruption of alveolar development with fewer but larger alveoli, then infants and toddlers with CLDI would have a lower pulmonary diffusing capacity relative to their alveolar volume when compared with infants and toddlers born at full term. Some of the results of this study have previously been reported in the form of an abstract (8).
METHODS
Subjects
CLDI.
Subjects born prematurely (24–29 wk of gestation) and having a diagnosis of BPD were recruited from the Neonatal Intensive Care Unit or the Pediatric Pulmonary Clinic of James Whitcomb Riley Hospital for Children (Indianapolis, IN) between 2007 and 2009. BPD was defined as an oxygen requirement at 36 weeks of gestation. Evaluated subjects were clinically stable outpatients with no oxygen requirement at the time of testing and no acute respiratory symptoms for at least 3 weeks.
Healthy full-term control subjects.
Full-term (≥37 wk of gestation) subjects were recruited on the basis of advertisements in local publications in Indianapolis, Indiana. All subjects had a respiratory history negative for wheezing, asthma, heart disease, or hospitalization for a respiratory illness. Data from 54 control infants were previously reported (8). Subjects were evaluated between 2007 and 2009.
Pulmonary Diffusing Capacity and Alveolar Volume
Subjects were evaluated at James Whitcomb Riley Hospital for Children (Indianapolis, IN) while sleeping in a supine position after chloral hydrate sedation (50–100 mg/kg); oxygen saturation and heart rate were continuously monitored. The study was approved by the institutional review boards at Indiana University and informed parental consent was obtained.
Pulmonary diffusing capacity (DlCO) and alveolar volume (Va) were measured at an elevated lung volume defined by an airway pressure of 30 cm of H2O using a single breath-hold technique (9). The inspiratory test gas contained 4% He, 0.3% C18O, 20% O2, and balance N2 and the alveolar concentrations of C18O and He were calculated as the average concentrations between 60 and 80% of the passive expired volume following the 4-second breath hold. Va and DlCO were expressed as the averages of two or three measurements within 10% of each other. A blood sample from a finger stick was obtained for hemoglobin (Hb) concentration (Hb 201; HemoCue, Lake Forest, CA); DlCO was adjusted for Hb, as recommended by the American Thoracic Society/European Respiratory Society Task Force (10). As an assessment of ventilation inhomogeneity, which can result in lower values for alveolar volume and diffusing capacity, we calculated the slope of the percent helium concentration versus expired volume over the 60–80% volume range in which alveolar gas concentrations were measured (11). To adjust for differences in somatic size among subjects, a nondimensional slope was calculated by dividing the slope by the average expired helium concentration and the total expired volume (12, 13).
Analysis
Demographic variables and patient characteristics at test date were compared between CLDI and control groups, using two-sample t tests for continuous variables and chi-square tests for binary variables. Linear regression models were used to evaluate the effect of CLDI status, gestational ages on Va and DlCO. To evaluate the effect of CLDI status, we employed an analysis of covariance (ANCOVA) model with CLDI status, body length at test date as covariates. We also used linear regression models to study the gestational age effect on Va and DlCO and to adjust for body length at test. SAS 9.1.3 software (SAS Institute, Inc, Cary, NC) was used to conduct all of the analyses.
RESULTS
Subjects
Thirty-nine infants with CLDI at corrected ages between 4 and 18 months were evaluated between 2007 and 2009. The demographics for the subjects with CLDI and the 61 full-term control subjects are summarized in Table 1. Those with CLDI were all born premature and therefore they had significantly lower gestational ages and weights at birth; however, there were no significant differences in sex or race. At the time of testing, the subjects with CLDI had lower body weights and lengths, whereas they had a small, but significantly higher Hb level compared with full-term control subjects (12.7 vs. 12.1; P < 0.003). Subjects born premature were also more frequently exposed to maternal smoking during pregnancy compared with subjects born full term (P < 0.001).
TABLE 1.
DEMOGRAPHICS
| Variable | Subjects with CLDI [mean (SD)] | Full-term Subjects [mean (SD)] | P Value |
|---|---|---|---|
| Number of subjects | 39 | 61 | |
| Gestational age, wk | 26 (1.7) | 39 (0.9) | <0.001 |
| Corrected age at test date, mo | 11.6 (3.8) | 13.4 (6.1) | 0.082 |
| Birth weight, kg | 0.87 (0.24) | 3.45 (0.50) | <0.001 |
| Weight at test date, kg | 8.94 (1.74) | 9.77 (2.09) | 0.041 |
| Length at test date, cm | 71.7 (5.6) | 74.6 (7.7) | 0.028 |
| Weight at test date, Z-score | −0.20 (1.22) | 0.29 (0.95) | 0.027 |
| Length at test date, Z-score | −0.90 (1.04) | −0.40 (0.92) | 0.013 |
| Hb, g/dl | 12.7 (1.16) | 12.1 (0.90) | 0.003 |
| Mechanical ventilation, d (range) | 19 (0–83) | ||
| CPAP, d (range) | 18 (0–49) | ||
| Supplemental oxygen, d (range) | 82 (33–170) | ||
| Female, n/N, % | 21/39 (53.8%) | 30/61 (49.2%) | 0.649 |
| White, n/N, % | 25/39 (64.1%) | 34/61 (55.7%) | 0.407 |
| Maternal smoking during pregnancy, n/N, % | 11/39 (28.2%) | 3/61 (4.9%) | 0.001 |
Definition of abbreviations: CLDI = chronic lung disease of infancy; CPAP = continuous positive airway pressure; Hb = hemoglobin.
Alveolar Volume and Pulmonary Diffusing Capacity
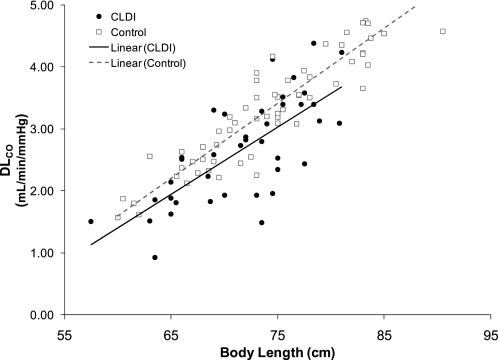
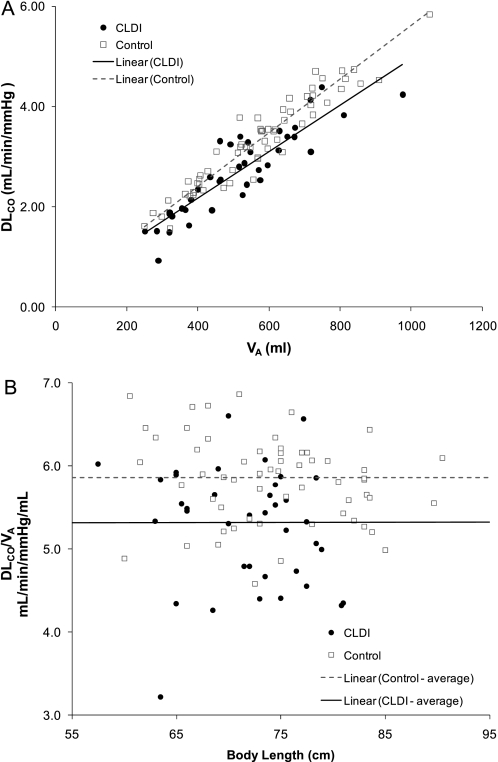
Alveolar volume increased with increasing body length for subjects with CLDI and control subjects (Figure 1). When adjusted for body length by ANCOVA, there was no significant difference for Va between the two groups (Va, 545 vs. 555 ml; P = 0.58). Pulmonary diffusing capacity adjusted for Hb also increased with increasing body length for both groups (Figure 2); however, subjects with CLDI had a significantly lower DlCO compared with control subjects when adjusting for body length by ANCOVA (DlCO, 2.88 vs. 3.23 ml/min/mm Hg; P = 0.0004). A similar difference in DlCO was present between the two groups when using values of DlCO unadjusted for Hb. Pulmonary diffusing capacity adjusted for alveolar volume by ANCOVA was significantly lower for subjects with CLDI compared with control subjects (2.7 vs. 3.4 ml/min/mm Hg; P < 0.0001) (Figure 3A). In addition, the ratio of pulmonary diffusing capacity to alveolar volume (DlCO/Va) was not related to body length for either group (Figure 3B), and subjects with CLDI had significantly lower values for DlCO/Va compared with control subjects (5.3 vs. 5.9 ml/min/mm Hg/ml; P < 0.0001). There was no significant difference between the nondimensional slopes for CLDI and control groups (0.146 ± 0.106 vs. 0.115 ± 0.071; P = 0.12). When maternal smoking during pregnancy, which differed for the two groups, was included in the model, it was not significant (P > 0.35–0.7), and did not alter the previously described results; therefore, smoking was not included in the model.
Figure 1.
Alveolar volume, Va (ml), versus body length (cm). Individual data for subjects with chronic lung disease of infancy (CLDI) (solid circles) and control subjects (open squares) are presented, as well as the linear regressions for each group. There was no significant difference in Va when subjects with CLDI were compared with control subjects when adjusted for body length by analysis of covariance (P = 0.58).
Figure 2.
Pulmonary diffusing capacity, DlCO (ml/min/mm Hg), versus body length (cm). Individual data for subjects with chronic lung disease of infancy (CLDI) (solid circles) and control subjects (open squares) are presented, as well as the linear regressions for each group. DlCO was significantly lower for subjects with CLDI compared with control subjects when adjusted for body length by analysis of covariance (P = 0.0004).
Figure 3.
(A) Pulmonary diffusing capacity, DlCO (ml/min/mm Hg), versus alveolar volume, Va (ml). Individual data for subjects with chronic lung disease of infancy (CLDI) (solid circles) and control subjects (open squares) are presented, as well as the linear regressions for each group. DlCO was significantly lower for subjects with CLDI compared with control subjects when adjusted for alveolar volume by analysis of covariance (P = 0.0004). (B) Ratio of pulmonary diffusing capacity to alveolar volume, DlCO/Va (ml/min/mm Hg/ml), was not related to body length. Individual data for subjects with CLDI (solid circles) and control subjects (open squares) are presented, as well as the average data for each group. DlCO/Va was significantly lower for subjects with CLDI compared with control subjects (P = 0.0004).
We also evaluated whether gestational age at birth could account for additional variability of alveolar volume and pulmonary diffusing capacity, after adjusting for body length at the time of testing. As the subjects with CLDI and full-term control subjects were significantly different for gestational age with no overlap, we analyzed the effect of gestational age in each group separately. For both the subjects with CLDI and full-term control subjects, gestational age at birth was a significant covariate with alveolar volume: the lower the gestational age at birth, the smaller the alveolar volume at the time of testing, after adjusting for body length at the time of testing. Table 2 summarizes the analysis with body length at time of testing and gestational age at birth included in the model. For DlCO, the subjects with CLDI also demonstrated that the lower the gestational age at birth, the smaller the pulmonary diffusing capacity at the time of testing, after adjusting for body length at the time of testing (Table 2). The full-term control subjects exhibited a similar relationship, although it did not achieve statistical significance (P < 0.10). Adjusting for exposure to maternal smoking and race showed little improvement in R2 and similar gestational age effects; therefore, maternal smoking and race were not included in the model.
TABLE 2.
ANALYSIS WITH BODY LENGTH AT TIME OF TESTING AND GESTATIONAL AGE AT BIRTH
| Subjects with CLDI |
Control Subjects |
|||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P Value | R2 | Estimate (95% CI) | P Value | R2 | |
| Va | ||||||
| Intercept | −1,774.3 (−2,379.5, −1,169.1) | <0.001 | 0.70 | −1,900.0 (−2,710.9, −1,089.1) | <0.001 | 0.86 |
| Body length at testing, cm | 23.2 (17.8, 28.6) | <0.001 | 20.5 (18.1, 22.9) | <0.001 | ||
| Gestational age at birth, wk | 24.0 (6.1, 41.9) | 0.010 | 24.2 (2.7, 45.7) | 0.028 | ||
| DlCO | ||||||
| Intercept | −9.10 (−12.56, −5.63) | <0.001 | 0.62 | −9.13 (−13.42, −4.84) | <0.001 | 0.87 |
| Body length at testing, cm | 0.11 (0.08, 0.14) | <0.001 | 0.12 (0.11, 0.13) | <0.001 | ||
| Gestational age at birth, wk | 0.16 (0.05, 0.26) | 0.004 | 0.09 (−0.02, 0.21) | 0.106 | ||
Definition of abbreviations: CI = confidence interval; DlCO = pulmonary diffusing capacity; Va = alveolar volume.
DISCUSSION
Our study is the first to demonstrate that clinically stable infants and toddlers with chronic lung disease of infancy after very premature birth have reduced pulmonary diffusing capacity when compared with healthy control subjects born full term. This finding is present when DlCO is adjusted for body length or alveolar volume. Although morphometric data from autopsy or biopsy specimens have reported fewer, but larger, alveoli from subjects with severe CLDI, our physiological data suggest that abnormal growth and development of the lung parenchyma occur even in clinically stable infants and toddlers with less severe lung disease.
We found that alveolar volume increased with body length for infants and toddlers with CLDI, as well as for full-term control subjects; however, there was no difference between the groups in alveolar volume adjusted for body length. When gestational age at birth and body length at time of evaluation were included as independent variables, alveolar volume increased with increasing gestational age, as well as with increasing body length when each group was analyzed separately. This finding suggests that the degree of lung parenchymal development at the time of birth may be an important determinant of subsequent alveolar development. Among our subjects with CLDI, this relationship could potentially be driven by lower gestational age reflecting more severe CLDI, as gestational age was highly correlated with exposure to mechanical ventilation and supplemental oxygen. However, gestational age was a significant covariate, not only among subjects with CLDI, but even among our healthy full-term control subjects, for whom there was a relatively limited range of gestational ages (37–40 wk). This observation in the full-term control subjects suggests that gestational age per se may be an important determinant for subsequent lung growth.
Similar to alveolar volume, DlCO increased with gestational age, as well as body length in subjects with CLDI. When the analysis was limited to full-term control subjects, there was a similar relationship, although it did not achieve statistical significance (P < 0.11), which may reflect the limited range of gestational ages in this group (37–40 wk). When analyzing DlCO/Va, subjects with CLDI had significantly lower values compared with control subjects; neither body length at testing nor gestational age at birth was a significant covariate. Our findings indicate that infants and toddlers who were born very premature and developed CLDI had significantly lower pulmonary diffusing capacities compared with full-term control subjects, whether adjusted for body length or alveolar volume. Although the differences in pulmonary diffusing capacity between the CLDI and full-term groups appear to be present over the age range we evaluated, we are not able to determine from our cross-sectional data whether subjects with CLDI exhibit catch-up in alveolar growth and development. When comparing DlCO for the two groups, approximately 25% of subjects with CLDI had low values for DlCO; however, many subjects with CLDI had values similar to those for healthy control subjects. The evaluation of genetic variants of factors that contribute to parenchymal development, as well as sex, as risk factors for worse CLDI will require of a much larger population of subjects.
There are several limitations to our study. Our population of premature subjects included only those with very premature birth and the development of CLDI. Future studies that include healthy premature infants between 30 and 36 weeks of gestation and not needing oxygen or mechanical ventilation will be required to address the independent effects of premature birth on lung growth and development. The importance of evaluating this population of healthy infants born premature has been highlighted by the lower lung function reported by several investigators (14–16). Second, several factors other than the alveolar–capillary unit can influence our measured values of DlCO. In our study of infants and toddlers, alveolar volume and pulmonary diffusing capacity were measured with a 4-second breath-hold technique; we cannot achieve a 10-second maneuver, as used in older children and adults. Although 4 seconds is adequate to obtain gas equilibration in healthy lungs (17), ventilation inhomogeneity may contribute to inadequate gas equilibration within the lung during the breath-hold maneuver, thus underestimating alveolar volume and pulmonary diffusing capacity (11). We found no significant difference between the CLDI and control groups for the nondimensional slopes over the region that alveolar gas concentration was estimated. This finding suggests marked ventilation inhomogeneity was not present in our CLDI group, which is consistent with data presented by Hülskamp and colleagues, who reported a small effect on lung clearance index (LCI) with neonatal oxygen exposure; 100 days of oxygen exposure during the neonatal period correlated with an increase in LCI from approximately 7.0 to 7.3 (18). This small increase in LCI in infants with CLDI contrasts to a much higher LCI observed in clinically stable outpatient infants with cystic fibrosis (CF), who have an LCI of 8.4 (19). The much higher LCI of infants with CF compared with those with CLDI is consistent with CF being primarily an airway disease with marked ventilation inhomogeneity, whereas CLDI or “new BPD” may be primarily a parenchymal disease. Asthma, which is primarily an airway disease, is associated with ventilation inhomogeneity and elevated LCI even in clinically stable outpatients compared with healthy nonasthmatic subjects (8.2 vs. 6.6). After induced bronchoconstriction and marked increase in ventilation inhomogeneity, Verbanck and coworkers reported that the measured DlCO declined by only 6% (11). Cardiac output and ventilation–perfusion inhomogeneity can also affect the measured DlCO. Although we did not measure cardiac output, our infants and toddlers with CLDI were clinically stable outpatients without oxygen requirement, they did not have a diagnosis of pulmonary hypertension, and they were not using cardiac medications. We do not anticipate that they would have lower cardiac outputs than the control subjects, particularly during sleep. Our subjects were also assessed when supine, which should minimize ventilation–perfusion inhomogeneity related to gravity. Therefore, we believe that our observed decrease in DlCO/Va for our subjects with CLDI reflects a decrease in gas transfer across the alveolar capillary unit, which may be secondary to abnormal development of the lung parenchyma. As we did not partition DlCO into its capillary membrane and pulmonary capillary bed components, we were not able to determine whether these two components were equally affected. If there was an arrested development of alveolar capillary units, we would anticipate that both components would be affected.
In summary, clinically stable infants and toddlers with CLDI have decreased pulmonary diffusing capacity when adjusted for body length or alveolar volume. These physiological findings are consistent with the morphometric data obtained from subjects with severe lung disease, which suggests an impairment of alveolar development after very premature birth.
Supported by NIH grant HL054062.
Originally Published in Press as DOI: 10.1164/rccm.200908-1190OC on February 4, 2010
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955. [DOI] [PubMed] [Google Scholar]
- 2.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. N Engl J Med 1967;267:357–368. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. [DOI] [PubMed] [Google Scholar]
- 4.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8:73–81. [DOI] [PubMed] [Google Scholar]
- 5.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 6.Margraf LR, Tomashefski JF Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 1991;143:391–400. [DOI] [PubMed] [Google Scholar]
- 7.Stocker JT. Pathologic features of long-standing “healed” bronchopulmonary dysplasia: a study of 28 3- to 40-month-old infants. Hum Pathol 1986;17:943–961. [DOI] [PubMed] [Google Scholar]
- 8.Balinotti J, Chakr V, Tiller C, Kimmel R, Coates C, Kisling J, Nguyen J, Yu Z, Tepper RS. Decreased pulmonary diffusing capacity in infants and toddlers born premature [abstract]. Am J Respir Crit Care Med 2009;179:A4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo A, Llapur CJ, Martinez T, Kisling J, Williams-Nkomo T, Coates C, Tepper RS. Measurement of single breath-hold carbon monoxide diffusing capacity in healthy infants and toddlers. Pediatr Pulmonol 2006;41:544–550. [DOI] [PubMed] [Google Scholar]
- 10.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735. [DOI] [PubMed] [Google Scholar]
- 11.Verbanck S, Schuermans D, Van MS, Vincken W, Thompson B. The effect of conductive ventilation heterogeneity on diffusing capacity measurement. J Appl Physiol 2008;104:1094–1100. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson PM. Peripheral airway involvement in CF and asthma compared by inert gas washout. Pediatr Pulmonol 2007;42:168–176. [DOI] [PubMed] [Google Scholar]
- 13.Macleod KA, Horsley AR, Bell NJ, Greening AP, Innes JA, Cunningham S. Ventilation heterogeneity in children with well controlled asthma with normal spirometry indicates residual airways disease. Thorax 2009;64:33–37. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich L, Pitrez PM, Stein RT, Goldani M, Tepper R, Jones MH. Growth rate of lung function in healthy preterm infants. Am J Respir Crit Care Med 2007;176:1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoo AF, Dezateux C, Henschen M, Costeloe K, Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr 2002;141:652–658. [DOI] [PubMed] [Google Scholar]
- 16.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med 2002;165:83–87. [DOI] [PubMed] [Google Scholar]
- 17.Turcotte RA, Perrault H, Marcotte JE, Beland M. A test for the measurement of pulmonary diffusion capacity during high-intensity exercise. J Sports Sci 1992;10:229–235. [DOI] [PubMed] [Google Scholar]
- 18.Hülskamp G, Lum S, Stocks J, Wade A, Hoo AF, Costeloe K, Hawdon J, Deeptha K, Pillow JJ. Association of prematurity, lung disease and body size with lung volume and ventilation inhomogeneity in unsedated neonates: a multicentre study. Thorax 2009;64:240–245. [DOI] [PubMed] [Google Scholar]
- 19.Lum S, Gustafsson P, Ljungberg H, Hülskamp G, Bush A, Carr SB, Castle R, Hoo AF, Price J, Ranganathan S, et al.; London Cystic Fibrosis Collaboration. Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax 2007;62:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]