Abstract
Rationale: Premature newborns frequently require manual ventilation for resuscitation during which lung injury occurs. Although surfactant protein (SP)-D regulates pulmonary inflammation, SP-D levels are low in the preterm lung. Commercial surfactants for treatment of respiratory distress syndrome do not contain SP-D.
Objectives: To determine whether addition of recombinant human SP-D (rhSP-D) to commercial surfactant influences lung inflammation in ventilated premature newborn lambs.
Methods: Prematurely delivered lambs (130 d gestation age) were resuscitated with 100% O2 and peak inspiratory pressure 40 cm H2O for 20 minutes and then treated with Survanta or Survanta containing rhSP-D. Ventilation was then changed to regulate tidal volume at 8 to 9 ml/kg. At 5 hours of age lambs were killed for sample collection.
Measurements and Main Results: Sequential blood gas and tidal volume were similar in lambs treated with or without rhSP-D, indicating that lung immaturity and ventilatory stress used to support premature lambs were comparable between the two groups. Ventilation caused pulmonary inflammation in lambs treated with surfactant alone. In contrast, surfactant containing rhSP-D decreased neutrophil numbers in bronchoalveolar lavage fluid and decreased neutrophil elastase activity in lung tissue. IL-8 mRNA and IL-8 protein were significantly decreased in the +rhSP-D group lamb lungs, to 20% of those in controls. The addition of rhSP-D also rendered Survanta more resistant to plasma protein inhibition of surfactant function.
Conclusions: Treatment with rhSP-D–containing surfactant inhibited lung inflammation and enhanced the resistance of surfactant to inhibition, supporting its potential usefulness for prevention of lung injury in the preterm newborn.
Keywords: respiratory distress syndrome, surfactant treatment, IL-8, newborn resuscitation, lung injury
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Premature newborns require manual ventilation for resuscitation during which lung injury can occur contributing to development of chronic lung disease. Although surfactant protein (SP)-D has antiinflammatory function, commercially available surfactants for treatment of respiratory distress syndrome do not contain SP-D.
What This Study Adds to the Field
Addition of recombinant human SP-D to commercial surfactant improved surfactant function by protecting the premature lung from inflammation induced by ventilation and by making it more resistant to protein inhibition of surfactant function. Treatment with surfactant containing recombinant human SP-D represents a potential therapy for lung disease associated with prematurity.
Surfactant protein (SP)-D is a member of the collectin family of innate host defense proteins. SP-D has antiinflammatory functions and inhibits production of proinflammatory cytokines and chemokines in the lung (1). In the premature newborn lung with respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD), SP-D levels are low (2, 3). Commercially available surfactants for prevention and treatment of RDS contain phospholipids, SP-B, and/or SP-C, but do not contain the collectin family members SP-A or SP-D. Although both SP-A and SP-D modulate a number of immune cell functions, studies have shown that they play diverse roles in the suppression of lung inflammation (4). Addition of SP-A to surfactant for treatment was not effective in reducing lung inflammation in the ventilated premature newborn lamb (5). Premature newborns are routinely resuscitated by manual ventilation in the delivery room followed by mechanical ventilation and surfactant treatment in the neonatal intensive care unit. The premature lung requires high inflating pressures and oxygen for adequate ventilation and oxygenation and is highly susceptible to injury because of its structural immaturity, surfactant deficiency, presence of fetal lung fluid, and immature immune system, factors that are likely to contribute to the development of the chronic lung disease, BPD. Surfactant treatment is routinely given to extremely preterm infants as early as possible after birth to minimize lung injury, improve surfactant distribution, and minimize inhibition of surfactant function by leaked proteins (6). In clinical practice surfactant treatment is typically administered within 20 minutes after birth, a time during which lung injury may occur. Because susceptibility of the premature newborn lung to ventilation is generally accepted, lung-protective ventilatory strategies are increasingly used for resuscitation. Despite surfactant replacement and changes in ventilation techniques, the incidence of BPD remains a significant clinical problem. We hypothesized that the addition of recombinant human SP-D (rhSP-D) to commercial surfactant for treatment would inhibit inflammation in the ventilated premature newborn lung. We used a moderate ventilation style to support the prematurely delivered lamb model of RDS and assessed the efficacy of rhSP-D–containing surfactant treatment given at 20 minutes of age to inhibit lung inflammation.
Development of superior surfactant preparations that protect the premature lung from inflammation caused by ventilation and hyperoxia, and are more resistant to plasma protein inhibition, may be useful in protecting the preterm lung from injury and BPD.
METHODS
Delivery, Resuscitation, Surfactant Treatment, and Ventilation of the Premature Lamb
Protocols were approved by the Animal Care and Use Committee of the Cincinnati Children's Research Foundation. Premature lambs were delivered by cesarean section at 130 days' gestation age (GA) (term 150 d GA) and tracheostomized as described previously (5, 7). Premature newborn lambs were resuscitated with 100% O2, peak inspiratory pressure (PIP) of 40 cm H2O, 4 cm H2O positive end-expiratory pressure (PEEP), and respiratory rate of 40/min using pressure-limited ventilator (Sechrist Industries, Anaheim, CA). To avoid overstretch of the premature newborn infant lung during manual ventilation, the clinical resuscitation bag has a pressure relief valve set at 40 cm H2O and therefore PIP for resuscitation was limited to 40 cm H2O for the study. Premature lambs at this GA require surfactant treatment to survive. At 20 minutes of age, two groups of lambs were treated with Survanta (Abbott Laboratories, Columbus, OH) mixed with rhSP-D (+rhSP-D group) or buffer (control group), using two boluses for instillation (8). rhSP-D was synthesized as previously described (7, 9, 10). Seven milligrams of rhSP-D in 5 ml buffer (20 mM Tris, 200 mM NaCl, 1 mM ethylenediaminetetraacetic acid, pH 7.4) or 5 ml buffer alone were mixed with a clinical treatment dose of Survanta (100 mg/4 ml/kg), amounts that are similar to both SP-D and surfactant lipid pool sizes in the normal term newborn lung (10, 11). The surfactant mixture was prepared by one investigator and the study was performed blindly. After surfactant treatment, the PIP was decreased to regulate tidal volume (Vt) at 8 to 9 ml/kg (Bicore Monitoring Systems, Anaheim, CA) and Fio2 was adjusted to maintain a target Po2 of 100 to 150 mm Hg. Ventilatory rate, inspiratory time of 0.6 seconds, and PEEP were not changed.
Based on our previous experience, the 5-hour study period was chosen to detect changes in proinflammatory cytokine mRNAs induced by initial ventilation (12). After 5 hours, lambs were ventilated with Fio2 = 1 for 5 minutes, then given 100 mg pentobarbital intravascularly, after which the endotracheal tube was clamped to permit oxygen absorption atelectasis (7, 13). After the thorax was opened, the deflation limb of pressure-volume curve was measured (5, 7). Lung tissue of the right lower lobe was frozen in liquid nitrogen for RNA isolation and measurement of neutrophil elastase (NE) activity (14, 15). NE activity was assessed by a spectrophotometric assay using chromogenic substrate specific for NE: N-methoxy-succinyl-Ala-Ala-Pro-Val pNA (16). Sequences of primers for quantitative reverse transcriptase–polymerase chain reaction are:
For IL-8: 5′-TGGCCAGGATTCACGAGTTC and 5′-TCTGTGAGGTAGAAAGATGACTGAGATATT
For IL-6: 5′-GGAGGAAAAAGATGGATGCTTCCAA and 5′-CAGCAGTGGTTTTGATCAAGCAA
For IL-1β: 5′-GGCTCTCCACCTCCTCTCA and 5′-AGCTCATGCAGAACACCTT
For tumor necrosis factor (TNF)-α: 5′-GCCGGAATACCTGGACTATGC and 5′-CAGGGCGATGATCCCAAAGTAG
For keratinocyte-derived chemokine (KC): 5′-TGCCAGTGCCTGCAGAC and 5′-AGTGGCTATGACTTCGGTTTGG
For monocyte chemotactic protein 1 (MCP1): 5′-CCCCGACTATCTGTTTCCACAAC and 5′-CCTGGAAGGGCTTCTGATCTG
For ovine ribosomal protein L32 5′-GCAGAAGATTCAAGGGCCAGATC and 5′-GGTTTTCTTGTTGCTCCCGTAAC
Lung tissue of the right middle lobe was homogenized in 0.9% NaCl and supernatant after centrifugation at 1,000 × g for 15 minutes was frozen for ELISA of proinflammatory cytokine proteins (5, 7). Bronchoalveolar lavage fluid (BALF) was recovered from the left lung (7) for further analyses. Total proteins were analyzed (14) in the supernatant of BALF after 10 minutes of centrifugation at 284 × g. The right upper lobe was inflation-fixed at 30 cm H2O for morphology (10). The amount of rhSP-D in aliquots of BALF was analyzed by ELISA (7).
Surfactant Surface Activity and Resistance against Protein Inhibition
Surface tension was measured by captive bubble surfactometer (17) on 3 μL of samples containing 15 μg/μl Survanta and 2% rhSP-D or buffer in the presence or absence of surfactant inhibitor (21 μg/μl plasma protein) (18). This amount of plasma protein relative to Survanta was 30% lower than the concentration that is known to inhibit the activity of Survanta in the ventilated premature newborn lamb lung in vivo (19). The influence of rhSP-D on the ultrastructure of Survanta was studied as previously described (20).
Data Analysis
Results are given as means ± SEM. Comparisons between +rhSP-D and control groups were made with two-tailed unpaired t tests. For multiple groups, one-way analysis of variance (ANOVA) followed by Bonferroni-Dunn test, or two-way repeated measures ANOVA were used. Significance was accepted at P less than 0.05.
RESULTS
rhSP-D Treatment Did Not Alter Lung Function
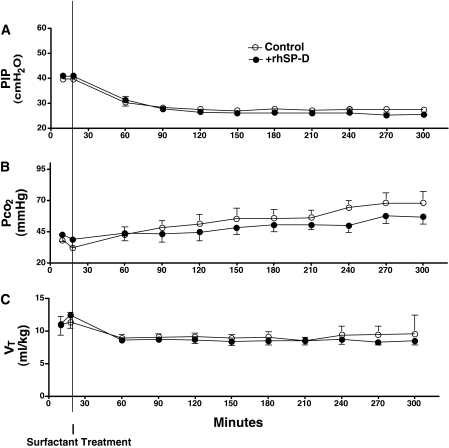
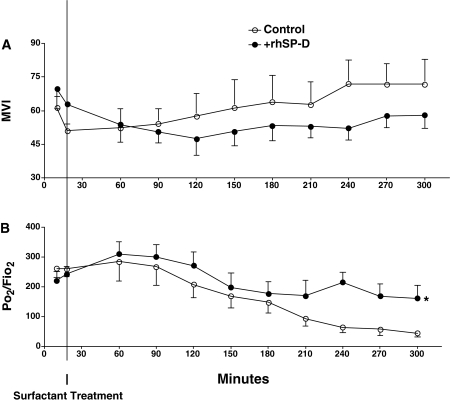
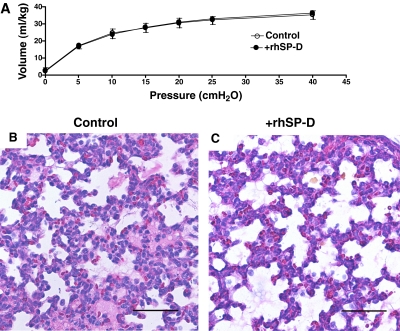
Six control and six rhSP-D–treated lambs were studied. Sex (three male and three female per group), cord blood pH (7.36 ± 0.04 [control], 7.36 ± 0.05 [+SP-D]), body weight (3.0 ± 0.3 [control], 3.0 ± 0.1 kg [+SP-D]), and lung weight (116 ± 15 [control], 115 ± 5 g [+SP-D]) were similar between +rhSP-D and control groups. Blood pressure, heart rate, hematocrit, and glucose, sodium, potassium, and calcium in the blood samples were recorded every 30 minutes and were normal throughout the study period (data not shown). Rectal temperature was maintained at the normal body temperature for sheep (38.5°C) by means of heating pads, radiant heat, and plastic body-covering wrap. Ventilation was regulated well for both groups according to the protocol. Lambs were resuscitated with PIP 40 cm H2O for 20 minutes after birth (Figure 1A), which resulted in mean Pco2 40 mm Hg (Figure 1B) and Vt 11 ml/kg (Figure 1C) for both groups. After surfactant treatment given at 20 minutes of age, ventilation was changed to regulate Vt at 8 to 9 ml/kg (Figure 1C) and required a mean PIP of 27 cm H2O (Figure 1A) for both groups. These results indicated that lung immaturity, as well as ventilatory stress used to support premature lambs, were comparable between the groups. A modified ventilation index was calculated as PIP × Pco2 × respiratory rate/1,000 (21). Although it did not reach statistical significance, mean modified ventilation index was better for the +rhSP-D group after 240 minutes (Figure 2A). High Fio2 (0.75–1.0) was required for both groups to maintain Po2 at the target. Premature lambs at this GA have patent ductus arteriosis, and Po2/Fio2 may not be directly associated with lung function. Nevertheless, Po2/Fio2 was higher in the +rhSP-D group than control group (P < 0.01 by two-way repeated measures ANOVA) (Figure 2B). Po2/Fio2 was significantly decreased with time after 210 minutes (P < 0.05 by one-way ANOVA) in the control group. Deflation limb of pressure-volume curves was not different between the groups (Figure 3A). Likewise, lung morphology was similar for both groups with typical findings consistent with immaturity, including thickened alveolar septal walls and patchy atelectasis. More fluid was noted in alveoli of the control lambs compared with the +rhSP-D lambs (Figures 3B and 3C).
Figure 1.
Lung physiology. Per protocol, ventilation was carefully regulated for both groups. (A) Premature newborn lambs were resuscitated after birth by ventilation with peak inspiratory pressure (PIP) 40 cm H2O, resulting in (B) mean Pco2 40 mm Hg and (C) mean Vt 11 ml/kg for both groups. Surfactant was given at 20 minutes of age and ventilation was changed (C) to regulate Vt at 8 to 9 ml/kg, requiring (A) a mean PIP of 27 cm H2O for both groups. These data indicate that lung immaturity, as well as ventilatory support of the premature lambs, was comparable between control lambs and lambs treated with recombinant human SP-D (+rhSP-D). Some error bars are within symbols.
Figure 2.
Modified ventilation index (MVI) and Po2/Fio2. (A) MVI was calculated as peak inspiratory pressure × Pco2 × respiratory rate/1,000. Although not significant, MVI tends to be better (lower) for the group treated with recombinant human surfactant protein D (+rhSP-D) group at later times. (B) Po2/Fio2 was higher in +rhSP-D group compared with control. *P < 0.01 by two-way repeated measures analysis of variance (ANOVA) (overall comparison of control versus +rhSP-D group). Po2/Fio2 was significantly decreased with time after 210 minutes in control group (P < 0.05 vs. 18 min by one-way ANOVA).
Figure 3.
Pressure-volume curves and lung histology. (A) The deflation limbs of pressure-volume curves were not different between the groups. (B, C) Lung histology assessed after staining with hematoxylin and eosin was similar for both groups. Histology was typical of immature lung, including thickened alveolar septal walls and patchy atelectasis. More alveolar fluid was observed in control lambs than in lambs treated with recombinant human surfactant protein D (+rhSP-D). Scale bar: 100 μm.
rhSP-D Treatment Decreased Pulmonary Inflammation
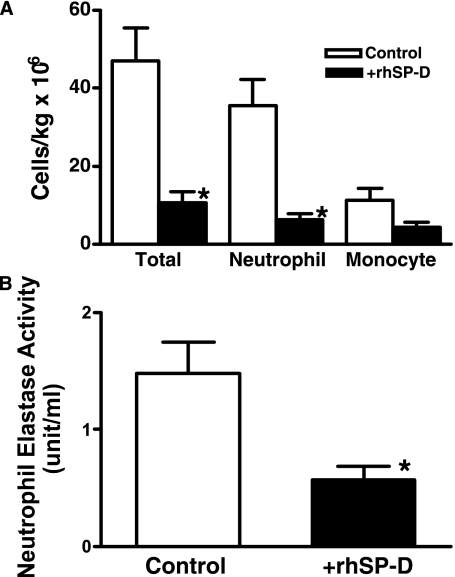
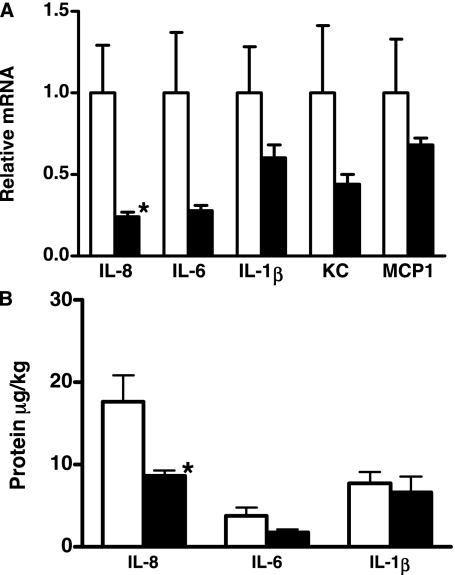
Our previous studies indicated a lack of inflammation, detected in BALF and lung tissue from 130-day GA lambs killed at delivery without ventilation (12, 13). Despite use of a lung-protective ventilatory strategy, 5 hours of ventilation was associated with lung inflammation in the control lambs. In contrast, lung inflammation was decreased in all the lambs treated with rhSP-D. Inflammatory cells in the pellets collected by centrifugation were counted using trypan blue and differential cell counts were performed on the stained cytospin preparation (5, 12). Total inflammatory cell numbers as well as neutrophils were significantly decreased by rhSP-D (Figure 4A). Increased NE activity has been associated with development of BPD (15, 16). The addition of rhSP-D to Survanta decreased NE activity in the lung (Figure 4B). Expression of IL-8, IL-6, IL-1β, TNF-α, KC, and MCP1 mRNA were analyzed by reverse transcriptase–polymerase chain reaction (Figure 5A) and IL-8, IL-6, and IL-1β proteins in the supernatants of lung homogenates were measured by ELISA (Figure 5B). Ovine ribosomal protein L32 was used as a reference RNA. Proinflammatory cytokine IL-8 (mRNA and protein), which plays a major role in neutrophil recruitment, was significantly decreased in the lung of rhSP-D–treated lambs. Although not statistically significant, mean IL-6 mRNA expression (P = 0.06), and IL-6 protein (P = 0.1) in the lung were lower in the +rhSP-D group. IL-1β protein and mRNA were not significantly influenced by rhSP-D treatment. Expression of TNF-α mRNA was similarly present at low levels in both groups (data not shown). KC, a functional homolog of IL-8, is critical for neutrophil recruitment and known to increase in ventilation-induced lung injury in adults (22). MCP1 possesses potent chemotactic activity for monocytes. Because of the large variation in lung inflammation in the control lambs, KC and MCP1 mRNA in the lung were not significantly different between the two groups, although mean levels were decreased by rhSP-D treatment.
Figure 4.
Inflammatory cells in bronchoalveolar lavage fluid (BALF) and neutrophil elastase (NE) activity in lung homogenates. (A) Increased total inflammatory cells and neutrophils in BALF induced by ventilation were suppressed by recombinant human surfactant protein D (rhSP-D). (B) NE activity was assessed by a spectrophotometric assay using a chromogenic substrate specific for NE as described in Methods. Treatment with rhSP-D–containing Survanta decreased NE activity. *P < 0.05 versus control.
Figure 5.
Proinflammatory cytokines, keratinocyte-derived chemokine (KC), and monocyte chemotactic protein 1 (MCP1) in lung homogenates. (A) Increased expression of IL-8 mRNA was significantly suppressed by recombinant human surfactant protein D (rhSP-D) treatment. Although not statistically significant due to the large variation in the control group, mean values of IL-6, IL-1β, KC, and MCP1 were generally lower in the +rhSP-D group. (B) IL-8 protein in lung homogenates was significantly decreased by rhSP-D treatment. IL-1β was not influenced by rhSP-D treatment. *P < 0.05 versus control.
The CD45 antibody recognizes the leukocyte common antigen and is present on cells of hematopoietic origin, except for erythroid cells and platelets. CD45-positive cells were isolated from BALF using magnetic cell separation (Miltenyi Biotech Inc., Auburn, CA) and CD14, CD11b, and CD44 were analyzed by flow cytometry (data not shown). CD14-positive cells were not detected in either group, suggesting that lung inflammation was not associated with infection. Both CD11b and CD44 influence vascular-to-tissue migration of neutrophils and monocytes to the sites of inflammation (23). Treatment with rhSP-D did not influence expression of CD11b or CD44, suggesting that suppression of neutrophil recruitment by rhSP-D in the lung was independent of changes in CD11b and CD44.
After instillation of 7 mg of rhSP-D, 6.7 ± 0.2 mg rhSP-D was recovered in BALF 4.7 hours after treatment. The slow clearance of exogenous rhSP-D from the lung is consistent with previous findings (11) supporting the low rate of surfactant clearance in the preterm lung. Human SP-D was not detected in BALF from control lambs.
Addition of rhSP-D to Surfactant Increased Resistance against Surfactant Inhibition
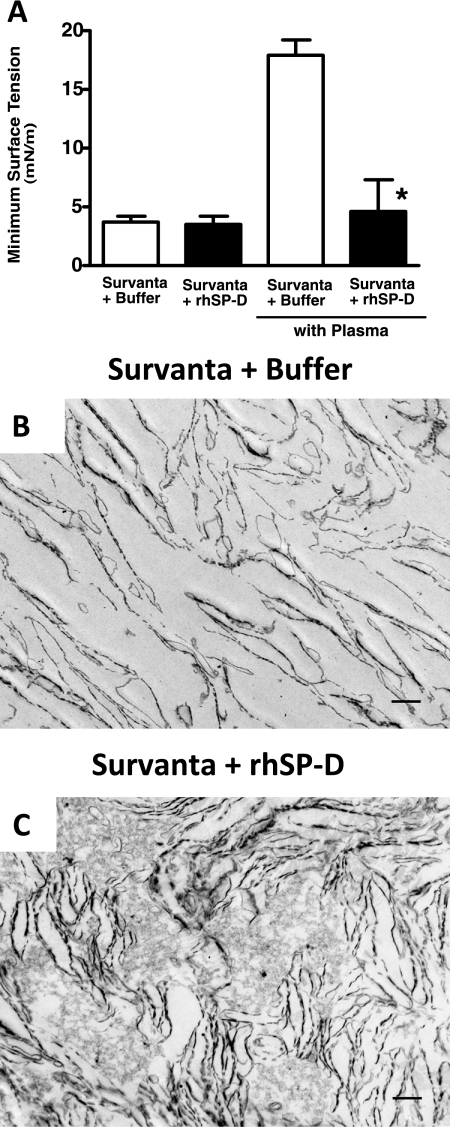
Minimum surface tension of Survanta with (+rhSP-D) or without rhSP-D (+buffer) in the presence or absence of a surfactant inhibitor (plasma protein) was measured with a captive bubble surfactometer (Figure 6A). The finding that minimum surface tension was low with or without rhSP-D is consistent with the high surface activity of Survanta and similarity of lung function and pressure-volume curves seen in both +rhSP-D–treated and control lambs. Immediately after mixing with plasma, surfactant mixtures were applied to the bubble. Plasma proteins inhibited the surface tension–lowering properties of Survanta, minimum surface tension being increased to greater than 15 mN/m. The addition of 2% rhSP-D to Survanta rendered the Survanta more resistant to plasma protein inhibition, the minimum surface tension remaining low in the presence of plasma proteins. Because SP-D influences surfactant ultrastructure in the alveolus by causing lysis of surfactant lipid layers (10), we assessed the ultrastructure of the surfactant mixtures used for treatment. The simple lipid layers formed by Survanta (Figure 6B) were changed by the addition of rhSP-D causing the formation of lipid aggregates and multilayers (Figure 6C). These changes in the ultrastructure of Survanta caused by rhSP-D may be related to its resistance to inhibition of surface activity by plasma protein. Although proteins in BALF in both control (60 ± 7 mg/kg) and +rhSP-D groups (57 ± 11 mg/kg) were threefold higher than that in nonventilated premature lambs seen in our previous studies (12, 13), they were not high enough to inhibit the function of the large amount of Survanta given to the lambs. Inhibition of surfactant function by plasma protein occurs when alveolar proteins are increased above 200 mg/kg (19).
Figure 6.
Resistance to surfactant inhibition and effects on the ultrastructure of Survanta. (A) Surface tension was measured by a captive bubble surfactometer. Survanta (+ buffer) had high surface activity and minimum surface tension was low and was not influenced by addition of recombinant human surfactant protein D (rhSP-D). Plasma protein inhibited surface tension–lowering properties of Survanta and minimum surface tension was increased. The addition of rhSP-D rendered the Survanta more resistant to plasma protein inhibition. Minimum surface tension was low in the presence of plasma protein. n = 3, *P < 0.05 versus Survanta + buffer with plasma. (B, C) Representative electron micrographs of Survanta mixed with buffer or rhSP-D. Addition of rhSP-D changed ultrastructure of Survanta from simple lipid layers to the mixture of multiple lipid layers and lipid aggregates. n = 3 per group. Scale bar: 500 nm.
DISCUSSION
Premature newborn infants often require high ventilatory pressure and hyperoxia for resuscitation, a process that induces lung injury and that may contribute to acute and chronic lung disease (15, 24) regardless of routine treatment with surfactant (25). The causes and mechanisms of ventilation-induced lung injury have been studied in adult animal models and are believed to be initiated primarily by alveolar macrophages (26). Mechanisms of ventilation injury in the preterm lung likely differ from those affecting the adult lung. The preterm lung contains essentially no mature alveolar macrophages and few monocytes (27) and PMNs, and very low levels of the innate defense proteins, including SP-D. Previous in vitro studies demonstrated that SP-D directly interacts with neutrophils, monocytes, and macrophages to enhance host defense and prevent inflammation (28, 29). In the premature lung, both lung stretch (24) and hyperoxia (30) induce the release of inflammatory mediators such as IL-8, KC, MCP1, and IL-6, causing inflammation, neutrophil recruitment, and disruption of cell contacts resulting in protein leak, edema, microvascular injury, and epithelial cell apoptosis.
Barotrauma or stretch can contribute to lung injury and are related to the Vt used for ventilation. Previous premature newborn animal studies used to assess lung injury during resuscitation were ventilated with a target Vt of more than 15 ml/kg (19, 24, 31). In clinical practice, resuscitation of manually ventilated premature newborns is often performed by observing chest wall movement without monitoring Vt. Chest wall movement in the preterm may require considerable pressure during resuscitation. To minimize the lung injury caused by manual ventilation in the delivery room, the resuscitation bag for newborns has a pressure relief system set at 40 cm H2O. The importance of the use of adequate PEEP for resuscitation to minimize lung injury has been demonstrated by studies with premature newborn animal models (12, 32). Taking into account these concerns regarding ventilation for newborns, in this study we resuscitated the premature lambs by targeting PIP at 40 cm H2O and PEEP of 4 cm H2O. The resulting Pco2 and Vt indicated adequate ventilation, rather than overventilation, yet lung inflammation still occurred in the control lambs.
In the present preterm newborn lamb model, treatment with rhSP-D–containing surfactant was associated with decreased inflammatory cells, IL-8, and neutrophil elastase activity in the lung without influencing lung function. IL-8 is a potent neutrophil chemoattractant that increases neutrophils and other inflammatory cells in the alveoli that are believed to contribute to lung damage (33). Increases in IL-8 were associated with an influx of neutrophils in airway aspirates from premature newborns with RDS who later developed BPD (34, 35). Taken together, rhSP-D treatment decreased IL-8, which suppressed neutrophil recruitment in the ventilated premature newborn lamb lung.
Lung inflammation appears to be a common factor associated with BPD (25), whether related to prematurity, ventilation-induced injury, or infection. The present study demonstrates that treatment with rhSP-D suppresses NE activity in the ventilated preterm newborn lamb lung. NE is a potent serine proteinase, responsible for tissue destruction in adult lung with emphysema (36). In the preterm newborn lung, increased NE activity affects lung remodeling and increases alveolar epithelial apoptosis and the development of BPD (15, 16).
SP-D is a multimeric glycoprotein of the collectin family of innate immune molecules. Initially, these are trimeric subunits stabilized by disulfide bonds; subsequently higher-order multimers produce a wheel-like dodecameric structure stabilized by interchain sulfhydryl bonds (37, 38). SP-D structure is critical to its function (39). Although the carbohydrate recognition domain mediates its interactions with pathogens, several in vivo studies in which mutant or full-length SP-D were expressed in Sftpd−/− mice lungs suggest that the full function of SP-D is dependent on a full-length, multimeric protein (40–46). The rhSP-D used in the present study is a full-length SP-D. Findings to date suggest that this rhSP-D recapitulates the full functionality of SP-D, including host defense against lipopolysaccharides, binding to newly secreted surfactant, lysing surfactant lipid layers, converting surfactant ultrastructure in the alveolus, and correcting the abnormal surfactant metabolism of Sftpd−/− mice (7, 9, 10, 47).
More than 90% of SP-D in BALF is soluble (non–lipid associated). The remaining small amount of SP-D associates with surfactant lipid and influences surfactant ultrastructure, at least in part, by its phosphatidylinositol-dependent lytic activity on phospholipid membranes, which is critical for surfactant homeostasis (10). In SP-D–deficient mice, the abnormally large surfactant forms are inefficiently taken up by type II cells, resulting in a threefold increase in the surfactant pool size (47, 48). Survanta is a lipid extract of minced bovine lung, supplemented with dipalmitoyl-phosphatidylcholine, palmitic acid, and tripalmitin to favorably alter in vitro surface properties. Survanta contains SP-C and trace amounts of SP-B, but does not contain SP-A or SP-D. Thus, Survanta is deficient of surfactant proteins required for normal surfactant forms, including lamellated structure and tubular myelin. The ultrastructure of Survanta consisted of simple lipid layers that were altered by rhSP-D addition, resulting in a mixture of lipid aggregates and lipid multilayers. SP-D–mediated changes in ultrastructure may influence surfactant metabolism and function in the preterm newborn lung.
Immature alveolar type II cells and macrophages in premature newborns are associated with the slow clearance of exogenously instilled surfactant from the lung (11). More than 90% of exogenous surfactant remains in the lung 24 hours after surfactant treatment. Likewise, in the present study, 96% of rhSP-D was detected in the preterm lamb lung 4.7 hours after treatment. Thus, the clinical requirement for multiple surfactant treatments in some cases results not from surfactant deficiency but from inhibition of surfactant function by serum proteins leaking from the alveolar capillary to the airspace (49). Addition of rhSP-D to Survanta influenced the ultrastructure of surfactant lipid and increased its resistance to surfactant inhibition by plasma protein in vitro.
The potential usefulness of rhSP-D supplement is also suggested by previous findings regarding the prevention of systemic sepsis following intratracheal endotoxin in the preterm lamb model (7). The present study also demonstrates that rhSP-D–containing surfactant is effective in suppressing lung inflammation in the ventilated premature newborn lamb and increasing resistance against surfactant inhibitor. Neonatal sepsis syndrome is a common cause of neonatal morbidity and mortality in both term and preterm infants. Use of rhSP-D–containing surfactant for treatment of RDS may be beneficial for preventing neonatal sepsis syndrome and BPD that remain major clinical problems affecting preterm newborn infants.
Acknowledgments
The authors thank Shawn Grant, Angelica Schehr, and Michael Minnick for expert technical support. Survanta was supplied by Abbott Laboratories.
Supported by the March of Dimes Foundation #6-FY109–235 (M.I.), Uehara Memorial Foundation Research Fellowship (A.S.), and National Institutes of Health grant HL085610 (J.A.W.).
Originally Published in Press as DOI: 10.1164/rccm.200912-1818OC on February 4, 2010
Conflict of Interest Statement: A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.W. owns patents (US2006043055) for the use of SP-D for treatment of lung disease (Cincinnati Children's Hospital Medical Center). R.K.S. is employed by Genzyme Corporation. M.I. hold a patent regarding the use of SP-D (US2006043055). Genzyme Corporation has previously (2005–2006) taken a short-term license to explore the usefulness of SP-D and the rhSP-D was synthesized by the collaboration with Genzyme. There is no financial support from Genzyme for this study.
References
- 1.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23. [DOI] [PubMed] [Google Scholar]
- 2.Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, MacPherson P, Lopez Bernal A. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1994;1210:303–307. [DOI] [PubMed] [Google Scholar]
- 3.Awasthi S, Coalson JJ, Crouch E, Yang F, King RJ. Surfactant proteins A and D in premature baboons with chronic lung injury. Evidence for an inhibition of secretion. Am J Respir Crit Care Med 1999;160:942–949. [DOI] [PubMed] [Google Scholar]
- 4.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 2002;109:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer BW, Jobe AH, Bachurski CJ, Ikegami M. Surfactant protein A recruits neutrophils into the lungs of ventilated preterm lambs. Am J Respir Crit Care Med 2001;163:158–165. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami M, Carter K, Bishop K, Yadav A, Masterjohn E, Brondyk W, Scheule RK, Whitsett JA. Intratracheal recombinant surfactant protein D prevents endotoxin shock in the newborn preterm lamb. Am J Respir Crit Care Med 2006;173:1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda T, Ikegami M, Rider ED, Jobe AH. Distribution of surfactant and ventilation in surfactant-treated preterm lambs. J Appl Physiol 1994;76:45–55. [DOI] [PubMed] [Google Scholar]
- 9.Ikegami M, Scoville EA, Grant S, Korfhagen T, Brondyx W, Scheule RK, Whitsett JA. Surfactant protein-D and surfactant inhibit endotoxin induced pulmonary inflammation. Chest 2007;132:1447–1454. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J Appl Physiol 2009;106:1545–1552. [DOI] [PubMed] [Google Scholar]
- 11.Ikegami M, Jobe AH. Surfactant metabolism. Semin Perinatol 1993;17:233–240. [PubMed] [Google Scholar]
- 12.Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, Ikegami M. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med 2001;164:494–498. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami M, Jobe A. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to E.coli endotoxin. Pediatr Res 2002;52:356–362. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Watterberg KL, Carmichael DF, Gerdes JS, Werner S, Backstrom C, Murphy S. Secretory leukocyte protease inhibitor and lung inflammation in developing bronchopulmonary dysplasia. J Pediatr 1994;125:264–269. [DOI] [PubMed] [Google Scholar]
- 16.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2006;291:L619–L627. [DOI] [PubMed] [Google Scholar]
- 17.Schoel WM, Schürch S, Goerke J. The captive bubble method for the evaluation of pulmonary surfactant: surface tension, area, and volume calculations. Biochim Biophys Acta 1994;1200:281–290. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami M, Korfhagen TR, Whitsett JA, Bruno MD, Wert SE, Wada K, Jobe AH. Characteristics of surfactant from SP-A deficient mice. Am J Physiol 1998;275:L247–L258. [DOI] [PubMed] [Google Scholar]
- 19.Wada K, Jobe AH, Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol 1997;83:1054–1061. [DOI] [PubMed] [Google Scholar]
- 20.Schmiedl A, Krug N, Hohlfeld JM. Influence of plasma and inflammatory proteins on the ultrastructure of exogenous surfactant. J Electron Microsc (Tokyo) 2004;53:407–416. [DOI] [PubMed] [Google Scholar]
- 21.Norden MA, Butt W, McDougall P. Predictors of survival for infants with congenital diaphragmatic hernia. J Pediatr Surg 1994;29:1442–1446. [DOI] [PubMed] [Google Scholar]
- 22.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002;110:1703–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weirich E, Rabin RL, Maldonado Y, Benitz W, Modler S, Herzenberg LA. Neutrophil CD11b expression as a diagnostic marker for early-onset neonatal infection. J Pediatr 1998;132:445–451. [DOI] [PubMed] [Google Scholar]
- 24.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 2007;176:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha GS, Mezzacappa-Filho F, Ribeiro JD. Risk factors for bronchopulmonary dysplasia in very low birth weight newborns treated with mechanical ventilation in the first week of life. J Trop Pediatr 2005;51:334–340. [DOI] [PubMed] [Google Scholar]
- 26.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 1990;141:471–501. [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res 2003;54:52–57. [DOI] [PubMed] [Google Scholar]
- 28.Cai GZ, Griffin GL, Senior RM, Longmore WJ, Moxley MA. Recombinant SP-D carbohydrate recognition domain is a chemoattractant for human neutrophils. Am J Physiol 1999;276:L131–L136. [DOI] [PubMed] [Google Scholar]
- 29.Crouch EC, Persson A, Griffin GL, Chang D, Senior RM. Interactions of pulmonary surfactant protein D (SP-D) with human blood leukocytes. Am J Respir Cell Mol Biol 1995;12:410–415. [DOI] [PubMed] [Google Scholar]
- 30.Chambers HM, van Velzen D. Ventilator-related pathology in the extremely immature lung. Pathology 1989;21:79–83. [DOI] [PubMed] [Google Scholar]
- 31.Wallace MJ, Probyn ME, Zahra VA, Crossley K, Cole TJ, Davis PG, Morley CJ, Hooper SB. Early biomarkers and potential mediators of ventilation-induced lung injury in very preterm lambs. Respir Res 2009;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siew ML, Te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, Morley CJ, Davis PG, Yagi N, Uesugi K, et al. Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. J Appl Physiol 2009;106:1487–1493. [DOI] [PubMed] [Google Scholar]
- 33.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994;56:559–564. [PubMed] [Google Scholar]
- 34.Munshi UK, Niu JO, Siddiq MM, Parton LA. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol 1997;24:331–336. [DOI] [PubMed] [Google Scholar]
- 35.Beresford MW, Shaw NJ. Detectable IL-8 and IL-10 in bronchoalveolar lavage fluid from preterm infants ventilated for respiratory distress syndrome. Pediatr Res 2002;52:973–978. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 2003;163:2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol Lung Cell Mol Physiol 1998;275:L1–L13. [DOI] [PubMed] [Google Scholar]
- 38.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol 1998;19:177–201. [DOI] [PubMed] [Google Scholar]
- 39.Yamazoe M, Nishitani C, Takahashi M, Katoh T, Ariki S, Shimizu T, Mitsuzawa H, Sawada K, Voelker DR, Takahashi H, et al. Pulmonary surfactant protein D inhibits lipopolysaccharide (LPS)-induced inflammatory cell responses by altering LPS binding to its receptors. J Biol Chem 2008;283:35878–35888. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Ikegami M, Dey CR, Korfhagen TR, Whitsett JA. Reversibility of pulmonary abnormalities by conditional replacement of surfactant protein D (SP-D) in vivo. J Biol Chem 2002;277:38709–38713. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA. Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure. J Biol Chem 2001;276:19214–19219. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Hartshorn KL, Crouch EC, Ikegami M, Whitsett JA. Complementation of pulmonary abnormalities in SP-D(−/−) mice with an SP-D/conglutinin fusion protein. J Biol Chem 2002;277:22453–22459. [DOI] [PubMed] [Google Scholar]
- 43.Palaniyar N, Zhang L, Kuzmenko A, Ikegami M, Wan S, Wu H, Korfhagen TR, Whitsett JA, McCormack FX. The role of pulmonary collectin N-terminal domains in surfactant structure, function, and homeostasis in vivo. J Biol Chem 2002;277:26971–26979. [DOI] [PubMed] [Google Scholar]
- 44.Fisher JH, Sheftelyevich V, Ho YS, Fligiel S, McCormack FX, Korfhagen TR, Whitsett JA, Ikegami M. Pulmonary-specific expression of SP-D corrects pulmonary lipid accumulation in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L365–L373. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Ikegami M, Korfhagen TR, McCormack FX, Yoshida M, Senior RM, Shipley JM, Shapiro SD, Whitsett JA. Neither SP-A nor the N-terminal domains of SP-A can substitute for SP-D in the regulation of alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol 2006;291:L181–L190. [DOI] [PubMed] [Google Scholar]
- 46.Kingma PS, Zhang L, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd−/− mice requires the collagenous domain of surfactant protein D. J Biol Chem 2006;281:24496–24505. [DOI] [PubMed] [Google Scholar]
- 47.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L552–L561. [DOI] [PubMed] [Google Scholar]
- 48.Ikegami M, Whitsett JA, Jobe AH, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene ablated mice. Am J Physiol Lung Cell Mol Physiol 2000;279:L468–L476. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami M, Jacobs H, Jobe AH. Surfactant function in the respiratory distress syndrome. J Pediatr 1983;102:443–447. [DOI] [PubMed] [Google Scholar]