Abstract
Rationale: In animal models of pulmonary hypertension, simvastatin has been shown to reduce pulmonary artery pressure and induce regression of associated right ventricular (RV) hypertrophy.
Objectives: To assess the therapeutic value of simvastatin in patients with pulmonary arterial hypertension (PAH).
Methods: Forty-two patients with PAH were randomized to receive either simvastatin (80 mg/d) or placebo in addition to current care for 6 months, and thereafter offered open-label simvastatin. The primary outcome was change in RV mass, assessed by cardiac magnetic resonance (CMR).
Measurements and Main Results: At 6 months, RV mass decreased by 5.2 ± 11 g in the statin group (P = 0.045) and increased 3.9 ± 14 g in the placebo group. The treatment effect was −9.1 g (P = 0.028). N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels decreased significantly in the statin group (−75 ± 167 fmol/ml; P = 0.02) but not the placebo group (49 ± 224 fmol/ml; P = 0.43; overall treatment effect −124 fmol/ml; P = 0.041). There were no significant changes in other outcome measures (including 6-minute walk test, cardiac index, and circulating cytokines). From 6 to 12 months, both RV mass and NT-proBNP increased toward baseline values in 16 patients on active treatment who continued with simvastatin but remained stable in 18 patients who switched from placebo to simvastatin. Two patients required a reduction in dose but not cessation of simvastatin.
Conclusions: Simvastatin added to conventional therapy produces a small and transient early reduction in RV mass and NT-proBNP levels in patients with PAH, but this is not sustained over 12 months.
Clinical trial registered with www.clinicaltrials.gov (NCT00180713).
Keywords: hypertension, pulmonary; simvastatin; MRI; hypertrophy
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Statins have shown impressive efficacy in reducing and reversing pulmonary hypertension and right ventricular (RV) hypertrophy in animal models, but its effects in patients with pulmonary hypertension have yet to be assessed.
What This Study Adds to the Field
Simvastatin reduced RV mass and N-terminal pro–B-type natriuretic peptide levels compared with placebo in patients with pulmonary hypertension over 6 months. However, these reductions were not sustained at 12 months.
Pulmonary arterial hypertension (PAH) is characterized by increased pulmonary vascular resistance due to vasoconstriction, inflammation, thrombosis, and structural remodeling of pulmonary arterioles, with occlusion of the lumen of some vessels (1). Untreated, pressure overload of the right ventricle leads to progressive hypertrophy and dilatation, and end-stage heart failure within 2 to 3 years. The options for therapeutic intervention have increased, and include endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, and prostacyclin analogs, but the prognosis remains poor. Survival in specialist centers offering best care is around 85% at 2 years (2).
Statins offer a novel approach to the treatment of PAH. In addition to lowering cholesterol, statins have been shown to have antiproliferative, antithrombotic, antiinflammatory, and antioxidant effects (3). This spectrum of activity arises from the inhibition of isoprenoids (geranylgeranylpyrophosphate and farnesylpyrophosphate), which are essential for the post-translational isoprenylation of Rho and Ras family GTPases. Statins, in particular simvastatin, have been reported to attenuate the development of pulmonary hypertension in a number of experimental animal models (4–12) and to regress established pulmonary hypertension and vascular remodeling induced by either pneumonectomy and monocrotaline treatment (13) or chronic hypoxia (14). There is evidence that this is achieved through increased apoptosis as well as reduced proliferation of smooth muscle cells in obstructive vascular lesions. Of interest, not all studies report a reduction in pulmonary artery pressure (12, 15, 16), but a reduction in right ventricular (RV) hypertrophy appears to be a consistent finding (4–9, 12–16).
Data in humans are fewer. An open-label observational study reported the use of simvastatin as an adjunctive therapy in 16 patients with pulmonary hypertension of mixed etiologies and suggested that the drug was well tolerated (17). Given the consensus that the RV is an important determinant of clinical outcome in PAH (18–21), we conducted a double-blind, randomized, placebo-controlled study of the effects of simvastatin added to optimized conventional care on RV mass and function, measured using cardiac magnetic resonance (CMR), in patients with PAH in functional class II and III. In addition we measured circulating N-terminal pro–B-type natriuretic peptide (NT-proBNP) and growth and differentiation factor-15 (GDF-15) levels as prognostic biomarkers in PAH (22); exercise capacity, assessed by 6-minute walk distance; plasma nitric oxide metabolites (NOx) and cytokine levels as exploratory biomarkers; and safety and tolerability.
METHODS
Selection of Patients
Patients attending Hammersmith Hospital or Giessen University Hospital between April 2005 and September 2008 with PAH—idiopathic, heritable, or associated with an atrial-septal defect or connective tissue disease (scleroderma or systemic lupus erythematosus)—were considered for the study. PAH was diagnosed on the basis of cardiac catheter data (mean pulmonary artery pressure at rest >25 mm Hg, pulmonary capillary wedge pressure <15 mm Hg and pulmonary vascular resistance >3 Wood units) and the results of extensive screening for other causes according to current guidelines (2, 23, 24). Patients had to be stable (i.e., no change in therapy) on a phosphodiesterase type 5 inhibitor and/or endothelin receptor antagonist plus background therapy (diuretics and anticoagulants, plus/minus digoxin) for at least 2 months with a 6-minute walk distance between 150 and 500 m at entry. Exclusion criteria were: elevated baseline liver enzymes (more than three times the upper limit of the normal range), already taking or contraindication to statin therapy, and already receiving or urgent need for prostanoid therapy on clinical grounds.
The study was performed according to the 1975 Declaration of Helsinki (modified in 1983) and in adherence to local guidelines for good clinical practice. The local ethics review committee approved the protocol, and written informed consent was obtained from all patients.
Study Design
Patients were randomized, double-blind, to receive either simvastatin or matching placebo for 24 weeks and thereafter offered open-label simvastatin. The blinding and randomization were performed by Nova Labs (Leicester, UK). Patients started on 1 tablet (simvastatin 40 mg or placebo) at night for the first 4 weeks and then 2 tablets daily. Blinding was maintained during Month 7 to allow commencement and up-titration of simvastatin in patients previously on placebo. There was provision to reduce the dose of study medication from 2 to 1 tablet daily if the patient was not tolerating the higher dose. Data from blood tests (including serum lipid levels and NT-proBNP), CMR, echocardiography, and 6-minute walk were gathered separately by different members of the team and only collated at the time of unblinding to reduce the chance of inadvertent unblinding of the study.
Outcome Measures
The following tests were performed at baseline, 24, and 52 weeks: CMR, transthoracic echocardiogram, 6-minute walk distance (with pre and post exhaled nitric oxide measurements), Borg dyspnea index (performed immediately after the 6-min walk), and Cambridge Pulmonary Hypertension Outcome Review quality of life questionnaire. Blood sampling for hormone, nitric oxide metabolites (NOx), and cytokine levels was performed at baseline, 4, 24, and 52 weeks and for routine biochemistry, lipid profile, and hematology at baseline and regular intervals.
This is an exploratory study. The primary efficacy measure was predefined as the placebo-corrected change in RV mass from baseline to 6 months as measured by CMR. This was selected on the basis that a change in RV mass is a robust measure of treatment efficacy in animal models and a useful bridging biomarker for human studies. A change in RV mass can be detected in a relatively small number of patients and a positive effect would support further investigation; conversely, lack of effect would suggest simvastatin is unlikely to have a major beneficial effect in pulmonary hypertension. To facilitate interpretation, secondary measures of efficacy were chosen to capture functional and mechanistic endpoints. These were: change in 6-minute walk distance, cardiac index, Borg Dyspnea Index on the completion of the 6-minute walk test (where 0 = no dyspnea and 10 = maximum dyspnea), quality of life, plasma NT-proBNP levels, GDF-15, and a range of cytokines from baseline.
Imaging
CMR images were obtained using a 1.5-T scanner (Sonata; Siemens, Erlangen, Germany), with six-element coils placed anteriorly and posteriorly. Steady-state free-precession cines (retrospective gating, 25 frames, 7-mm slice thickness, 3-mm interslice gap, breath-hold 8–11 s) were acquired. True cardiac axes were defined by iterative cross-cuts and the basal short-axis slice was piloted from the first frame of the horizontal and vertical long-axis acquisitions, with the trailing edge of the basal slice contacting the ventricular border of the AV groove. The entirety of right and left ventricles was then imaged (25). Manual analysis was performed with a graphics tablet and CMRTools (Imperial College, London, UK) by a single operator to produce biventricular end-diastolic volume, end-systolic volume, stroke volume, ejection fraction, and mass. The first frame was treated as end-diastole. End-systole was defined as the smallest frame. The interventricular septum was portioned so any hypertrophy was attributed to the RV. Cardiac index was calculated from phase-velocity mapping of the aorta to produce cardiac output. Cardiac output and RV mass were indexed to body surface area to give cardiac index and RV mass index, respectively (26).
Two-dimensional and Doppler echocardiography were performed using standard techniques on commercially available equipment with a predefined imaging protocol. The maximal tricuspid regurgitant jet velocity (m/s), derived from the continuous wave signal, was added to estimated right atrial pressure (RAP) (based on inferior vena cava size and its changes with inspiration) in the modified Bernoulli equation to estimate systolic pulmonary artery pressure. The right atrial volume was calculated at end-systole from the apical four-chamber view using the single-plane diameter-length method (Volume = [πD2L]/6, where D = minor axis [cm], L = major or long axis [cm]). The left ventricular (LV) eccentricity index was measured at end-diastole and end-systole at the mid-ventricle level from the parasternal short-axis projection (27) and calculated as the ratio of the major axis of the LV parallel to the septum at the level of the chordae (a), divided by minor-axis perpendicular to and bisecting the septum at the same section (b) (eccentricity index = a/b). The RV Doppler (Tei) (28) index was calculated as described previously using the length of two time intervals in the formula (a−b)/b, where a equals the time between the onset of QRS complex and onset of tricuspid inflow and b equals the ejection time of RV outflow.
All measurements from images were made by an experienced operator who was unaware of each patient's clinical information.
Assays
Plasma NT-proBNP was determined using a noncompetitive assay as previously described (29, 30). Combined concentrations (NOx) of plasma nitrite (NO2−) and nitrate (NO3−), the stable oxidation products of nitric oxide (NO), were determined with an NO chemiluminescence detector (Sievers NOA 280; Analytix Ltd, Peterlee, UK) as described (31). GDF-15 was determined by ELISA using a DuoSet Sandwich ELISA kit (R&D Systems, Abingdon, UK). Plasma cytokines and growth factors were measured using a Bio-Plex Human Group I 27-plex Panel, with a detection range of 1.95 to 32,000 pg/ml (171-A11127; Bio-Rad Laboratories Ltd, Hemel Hempstead, Herts, UK).
Statistical Analysis
The study was powered to detect an 8.5-g difference in the change in RV mass between the statin and placebo groups at 24 weeks based on repeatability measurements in patients with PAH. Analysis was performed by intention to treat at 6 months and per protocol at 12 months. Missing variables were replaced with medians or means (for variables missing at baseline) or with expected variables calculated on the percentage change between baseline and 24 weeks observed for the group (placebo or statin) as a whole (a technique called imputation). Missing variables accounted for less than 5% of the data and there were no missing CMR data at baseline. Baseline patient characteristics of the two treatment groups were compared with t tests. Treatment effects were assessed by repeated measures ANOVA (32), with Wilks' lambda P values reported. RV mass, RV mass index, RV end-diastolic volume, RV end-systolic volume, eccentricity index end-diastole, eccentricity index end-systole, right atrial (RA) volume, plasma NOx, and GDF-15 were log transformed and NT-proBNP values were square rooted to achieve normality, verified by Kolmogorov-Smirnov tests. All P values were two-tailed. Differences in categorical distributions were assessed using the Fisher exact test. Analyses were performed using STATA 8 software (Stata Inc., College Station, Texas) and SPSS Statistics 17.0 (SPSS, Inc., Chicago, IL).
RESULTS
Forty-two patients were recruited to the study (38 from London and 4 from Giessen) (Table 1). Twenty-two (52%) patients were in World Health Organization functional class II and 20 (48%) were in World Health Organization functional class III. The majority (33/42) of patients had idiopathic or heritable PAH. Nineteen patients were assigned to simvastatin and 23 received placebo (Figure 1). There were differences between the two treatment groups in some baseline characteristics (Table 1). During the first 6 months, two patients assigned placebo discontinued the study at 8 weeks (own volition) and one patient was commenced on a statin at Week 12 due to a stroke (but analyzed as part of the placebo group on intention-to-treat principle). In the simvastatin treatment group, one patient deteriorated at 12 weeks requiring up-titration of sildenafil from 25 mg to 50 mg and was withdrawn from the study. The drug dose was reduced to 40 mg at 8 weeks in one patient due to muscle aches and an elevated creatine kinase (peak value 3,579 IU/L, normal range 0 to 150 IU/L). Creatine kinase returned to normal and this patient completed the study. Blinding was not broken.
TABLE 1.
PATIENT CHARACTERISTICS AT BASELINE
| Characteristic | Placebo (n = 23) | Simvastatin (n = 19) |
|---|---|---|
| Demographic variables | ||
| Women:men | 15:8* | 17:2 |
| Age in yr, mean (range) | 49.1 (24–73) | 43.2 (19–67) |
| BMI, kg/m2 | 26.3 ± 3.3 | 24.4 ± 4.7 |
| White/black/other (no.) | 21*/0/2* | 11/1/7 |
| Cause of pulmonary hypertension | ||
| Idiopathic/heritable | 18/2 | 12/1 |
| Associated with CTD | 2 | 3 |
| Associated with ASD | 1 | 3 |
| Mean pulmonary artery pressure at diagnosis, mm Hg (range) | 55.7 ± 12.5 (30–81) | 55.8 ± 10.3 (42–71) |
| Months since diagnosis | 43.5 ± 73 | 58.6 ± 62 |
| Current treatment, no. | ||
| Warfarin | 19 | 16 |
| Diuretics | 15 | 12 |
| Digoxin | 8 | 4 |
| Calcium antagonist | 4 | 6 |
| Sildenafil | 4 | 6 |
| Bosentan | 19* | 9 |
| Sildenafil + bosentan | 0* | 4 |
| Functional status | ||
| Functional class II/III | 11/12 | 11/8 |
| 6-min walk distance, m, mean ± SD (range) | 386 ± 110 (120–600) | 381 ± 69 (198–498) |
| Borg index at rest, median (interquartile range) | 1 (2) | 1 (2) |
| Borg index post 6-min walk, median (interquartile range) | 5 (4) | 7 (2) |
| NT- proBNP, fmol/ml | 444 ± 447 | 570 ± 579 |
| GDF-15, pg/ml | 369 ± 265 | 444 ± 331 |
| Plasma NOx, μmol/L | 62.6 ± 36.1 | 49.1 ± 21.7 |
| Hemodynamic variables | ||
| PA systolic pressure, mm Hg† | 92 ± 27.2 | 81.6 ± 21.2 |
| RV systolic pressure, mm Hg† | 90.4 ± 5.9 | 74.1 ± 6.1 |
| Systolic blood pressure, mm Hg | 117 ± 13 | 117 ± 15 |
| Diastolic blood pressure, mm Hg | 71 ± 8 | 67 ± 13 |
| Heart rate, beats/min | 75 ± 10 | 74 ± 15 |
| TRV velocity, m/s† | 4.5 ± 0.7 | 4.1 ± 0.7 |
| Tei Index† | 0.74 ± 0.3 | 0.6 ± 0.2 |
| RA volume, ml† | 108 ± 56 | 94 ± 34 |
| CMR data | ||
| RV mass, g | 102 ± 63 | 99.3 ± 41 |
| RV mass index, g/m2 | 56.4 ± 32.7 | 58.7 ± 22 |
| LV mass, g | 97 ± 30.5 | 84.7 ± 21.6 |
| LV mass index, g/m2 | 53.3 ± 14 | 50 ± 9.1 |
| RVedv, ml | 205 ± 91 | 203.9 ± 70.9 |
| RVesv, ml | 71.6 ± 44.1 | 75.1 ± 41.3 |
| RV ejection fraction, % | 40.5 ± 15.6 | 41.3 ± 15.8 |
| Cardiac index, L/min/m2 | 2.5 ± 0.7 | 2.6 ± 0.65 |
Definition of abbreviations: ASD = atrial septal defect; BMI = body mass index; CMR = cardiac magnetic resonance; CTD = connective tissue disease; GDF-15 = growth and differentiation factor-15; LV = left ventricular; NOx = nitric oxide metabolites; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PA = pulmonary artery; RA = right atrial; RV = right ventricular; RVedv = RV end-diastolic volume; RVesv = RV end-systolic volume; TRV = tricuspid regurgitant jet velocity.
Data are mean ± SD unless otherwise indicated.
P < 0.05.
Determined by echocardiography.
Figure 1.
Schematic overview of study. PAH = pulmonary arterial hypertension.
Sixteen patients on active treatment and 18 patients on placebo continued open-label simvastatin (32 patients on 80 mg, 2 patients on 40 mg) to 12 months (Figure 1). Two patients in the simvastatin treatment group withdrew at 24 weeks, one due to drug-related side effects (abdominal discomfort) and one due to clinical deterioration that required an escalation of PAH-specific therapies. In the placebo group, three patients withdrew at 24 weeks, two suffered clinical deterioration requiring escalation of therapies between 24 and 36 weeks, and the patient commenced on a statin at Week 12 due to a stroke was withdrawn from the study at Week 24. One patient had elevated creatine kinase levels at Week 36 (peak 13,346 IU/L), requiring withdrawal of the statin for 2 weeks and reintroduction at a reduced dose of 40 mg. The patient already on simvastatin 40 mg from the blinded part of the study continued on this dose to 12 months.
Cardiac Mass and Function
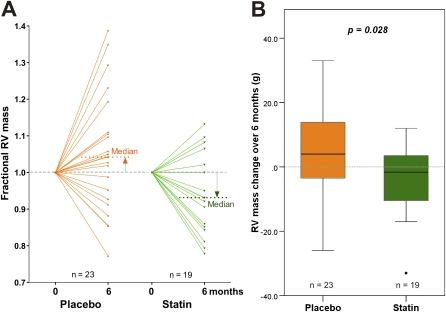
Baseline RV mass was markedly elevated in both treatment groups compared with healthy individuals of a similar age (mean 48; 95% confidence interval, 23–73 g) (25). Over the first 6-month period, RV mass increased (3.9 ± 13.9 g) in the placebo group and significantly decreased (−5.2 ± 11.3 g; P = 0.045) in the statin group (Table 2). The treatment effect was −9.1 g (P = 0.028). A similar trend in RV mass was seen if all the patients on sildenafil were excluded, reaching borderline significance (placebo: 4.5 ± 3.2 g; simvastatin: −5.4 ± 4.2 g; P = 0.07). A significant divergence in response was also seen in fractional change in RV mass from baseline (actual change/baseline mass) and RV mass index. Individual responses are shown in Figure 2A and the group changes in Figure 2B. No change was seen in LV mass. There were no significant changes in RV ejection fraction or Tei index. RV systolic pressure as estimated by echocardiography was not significantly affected by simvastatin.
TABLE 2.
RESPONSE TO ADDITION OF SIMVASTATIN TO USUAL THERAPY
| Change from Baseline at 24 wk |
||||||
|---|---|---|---|---|---|---|
| Placebo (n = 23) |
Statin (n = 19) |
Overall Mean Difference | ||||
| Variable | Mean | SD | Mean | SD | P value | |
| RV mass, g | 3.9 | 13.9 | −5.2 | 11.3 | −9.1 | 0.028 |
| Fractional RV mass | 0.05 | 0.16 | −0.05 | 0.11 | −0.10 | 0.026 |
| RV mass index, g/m2 | 1.9 | 7.8 | −2.8 | 6.7 | −4.7 | 0.049 |
| Fractional RV mass index | 0.05 | 0.16 | −0.05 | 0.11 | −0.09 | 0.042 |
| LV mass, g | −1.3 | 10.9 | 1.7 | 12.3 | 3.05 | 0.40 |
| Cardiac index, L/min/m2 | −0.05 | 0.59 | 0.12 | 0.58 | 0.17 | 0.37 |
| RV systolic pressure, mm Hg | −4.0 | 4.3 | 4.1 | 3.68 | 8.1 | 0.17 |
| RVedv, ml | 8.7 | 22.4 | 7.3 | 16.9 | −1.4 | 0.65 |
| RVesv, ml | 3.7 | 14.4 | 2.5 | 7.6 | −1.3 | 0.53 |
| RVEF, % | −1.0 | 5.3 | 0.7 | 6.1 | 1.7 | 0.35 |
| EIED | −0.16 | 0.34 | −0.06 | 0.50 | 0.11 | 0.56 |
| EIES | 0.36 | 0.89 | 0.48 | 1.29 | 0.12 | 0.90 |
| RA volume on echocardiogram, ml | −6.3 | 30.6 | −2.9 | 23.2 | 3.4 | 0.67 |
| Tei index | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.49 |
| 6MW test, m | 1.0 | 57.0 | 3.1 | 34.5 | 2.10 | 0.86 |
| Pre-6MW systolic BP, mm Hg | −9.1 | 47.5 | −7.5 | 17.5 | 1.6 | 0.76 |
| Pre-6MW diastolic BP, mm Hg | −4.6 | 28.7 | 6.1 | 24.2 | 10.7 | 0.38 |
| Pre-6MW HR, bpm | −1.7 | 30.1 | 0.0 | 12.5 | 1.7 | 0.62 |
| Post-6MW HR, bpm | −6.1 | 42.2 | −1.8 | 14.2 | 4.4 | 0.98 |
| NT-proBNP, fmol/ml | 49 | 224 | −75 | 167 | −124 | 0.041 |
| Plasma NOx, μmol/L | 21.1 | 70.6 | 15.7 | 19.8 | −5.4 | 0.89 |
| GDF-15, pg/ml | 158 | 270 | 33 | 397 | −125 | 0.09 |
| Symptoms score /25 | −0.6 | 3.2 | −1.9 | 4.1 | −1.4 | 0.25 |
| QoL score /25 | 0 | 4.7 | −1.6 | 4 | −1.7 | 0.26 |
Definition of abbreviations: BP = blood pressure; EIED = eccentricity index end-diastole; EIES = eccentricity index end-systole; GDF-15 = growth and differentiation factor-15; HR = heart rate; LV = left ventricular; NOx = nitric oxide metabolites; NT-proBNP = N-terminal pro–B-type natriuretic peptide; QoL = quality of life; RA = right atrial; RV = right ventricular; RVedv = RV end-diastolic volume; RVEF = RV ejection fraction; RVesv = RV end-systolic volume; EIED = eccentricity index end-diastole; EIES = eccentricity index end-systole; 6MW = 6-min walk.
Figure 2.
Analysis of RV (right ventricular) mass changes over initial 6 months by intention to treat. (A) Change in fractional RV mass (g) after 6 months of treatment with placebo or simvastatin added to usual care. (B) Box and whisker plot of change in RV mass from baseline in placebo- (n = 23) and simvastatin- (n = 19) treated patients.
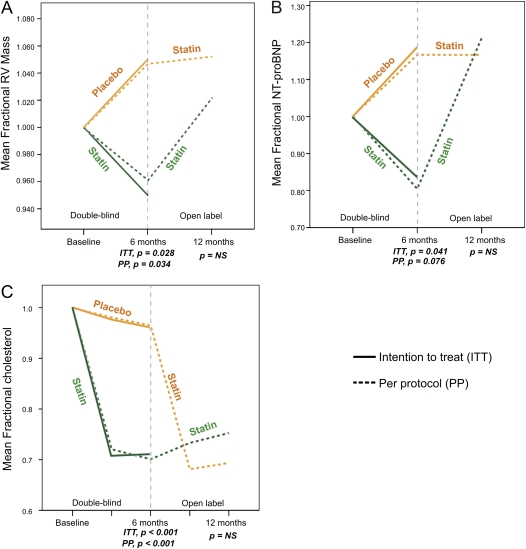
At 12 months, the decrease in RV mass seen on active treatment appeared to have reversed in those who continued on simvastatin: RV mass increased from 6 to 12 months (6.0 ± 13.4 g; P = 0.09) and no longer differed from baseline (mean change 0–12 mo, 1.9 ± 17.9 g; P = 0.605). In the patients who changed from placebo to simvastatin at 6 months, RV mass stabilized (mean increase from 6–12 mo, 0.1 ± 13.0 g; P = 0.97; Figure 3A).
Figure 3.
Change in (A) fractional right ventricular mass, (B) fractional N-terminal pro–B-type natriuretic peptide levels, and (C) fractional total cholesterol levels over 12 months. From 0 to 6 months represents double-blind randomization to simvastatin or placebo. From 6 to 12 months patients were on open-label simvastatin (80 mg). Dotted lines indicate per-protocol analysis; solid lines indicate intention-to-treat analysis. Measurements were made at discrete time points as indicated by the ticks of the x axis. ITT = intention to treat; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PP = per protocol; RV = right ventricular.
Exercise Capacity
The difference in 6-minute walk distance at 6 months in favor of simvastatin (+2.1 m) was not statistically significant (Table 2). More subjects in the placebo-treated group became tachycardic with exercise (heart rate > 100 beats per minute) than in the statin-treated group (43% compared with 16%; P = 0.05). Neither systolic nor diastolic blood pressure showed differential responses to exercise.
NT-proBNP Measurements
Baseline plasma NT-proBNP levels (Table 1) were elevated in both treatment groups (simvastatin: 570.0 [±579] fmol/ml vs. placebo: 444.2 [±447] fmol/ml) compared with those seen in healthy control subjects (<20 fmol/ml). Mean circulating NT-proBNP levels fell in the statin group (−75 ± 167 fmol/ml; P = 0.02) and showed a nonsignificant increase from baseline in the placebo group (49 ± 224 fmol/ml; P = 0.43) (Table 2). The overall treatment effect (−124 fmol/ml) was significant (P = 0.041). From 6 to 12 months, NT-proBNP levels increased in the patients who continued on statin throughout the study but remained stable in the patients who changed from placebo to simvastatin for this period (Figure 3B).
GDF-15 Measurements
Baseline and 6-month plasma GDF-15 levels were within normal range for 39 of the 42 subjects (22, 33). Mean circulating GDF-15 levels increased by 158 (±270) pg/ml in the placebo group (P = 0.01) and by 33 (±397) pg/ml in the treatment group (P = 0.72) (Tables 1 and 2).
Biochemical Measurements
There was no change in prewalk or postwalk exhaled NO levels but plasma NOx levels increased significantly over the first 6 months in the simvastatin group (Figure 4). There were no significant differences in hepatic enzymes, bilirubin, or international normalized ratio between the two treatment groups. No significant differences in the circulating levels of 27 cytokines and chemokines were found between the two groups at baseline or 6 months (see Table E1 in the online supplement).
Figure 4.
Change in plasma nitric oxide metabolites (μmol/L) before and after 6 months of treatment with simvastatin or placebo. Statistics shown are paired t tests. Box plots indicate median and quartiles. NOx = nitric oxide metabolites.
Baseline total cholesterol levels were 4.8 ± 1.1 mmol/L in the statin and 5.8 ± 1.8 mmol/L in the placebo-treated groups (P = 0.06). At 24 weeks these were 3.4 ± 0.8 and 5.7 ± 1.7 mmol/L (P < 0.0001), respectively, indicating good compliance with statin treatment. Total cholesterol levels remained reduced in the patients who continued simvastatin treatment to 12 months (3.6 ± 1.0 mmol/L) and decreased in the patients from the placebo group who switched to simvastatin treatment (4.0 ± 1.0 mmol/L; Figure 3C). Triglycerides at baseline were 1.45 ± 0.6 and 1.69 ± 0.78 mmol/L, respectively, and did not change significantly with treatment.
Quality of Life and Safety
Nonsignificant reductions in the 25-point symptom score (where score is proportional to symptom burden) were observed (mean reduction 0.6 points in the placebo group, P = 0.47, and 1.9 points in the statin-treated group, P = 0.08) in the first 6 months. Mean activity and quality-of-life scores did not change.
DISCUSSION
There is considerable interest in the use of statins for the treatment of PAH, with some patients and their physicians already electing to add a statin to their treatment program. Evidence of potential therapeutic benefit from this drug class comes from a series of animal studies but there are no robust data from studies in patients (4–14). Because the response of the RV to the increased afterload in PAH is an important determinant of patient outcome (18–21), this study examined prospectively the effect of simvastatin on RV mass and function in patients with PAH. The addition of high-dose (80 mg daily) simvastatin to standard care with sildenafil and/or endothelin antagonist treatment was associated with a 5% reduction in RV mass and a 13% decrease in NT-proBNP levels at 6 months, but no improvement in cardiac output or exercise capacity. Moreover, the effects on RV mass and NT-proBNP were not sustained, as both returned to baseline values in patients who continued with the statin for 12 months. Plasma NOx increased in the statin group but there was no significant change in a range of cytokines, potential antiinflammatory markers.
A reduction in RV hypertrophy in the statin-treated group was predicted from animal experiments, although animal models do not faithfully reproduce the human pathology (4–14). The observation that simvastatin inhibited further progression of RV hypertrophy in placebo-treated patients who changed to the statin supports the view that the introduction of simvastatin treatment has an early effect on RV mass. A reduction in RV mass without an increase in cardiac output should be interpreted with caution (20). Hypertrophy is the response of the RV to increased pulmonary vascular resistance. A reduction in RV mass when accompanied by a reduction in NT-proBNP levels may be a useful surrogate for a reduction in pressure load on the RV, although there was no measurable reduction in RV systolic pressure. On the other hand, statins have been shown to have a direct effect on cardiac myocytes and may reduce cardiac hypertrophy via a number of mechanisms (3, 34–36). A reduction in RV mass disproportionate to any reduction in pulmonary vascular remodeling may impair the capacity of the ventricle to meet demand. There was no evidence of impaired cardiac output or exercise capacity in this study but a disproportionate effect on the RV may be one explanation for the transient effect of the statin on RV mass; if the statin has little effect on pulmonary vascular remodeling, the disease will progress, increasing further the pressure-load on the RV, and this will eventually override any effect of the drug on the heart, resulting in progressive RV hypertrophy.
A larger long-term outcome study with this drug is required before judging whether simvastatin is useful as a specific treatment for PAH. It should be noted, however, that the treatment effect on 6-minute walk distance, an outcome measure accepted by regulatory authorities, at 6 months was small (+2 m) and a large number of patients would be required to show statistical significance. The study would have to be at least 12 months' duration to be sure of a sustained effect. The results reinforce the need to conduct studies of PAH treatments over an adequate duration of time to assess the medium term effects of therapy, as recently proposed (37).
Statins vary in their physicochemical properties, which influence their ability to exert pleiotropic effects in non-hepatic cells. Comparing the inhibitory effect of statins on vascular smooth muscle cell growth in culture suggests that lipophilic statins are more potent than hydrophilic agents in this class (O. Ali and J. Wharton, personal observations). Simvastatin daily was chosen for this study as it is the statin examined most commonly in animal models and has been most consistent in demonstrating beneficial effects. Simvastatin 80 mg is the maximum licensed dose for hypercholesterolemia; above this the risk of rhabdomyolysis becomes unacceptable (38). It is possible that a different lipophilic statin might have produced a different result.
There are several limitations to this exploratory study. (1) There are no data for pulmonary vascular resistance, so we do not know whether the reduction in RV mass is secondary to a reduction in pulmonary vascular resistance; (2) the study patients were stable, with a mean time from diagnosis of 4 to 5 years, which might reduce the potential to detect a beneficial effect of simvastatin in a relatively short study period; (3) the unequal distribution of sildenafil therapy in the simvastatin (10) and placebo (4) treatment groups is a potential confounding factor. In an earlier study sildenafil but not bosentan reduced RV mass significantly over a 16-week period (39). It seems unlikely that the additional six patients on sildenafil (four of whom were also taking bosentan) in the simvastatin group could significantly influence the results. But this cannot be discounted, (1) because the addition of simvastatin to sildenafil has been shown to be effective in an animal model (16), and (2) when considering the metabolism of these drugs. All three, simvastatin, sildenafil, and bosentan, are substrates for CYP3A4, whereas bosentan induces hepatic CYP3A4 expression. Simvastatin has no effect on bosentan levels but bosentan reduces simvastatin levels by up to 40% (40). Although there are no published data, simvastatin could theoretically increase sildenafil levels and vice versa. Thus, a combination of sildenafil and simvastatin may offer greater exposure to both drugs, whereas bosentan and simvastatin may reduce exposure to the statin. Despite this, when the patients on sildenafil were excluded from the analysis in this study, a similar trend in RV mass in the treatment and placebo groups was seen.
In summary, the addition of simvastatin to the treatment of patients with idiopathic/heritable PAH and PAH associated with atrial septal defect or connective tissue disease is safe and well tolerated and associated with an early reduction in RV mass and NT-proBNP, which is not sustained over 12 months.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the study and the staff of the Sir John McMichael Centre.
SiPHT Study Group: London: Doug Barker, Ines Cabrita, Gerry Coghlan, Alexandra Davidson, Julia Grappa, Adrian Hobbs, Sharon Meehan, Rick Wage, Farah Williams, Stephen J Wort. Germany: Henning Gall, Ekkehard Gruenig, Hannelore Mach, Hilde Riethmueller-Winzen.
Supported by project grants from the Medical Research Council, British Heart Foundation, and the PulmoTension Consortium.
A list of the members of the SiPHT Study Group can be found at the end of the article.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.2009111-699OC on January 28, 2010
Conflict of Interest Statement: M.R.W. served on the Board or Advisory Board of Bayer and received lecture fees from Bayer and BioMarin (up to $1,000). He received grant support from BioMarin and GlaxoSmithKline ($100,001 or more). O.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W. received lecture fees from Pfizer ($1,001–$5,000) and grant support ($10,001–$50,000). A.T. received lecture fees from Merck Sharp Dhome Chibret AG Switzerland ($1,001–$5,000). C.J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.A.G. served as a consultant, served on the Board or Advisory Board, and received lecture fees from Actelion, BayerSchering, Pfizer, Ergonex, and GlaxoSmithKline ($1,001–$5,000). He received grant support from Pfizer, BayerSchering, Aires, and Ergonex ($10,001–$50,000). L.H. served as a consultant for PAI ($10,001–$50,000) and served on the Board or Advisory Board for GlaxoSmithKline, Pfizer, and Bayer ($1,001–$5,000). He received lecture fees from Actelion ($5,001–$10,000), Pfizer, and GlaxoSmithKline ($1,001–$5,000). He received grant support from Pfizer and Actelion ($100,001 or more). P.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.H.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.R.G. served on the Board or Advisory Board of Actelion Pharmaceuticals, GlaxoSmithKline, Bayer Schering, Pfizer, and United Therapeutics ($1,001–$5,000) and received lecture fees from Actelion, Lily, GlaxoSmithKline, Bayer Schering, and Pfizer ($1,001–$5,000). He received grant support from United Therapeutics ($5,001–$10,000).
References
- 1.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10–S19. [DOI] [PubMed] [Google Scholar]
- 2.NationalPulmonary Hypertension Centres of the UK and Ireland. Consensus statement on the management of pulmonary hypertension in clinical practice in the UK and Ireland. Thorax 2008;63:ii1–ii41. [DOI] [PubMed] [Google Scholar]
- 3.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med 2008;14:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol 2003;285:H938–H945. [DOI] [PubMed] [Google Scholar]
- 5.Guerard P, Rakotoniaina Z, Goirand F, Rochette L, Dumas M, Lirussi F, Bardou M. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 2006;373:401–414. [DOI] [PubMed] [Google Scholar]
- 6.Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, Christians U, Weimann J, Kaisers U, Steudel W. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol 2007;293:L630–L638. [DOI] [PubMed] [Google Scholar]
- 7.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 2005;25:2335–2342. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med 2002;166:1403–1408. [DOI] [PubMed] [Google Scholar]
- 9.Rakotoniaina Z, Guerard P, Lirussi F, Goirand F, Rochette L, Dumas M, Bardou M. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn Schmiedebergs Arch Pharmacol 2006;374:195–206. [DOI] [PubMed] [Google Scholar]
- 10.Satoh K, Fukumoto Y, Nakano M, Sugimura K, Nawata J, Demachi J, Karibe A, Kagaya Y, Ishii N, Sugamura K, et al. Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell-derived factor-1. Cardiovasc Res 2009;81:226–234. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M, Satoh A. 3-Hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors and phosphodiesterase type V inhibitors attenuate right ventricular pressure and remodeling in a rat model of pulmonary hypertension. J Pharm Pharm Sci 2008;11:118s–130s. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 2008;294:H801–H809. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003;108:1640–1645. [DOI] [PubMed] [Google Scholar]
- 14.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol 2007;292:L1105–L1110. [DOI] [PubMed] [Google Scholar]
- 15.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol 2007;293:L933–L940. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Sebkhi A, Ali O, Wojciak-Stothard B, Mamanova L, Yang Q, Wharton J, Wilkins MR. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J 2009;34:948–957. [DOI] [PubMed] [Google Scholar]
- 17.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest 2005;127:1446–1452. [DOI] [PubMed] [Google Scholar]
- 18.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 19.Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, Guerrero ML. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 1994;89:1733–1744. [DOI] [PubMed] [Google Scholar]
- 20.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28:1250–1257. [DOI] [PubMed] [Google Scholar]
- 21.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114:1883–1891. [DOI] [PubMed] [Google Scholar]
- 22.Nickel N, Kempf T, Tapken H, Tongers J, Laenger F, Lehmann U, Golpon H, Olsson K, Wilkins MR, Gibbs JS, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;178:534–541. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009;119:2250–2294. [DOI] [PubMed] [Google Scholar]
- 24.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;34:1219–1263. [DOI] [PubMed] [Google Scholar]
- 25.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J 2006;27:2879–2888. [DOI] [PubMed] [Google Scholar]
- 26.Chai P, Mohiaddin R. How we perform cardiovascular magnetic resonance flow assessment using phase-contrast velocity mapping. J Cardiovasc Magn Reson 2005;7:705–716. [DOI] [PubMed] [Google Scholar]
- 27.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol 1985;5:918–927. [DOI] [PubMed] [Google Scholar]
- 28.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996;9:838–847. [DOI] [PubMed] [Google Scholar]
- 29.Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J 2009;30:1057–1065. [DOI] [PubMed] [Google Scholar]
- 30.Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, Hartford M, Caidahl K. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation 2002;106:2913–2918. [DOI] [PubMed] [Google Scholar]
- 31.Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. J Biol Chem 2005;280:10040–10046. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan LM. Repeated measures. Circulation 2008;117:1238–1243. [DOI] [PubMed] [Google Scholar]
- 33.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem 2007;53:284–291. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Shen Q, Wu Y. Simvastatin prevents cardiac hypertrophy in vitro and in vivo via JAK/STAT pathway. Life Sci 2008;82:991–996. [DOI] [PubMed] [Google Scholar]
- 35.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest 2001;108:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, Zhao L, Zheng Q, Shang F, Wang X, Wang L, Lang B. Simvastatin attenuates hypertrophic responses induced by cardiotrophin-1 via JAK-STAT pathway in cultured cardiomyocytes. Mol Cell Biochem 2006;284:65–71. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin VV, Badesch DB, Delcroix M, Fleming TR, Gaine SP, Galie N, Gibbs JS, Kim NH, Oudiz RJ, Peacock A, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S97–S107. [DOI] [PubMed] [Google Scholar]
- 38.Nissen SE. High-dose statins in acute coronary syndromes: not just lipid levels. JAMA 2004;292:1365–1367. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, Westwood MA, Stefanidis A, Ng LL, Pennell DJ, et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med 2005;171:1292–1297. [DOI] [PubMed] [Google Scholar]
- 40.Dingemanse J, Schaarschmidt D, van Giersbergen PL. Investigation of the mutual pharmacokinetic interactions between bosentan, a dual endothelin receptor antagonist, and simvastatin. Clin Pharmacokinet 2003;42:293–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.