Abstract
Rationale: Obstructive sleep apnea (OSA) is a highly prevalent disorder in children, in which enlarged adenotonsillar tissues (AT) play a major pathophysiologic role. Mechanisms leading to the proliferation and hypertrophy of AT in children who subsequently develop OSA remain unknown, and surgical extirpation of AT is associated with potential morbidity and mortality.
Objectives: We hypothesized that a computationally based analysis of gene expression in tonsils from children with OSA and children with recurrent tonsillitis without OSA can identify putative mechanistic pathways associated with tonsillar proliferation and hypertrophy in OSA.
Methods: Palatine tonsils from children with either polysomnographically documented OSA or recurrent infectious tonsillitis were subjected to whole-genome microarray and functional enrichment analyses followed by significance score ranking based on gene interaction networks. The latter enabled identification and confirmation of a candidate list of tonsil-proliferative genes in OSA.
Measurements and Main Results: In vitro studies using a mixed tonsil cell culture system targeting one of these candidates, phosphoserine phosphatase, revealed that it was more abundantly expressed in tonsils of children with OSA, and that pharmacological inhibition of phosphoserine phosphatase led to marked reductions in T- and B-lymphocyte cell proliferation and increased apoptosis.
Conclusions: A systems biology approach revealed a restricted set of candidate genes potentially underlying the heightened proliferative properties of AT in children with OSA. Furthermore, functional studies confirm a novel role for protein phosphatases in AT hypertrophy, and may provide a promising strategy for discovery of novel, nonsurgical therapeutic targets in pediatric OSA.
Keywords: sleep apnea, phosphatase, adenotonsillar, genetic network, microarray
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Untreated obstructive sleep apnea is associated with neurocognitive, metabolic, and cardiovascular morbidities in children and can affect up to 3% of the pediatric population. Enlargement of adenotonsillar tissue is the key etiological event in this disorder, yet the pathophysiological mechanisms leading to lymphadenoid proliferation are poorly understood.
What This Study Adds to the Field
In this work, we propose a network-based paradigm to systematically identify transcriptional programs regulating tonsillar hypertrophy in children with sleep apnea and demonstrate that selective targeting of a network node, phosphoserine phosphatase, reverses the proliferative state of adenotonsillar tissue. Our findings may result in nonsurgical alternatives for the treatment of pediatric sleep apnea.
Obstructive sleep apnea (OSA) is a prevalent disorder affecting up to 2 to 3% of children; it imposes substantial neurocognitive, behavioral, metabolic, and cardiovascular morbidities (1, 2). This condition is characterized by repeated events of partial or complete obstruction of the upper airways during sleep, leading to recurring episodes of hypercapnia, hypoxemia, and arousal throughout the night (3). Adenotonsillar hypertrophy is the major pathophysiological contributor to OSA in children (4, 5). However, the mechanisms underlying the regulation of benign follicular lymphoid proliferation, hypertrophy, and hyperplasia are poorly understood, severely limiting the prediction of children who are at risk for developing adenotonsillar enlargement and OSA. Several epidemiological studies have demonstrated that factors such as environmental smoking, allergies, and recurrent respiratory infections are associated with either transient or persistent hypertrophy of lymphadenoid tissue in the upper airways of snoring children (6–8). Interestingly, all of these risk factors involve the generation of an inflammatory response, suggesting that the latter may promote the onset and maintenance of proliferative signals to lymphadenoid tissues. In the vast majority of children diagnosed with OSA, the initial treatment approach is the surgical removal of enlarged tonsils and adenoids. Although 50 to 115 of every 10,000 children undergo tonsillectomy and adenoidectomy (9), the overall efficacy of this procedure has not been firmly established, with recent data suggesting that the success rate is significantly less than previously reported (10). Furthermore, this procedure is associated with pain, other morbidities, and substantial healthcare costs. Therefore, identification of a set of genes orchestrating the proliferation of adenotonsillar tissue in the upper airway of children with OSA may allow for development of novel, cost-effective, nonsurgical therapeutic strategies.
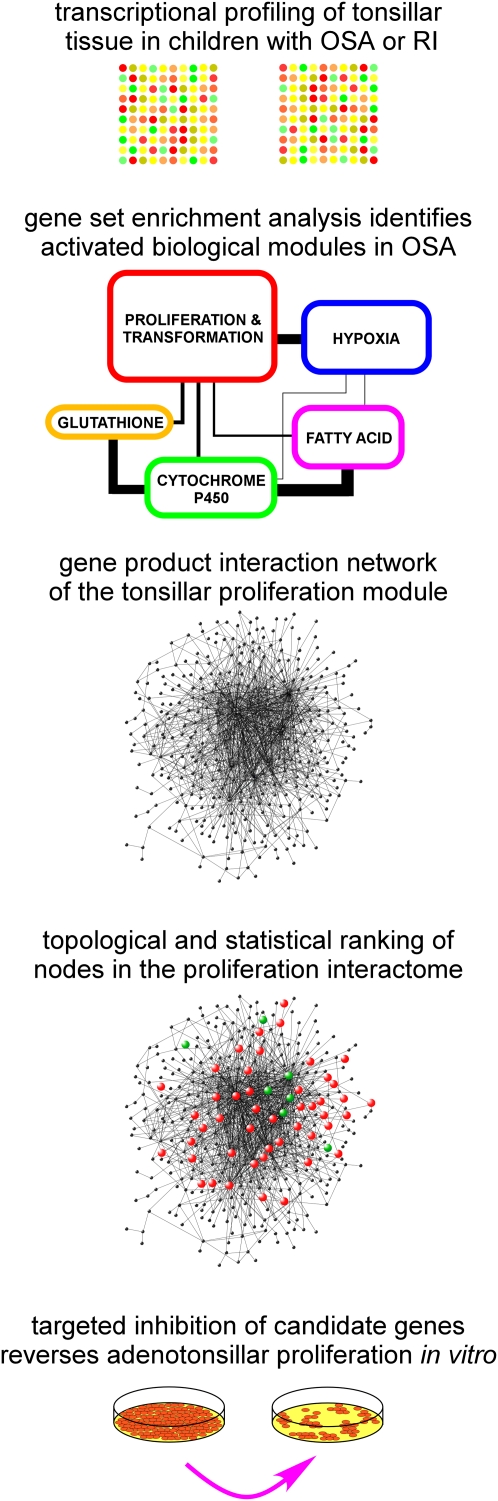
In this study, we integrated genome-wide expression analysis with novel bioinformatics methods to identify putative functional networks involved in the proliferation of tonsillar tissue in the upper airway of children with OSA (Figure 1). These analyses were conducted on tonsils from children with polysomnographically demonstrated OSA and from age-matched, sex-matched, and ethnicity-matched children without OSA who underwent tonsillectomy for recurrent tonsillar infections. We developed a computational framework to systematically narrow down differentially expressed genes to a restricted set derived from a network of proliferative pathways. To further validate this approach, the effect of antagonists directed against one of the network-associated candidate genes, phosphoserine phosphatase (PSPH), was assessed in a mixed tonsil cell culture system. We confirmed the pro-proliferative role played by PSPH in tonsillar tissue from children with OSA, but not in those derived from children with recurrent infections.
Figure 1.
Schematic overview of the experimental approach for identifying and confirming candidate targets in adenotonsillar hypertrophy of pediatric obstructive sleep apnea (OSA). RI = recurrent tonsillar infection.
METHODS
See online supplement for more details.
Patients
The study was approved by the University of Louisville Human Research Committee, and informed consent was obtained from the legal caregiver of each participant, with assent being obtained from children older than 7 years of age. Consecutive prepubertal nonobese children diagnosed with OSA at the University of Louisville Pediatric Sleep Research Center in Louisville, Kentucky, were invited to participate. Inclusion criteria were the presence of OSA according to polysomnographic criteria and age between 6 and 11 years. Exclusion criteria were chronic medical condition, receipt of medications, and any genetic or craniofacial syndromes.
Children with recurrent tonsillar infection (RI) were selected based on a history of at least five tonsillar infections requiring administration of antibiotic courses over a period of less than 6 months, as well as the absence of sleep-disordered breathing as assessed by an overnight sleep study. Subject characteristics are provided in Table 1.
TABLE 1.
DEMOGRAPHIC AND POLYSOMNOGRAPHIC CHARACTERISTICS OF CHILDREN WITH OBSTRUCTIVE SLEEP APNEA (OSA) AND RECURRENT TONSILLITIS (RI)
| OSA (n = 44) | RI (n = 44) | |
|---|---|---|
| Age, yr | 6.0 ± 1.6 | 5.5 ± 2.3 |
| Sex, F/M | 20/24 | 20/24 |
| African American, n | 14 | 14 |
| BMI, z score | 0.76 ± 0.07 | 0.54 ± 0.06 |
| TST, min | 487.5 ± 9.4 | 482.9 ± 8.3 |
| SWS, % TST | 27.5 ± 2.8 | 31.3 ± 3.6 |
| REM sleep, % TST | 19.5 ± 3.1 | 23.2 ± 2.8 |
| AHI, per h TST* | 12.7 ± 2.9 | 0.4 ± 0.1 |
| Nadir SaO2, %* | 83.5 ± 3.4 | 95.7 ± 0.3 |
| Arousal index, per h TST* | 17.4 ± 3.7 | 7.7 ± 0.5 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; F = female; M = male; TST = total sleep time; SWS = slow wave sleep.
All data are expressed as mean ± SD; data for the 18 initial subjects in each group and those for subsequent gene validation and proliferation studies were compared and found to be similar, and were therefore merged.
P < 0.001.
Polysomnographic Assessment
Overnight polysomnography was performed using standard measuring procedures. The diagnostic criteria for OSA included an obstructive apnea index greater than 1 per hour of total sleep time and/or an obstructive apnea-hypopnea index greater than 5 per hour of total sleep time with a nadir oxygen saturation value less than 92% (11).
Statistical Analysis
Results are presented as mean ± SD unless stated otherwise. Comparisons according to group assignment were made with independent t tests or analysis of variance followed by post hoc comparisons, with P values adjusted for unequal variances when appropriate (Levene test for equality of variances) or χ2 analyses with Fisher exact test (dichotomous outcomes). A two-tailed P value less than 0.05 was considered statistically significant.
Tonsillar Tissue Collection
Because tonsil cannot be obtained from normal children for obvious ethical reasons, consecutive children undergoing tonsillectomy at Kosair Children's Hospital for either OSA or RI were identified before surgery and recruited to the study. OSA and RI children were also required to have received their last dose of antibiotic therapy at least 6 weeks before the day of the surgery. Children with OSA were excluded if they suffered from RI. Tonsils were removed by a pediatric ear, nose, and throat specialist, and a portion of each tonsil was stabilized in RNALater (Applied Biosytems, Austin, TX) and stored at −80°C.
RNA Isolation and Microarray Hybridization
Total RNA from tonsils of 18 children with RI and 18 children with OSA (their clinical characteristics are shown in Table E1 in the online supplement) was isolated using RNeasy Lipid Tissue Mini Kit with DNase treatment (Qiagen, Valencia, CA), and hybridized to Agilent human whole-genome arrays containing 44,000 transcripts as previously described (14).
Identification of Enriched Pathways in Tonsillar Tissue
Gene expression intensities of all 36 subjects (OSA and RI), whose clinical characteristics are shown in Table E1, were normalized and log-transformed. Enriched pathways in tonsillar tissue from children with OSA were identified using gene set enrichment analysis (15) at a false discovery rate cutoff less than or equal to 10%.
Gene Interaction Network Analysis
Genes mapped to gene sets enriched in OSA subjects and involved in proliferative pathways were combined, and an interaction network was created using Ingenuity's knowledge base (16) and several publicly available gene product relationship databases (17–19). The topological characteristics of the interactome (i.e., the number of nodes and connectivity) were extracted.
Ranking of Network-associated Genes Based on Their Significance Score
A score based on the connectivity and the differential expression levels of the interactome's nodes was developed to identify the genes most likely to be important in tonsillar hypertrophy.
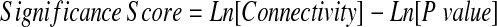 |
where the P value was based on a gene-by-gene intergroup comparison using the parametric t test (two-sided, unequal variance). Statistical cutoff values were determined by performing a random permutation analysis on the subjects and obtaining a null frequency distribution. Genes with scores above the 95th percentile of the null distribution were deemed significant.
Tonsil Immunohistochemistry
Tonsils were fixed overnight and sectioned on a freezing microtome. Coronal sections incubated with primary PSPH antisera (Abcam cta# ab58125; 1:1,000) followed by exposure to biotinylated anti-rabbit antibody and incubation with streptavidin-horseradish peroxidase. Sections were prepared from five sets of tonsils and of adenoids from either OSA or RI groups and visualized using a fluorescent microscope by an investigator blinded to the sample source.
Mixed Adenotonsillar Primary Cell Culture System
Surgically removed tonsils and adenoids were used for isolation of cells and establishment of a mixed cell culture as previously described (12). Cells were incubated to evaluate basal proliferation or treated with PSPH inhibitors such as okadaic acid, calyculin A, and protein phosphatase inhibitor 2 (PPI2) at 10−6 to 10−9 M concentrations.
Bromodeoxyuridine Cell Proliferation and Apoptosis Annexin V Assays with Flow Cytometry
To detect global cell, T-cell, and B-cell specific proliferation, we used bromodeoxyuridine pulsed proliferation analysis using flow cytometry (13). A similar approach was undertaken using annexin V mouse anti-human antibodies to quantify global, T-cell, or B-cell specific apoptosis (13). All data are expressed as the percentage of positive cell from the total cell population.
RESULTS
OSA Activates Distinct Biological Processes in Tonsillar Tissue of Children
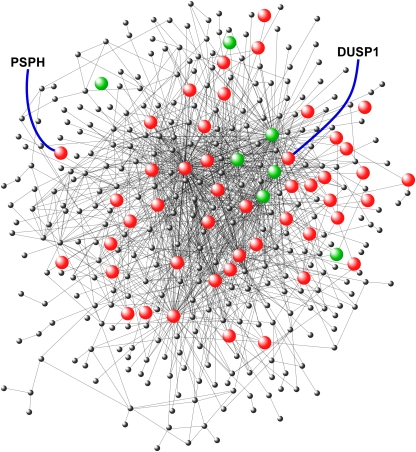
An overview of our approach is depicted in Figure 1. We applied gene set enrichment analysis (15) to the gene expression profiles in tonsils of children with OSA (n = 18) and RI (n = 18). Out of approximately 1,800 curated pathways sampled, 22 were enriched in the OSA phenotype at a false discovery rate less than or equal to 10%, whereas no gene sets were enriched in the RI group. The gene sets associated with OSA were functionally organized into five broad categories: proliferation, hypoxia, glutathione metabolism, cytochrome P450 activity, and fatty acid/steroid biosynthesis (Figure E1). The proliferation module contained the largest number of member genes (n = 564), and given its potential biological relevance to tonsillar hypertrophy, became the focus of subsequent analyses. Members of this module were linked together based on previously reported gene product interactions, resulting in a complex network composed of 361 nodes and 2,476 edges. Like many biological networks (20), the tonsillar hypertrophy interactome followed scale-free topological characteristics (Figures E2–E4).
An Integrative Scoring Strategy Identifies Putative Candidate Genes Mediating Tonsillar Hypertrophy in OSA
Using the connectivity matrix of the proliferation interactome and the differential expression of its nodes, we developed a significance scoring metric that ranked the gene members (score range: 0.01–11.35). A random permutation analysis was then used to determine a 95% significance cut-off value of 4.87, resulting in the selection of 69 genes (Table E2, Figure 2). Many of these gene candidates were involved in inflammation signaling (e.g., IL1B, IL1A, IL1F6, IL6, CCL19) and regulation (e.g., JUNB, FOS), and tissue growth and remodeling (e.g., TGFB1, TGFB2, HBEGF, CTGF, FN1). We have recently demonstrated that adenotonsillar tissue from children with sleep apnea express proinflammatory cytokines and is in a proliferative state (21). Several members of the carcinoembryonic gene family were among the top-ranked hits in our analysis, including CEACAM1, CEACAM5, and CEACAM6. These molecules are critical mediators of cell–cell adhesion and determinants of tissue architecture, aberrant growth, and hypertrophy (22–24). Two protein phosphatases, dual-specificity phosphatase 1 (DUSP1) and PSPH, were among the statistically significant network genes (score: 7.24, 5.92 respectively). Because little is known about the role of protein phosphatases in promoting tonsillar hypertrophy, we proceeded to investigate whether PSPH and DUSP1 represented novel therapeutic targets.
Figure 2.
Gene product interaction network in tonsillar proliferation of obstructive sleep apnea. Candidate genes reaching significance based on our scoring algorithm (cutoff > 4.86) are highlighted (red: up-regulated relative to RI; green: down-regulated relative to recurrent tonsillar infection). Phosphoserine phosphatase and dual-specificity phosphatase 1 have been labeled. See text for details.
Selective Inhibition of Phosphoserine Phosphatase Is Antiproliferative
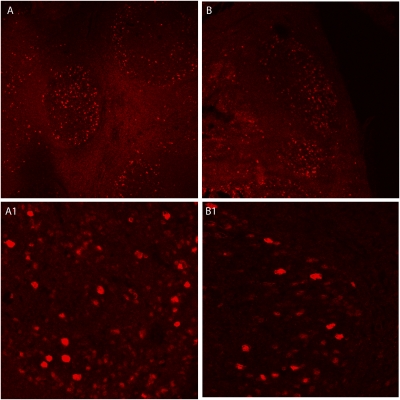
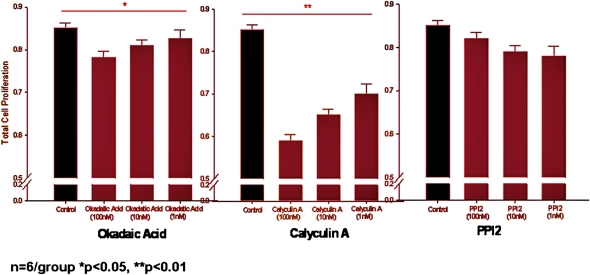
We initially determined that PSPH protein expression seemed to localize primarily to the germinal centers within the tonsil structure using immunohistochemistry and was qualitatively more abundant in children with OSA than those with RI (Figure 3). Next, we treated mixed cellular tonsil and adenoid cultures (12) with the phosphatase inhibitors okadaic acid, calyculin A, and PPI2 at increasing concentrations. Exposure to okadaic acid (which inhibits both PSPH and DUSP1) and calyculin A (a DUSP and protein phosphatase 1 and 2A inhibitor) reduced the proliferation of tonsillar/adenoid cell cultures harvested from children with OSA; however, this antiproliferative effect was not seen with PPI2 (a preferential protein phosphatase 2B antagonist; n = 6 for all experiments) (Figure 4). Much milder and nonsignificant effects were seen in tonsillar/adenoid cell cultures from children with recurrent infections (n = 6 for all experiments). Therefore, these findings confirm that phosphatases orchestrate proliferative signaling pathways in upper airway lymphadenoid tissues of children with obstructive sleep apnea. Our expression profiling–based strategy implies that important mechanisms regulating adenotonsillar proliferation occur at the transcriptional level, and targeted perturbation of gene expression can alter the production and activity of its corresponding protein, reversing the lymphadenoid hypertrophy associated with pediatric OSA.
Figure 3.
Immunohistochemical staining for phosphoserine phosphatase (PSPH) of palatine tonsils from (A, A1) a child with obstructive sleep apnea (OSA) and (B, B1) a matched child with recurrent tonsillar infection (RI). In children with OSA, PSPH preferentially localizes to germinal centers, where it is less abundant in children with RI.
Figure 4.
Proliferative rates in tonsil cell cultures from children with obstructive sleep apnea after administration of okadaic acid, calyculin A, and protein phosphatase inhibitor 2 (PPI2) at increasing concentrations. In recurrent tonsillar infection–derived tonsil cell cultures, no significant differences emerged for any of the compounds.
Inhibition of Phosphoserine Phosphatase Blocks Proliferation and Promotes Programmed Cell Death
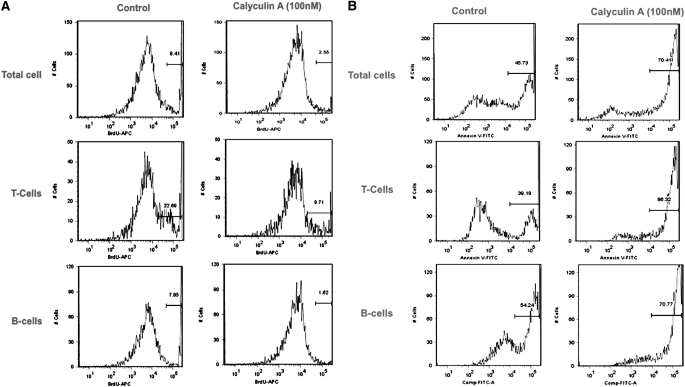
To confirm the antiproliferative effects of calyculin and the potential induction of apoptosis by this phosphatase inhibitor in the mixed-cell adenotonsillar cultures of OSA-derived tonsils, we performed flow cytometric analysis using bromodeoxyuridine and annexin V staining, respectively (13). Exposure of the cell cultures to 100 nM calyculin dramatically reduced cellular proliferation (Figure 5A, control versus calyculin A in total cells: 8.87 ± 0.35% vs. 3.06 ± 0.72%, P < 0.001; control versus calyculin A in T cells: 22.4 ± 1.78% vs. 9.8 ± 0.61%, P < 0.001; control versus calyculin A in B cells: 7.56 ± 0.56% vs. 1.78 ± 0.57%, P < 0.001) and resulted in a significantly higher rate of programmed cell death (Figure 5B, control vs. calyculin A in total cells: 45.5 ± 2.5% vs. 72.4 ± 3.40%, P < 0.001). The selective difference in apoptosis was further characterized by differentiating T-cell (sorted by CD3+) and B-cell subtypes (sorted by CD19+). Treatment with 100 nM calyculin elicited cell death in 85.7 ± 2.2% of T cells compared with control conditions (38.4 ± 2.2%; P < 0.001). Similarly, exposure to calyculin resulted in apoptosis among 71.2 ± 4.4% of B-cell lymphocytes compared with 53.7 ± 3.6% in untreated conditions (P < 0.001). These data imply that the antiproliferative effect of targeting phosphoserine phosphatase is mediated, in part, through induction of apoptosis in adenotonsillar cells. Furthermore, programmed cell death appears to be more prominent in T cells compared with B cells.
Figure 5.
(A) Representative example of bromodeoxyuridine (BrDU)-based proliferation assay in tonsil cell cultures from a 6-year-old child with obstructive sleep apnea (OSA) treated with vehicle (left) or calyculin (right). Marked reductions in global cell proliferation and in T-cell and B-cell proliferation are apparent. (B) Representative example of annexin V–based apoptosis assay in a 4-year-old child with OSA treated with vehicle (left) or calyculin (right). Marked increases in global cellular apoptosis and in T-cell and B-cell apoptosis are apparent.
Quantitative Reverse Transcriptase Polymerase Chain Reaction Validation of Selected Candidate Genes
Up-regulation of PSPH, DUSP1, and CEACAM5, in children with OSA was validated with quantitative reverse transcriptase–polymerase chain reaction in the original cohort of 36 subjects (see Table E3).
DISCUSSION
Pediatric sleep apnea is a common disorder primarily caused by enlarged tonsils and adenoids impinging on the patency of the upper airway during sleep. Mechanisms leading to the proliferation and enlargement of the tonsils and adenoids in children who subsequently develop OSA remain unknown. Furthermore, the usual treatment for pediatric sleep apnea (i.e., tonsillectomy and adenoidectomy) is costly and fraught with measurable adverse consequences ranging from mild events, such as pain, to serious complications, such as hemorrhage, infections, acute respiratory insufficiency, and potentially death (25). Therefore, effective nonsurgical strategies for the treatment of pediatric sleep apnea are imperatively needed and can dramatically impact clinical practice.
In the present study, we performed genome-wide transcriptional profiling of tonsillar tissues obtained from children with and without OSA and outlined a computational framework to identify novel candidate targets regulating adenotonsillar hypertrophy. We validated our unbiased approach by performing follow-up in vitro proliferation assays to demonstrate that selective targeting of two up-regulated candidate genes, PSPH and DUSP1, profoundly reverses the proliferative state of adenotonsillar tissue in OSA. Thus, phosphatases play a critical role in pediatric tonsillar hypertrophy and mediate the mechanisms leading to this proliferative state selectively in children with OSA but not in those with RI.
PSPH belongs to a subfamily of phosphotransferases and catalyzes the rate-limiting step in serine biosynthesis by converting l-phosphoserine to l-serine (26). The only reported case of PSPH deficiency in a child described pre- and postnatal growth retardation, moderate psychomotor retardation, facial abnormalities, and reduced PSPH activity in lymphoblasts and fibroblasts (27). Treatment with oral serine led to normalization of serine levels and some improvement in head growth. In mice, PSPH is abundantly expressed in proliferating embryonic and hematopoietic stem cells and in neural progenitors in the developing brain (28, 29). Furthermore, siRNA knockdown of PSPH expression inhibited neural stem cell proliferation (29), suggesting that this enzyme can be selectively targeted to affect cellular proliferation. To our knowledge, a role for PSPH in promoting tonsillar tissue hypertrophy has not been previously reported. We used several strategies to target PSPH expression and activity in adenotonsillar primary cell cultures, including pharmacological and siRNA inhibition (data not shown), resulting in significant antiproliferative effects specifically in cell cultures derived from children with OSA. Furthermore, inhibition of PSPH appears to promote programmed cell death in tonsillar cell cultures. Together, these observations suggest that PSPH is a logical therapeutic target in reversing the adenotonsillar enlargement of pediatric OSA.
Dual-specificity phosphatases have also been extensively investigated as critical modulators of lymphocyte function, proliferation, and apoptosis. Indeed, immune cell functions are intimately associated with the enzymatically catalyzed addition of phosphate to key tyrosine, threonine, and serine residues in proteins (30–32). Although the specific role of DUSP1 in tonsillar proliferation remains to be established in the context of pediatric OSA, our preliminary experiments using siRNA also support a regulatory role for this group of phosphatases in the regulation of proliferation and apoptosis among critical lymphocyte populations within tonsillar tissues.
We have previously demonstrated the effectiveness of nonsurgical approaches for treatment of mild sleep-disordered breathing in children, including the use of antiinflammatory agents, such as oral leukotriene modifiers (33) and intranasal steroids (34). Furthermore, we recently used adenotonsillar cell culture assays to confirm that these pharmacologic interventions promote an antiproliferative phenotype (13, 35). The current study provides a much broader framework for the selection of novel candidate pathways and critical mediators of adenotonsillar hypertrophy in children with OSA. Our computational network analysis identified several other putative targets with well-known roles in tissue remodeling and growth, including members of the carcinoembryonic gene family (CEACAM1, CEACAM5, CEACAM6), insulin growth factor binding protein 2 (IGFBP2), lipocalin 2 (LCN2), and connective tissue growth factor (CTGF), as well as many proinflammatory mediators (IL1B, IL1A, IL1F6, IL6, CCL19, JUN, FOS). Future work on the molecular mechanisms by which these candidates potentially regulate tonsillar proliferation will help elucidate the pathophysiology of OSA in children, and may result in the development of combinatorial therapies for this complex disorder.
There are several notable strengths in this work. First, because the data were generated from human tonsillar tissue samples, our findings are likely to be directly relevant to pediatric OSA. Second, in addition to the proliferation module, our unbiased approach identified several other biological modules selectively enriched in children with OSA, including those involved in hypoxia, fatty acid metabolism, and glutathione biosynthesis. Previous reports strongly suggest that exposure to intermittent hypoxia promotes the activation of oxidative stress pathways and can result in perturbations in lipid synthesis and metabolism (36). Therefore, a similar integrative network analysis may yield additional biologically relevant targets for treatment of pediatric OSA. Finally, our general computational methodology for target selection is applicable to any transcriptional or proteomic-based data acquisition from human tissue samples.
Our study has a number of limitations. Because tonsils cannot be obtained from normal, asymptomatic children for obvious ethical reasons, we compared enlarged tonsils in children with OSA with tonsils of children without OSA who suffered from a history of recurrent tonsillitis (RI). Although tonsils were removed at least 6 weeks after the last dose of antibiotics in the RI group, significant differences in gene expression may persist between the tonsillar tissue of RI subjects and healthy children. Additionally, intraoperative conditions, such as anesthesia and hypoxia, may alter gene expression, but such differences would cancel out because both groups of children, namely OSA and RI, underwent similar surgical and anesthetic procedures. The bioinformatics analyses in the present work are limited by our current state of knowledge in biological pathways and gene product relationships. Therefore, future iterations of the same data can yield different results. Furthermore, because our analyses are based on transcriptional signatures, we are unable to assess post-transcriptional effects. Finally, it is important to note that we confirmed the role of only a few selected putative candidate genes in mediating adenotonsillar proliferation. Future work is necessary to establish the biological relevance of the other candidates.
Despite its prevalence and associated morbidity, little is known about the pathophysiologic mechanisms leading to sleep apnea in children. Because adenotonsillar enlargement is the primary cause of pediatric OSA, our human-based approach provides a paradigm for systematically identifying targets for pharmacological reversal of adenotonsillar proliferation and avoiding surgical extirpation. Applying global gene expression analysis, bioinformatics, and network-based methods to clinically relevant phenotypes is a promising strategy for discovering novel therapeutic sites in complex human disorders.
Supplementary Material
Supported by the National Institutes of Health grants HL065270 and HL086662 (D.G.) and HL074223 (S.A.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200909-1398OC on January 21, 2010
Conflict of Interest Statement: A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.B. received $10,001–$50,000 from Jazz Pharmaceuticals for the Sleep Training Fellowship from July 1, 2008 to June 30, 2009. L.K-G. received more than $100,001 from Merck Co as an investigator-initiated grant. J.L.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G. received $10,001–$50,000 as a consultant on respiratory control from Galleon Pharmaceuticals and $5,001–$10,000 from Merck for lectures on pediatric asthma.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc 2008;5:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzumdar H, Arens R. Diagnostic issues in pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2003;167:65–70. [DOI] [PubMed] [Google Scholar]
- 5.Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, Agorogiannis E, Christodoulou S, Pantazidou A, Gourgoulianis K, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004;37:499–509. [DOI] [PubMed] [Google Scholar]
- 7.Teculescu DB, Caillier I, Perrin P, Rebstock E, Rauch A. Snoring in French preschool children. Pediatr Pulmonol 1992;13:239–244. [DOI] [PubMed] [Google Scholar]
- 8.Ersu R, Arman AR, Save D, Karadag B, Karakoc F, Berkem M, Dagli E. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest 2004;126:19–24. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Akker EH, Hoes AW, Burton MJ, Schilder AG. Large international differences in (adeno)tonsillectomy rates. Clin Otolaryngol Allied Sci 2004;29:161–164. [DOI] [PubMed] [Google Scholar]
- 10.Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O'Brien LM, Ivanenko A, Gozal D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 2006;149:803–808. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–753. [DOI] [PubMed] [Google Scholar]
- 12.Serpero LD, Kheirandish-Gozal L, Dayyat E, Goldman JL, Kim J, Gozal D. A mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitis. Laryngoscope 2009;119:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheirandish-Gozal L, Serpero LD, Dayyat E, Kim J, Goldman JL, Snow A, Bhattacharjee R, Gozal D. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J 2009;33:1077–1084. [DOI] [PubMed] [Google Scholar]
- 14.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med 2009;10:75–86. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. A network-based analysis of systemic inflammation in humans. Nature 2005;437:1032–1037. [DOI] [PubMed] [Google Scholar]
- 17.Alfarano C, Andrade CE, Anthony K, Bahroos N, Bajec M, Bantoft K, Betel D, Bobechko B, Boutilier K, Burgess E, et al. The biomolecular interaction network database and related tools 2005. update. Nucleic Acids Res 2005;33:D418–D424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peri S, Navarro JD, Kristiansen TZ, Amanchy R, Surendranath V, Muthusamy B, Gandhi TK, Chandrika KN, Deshpande N, Suresh S, et al. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res 2004;32:D497–D501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salwinski L, Miller CS, Smith AJ, Pettit FK, Bowie JU, Eisenberg D. The database of interacting proteins: 2004 update. Nucleic Acids Res 2004;32:D449–D451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barabasi AL, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet 2004;5:101–113. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Bhattacharjee R, Dayyat E, Snow AB, Kheirandish-Gozal L, Goldman JL, Li RC, Serpero LD, Clair HB, Gozal D. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res 2009;66:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, Neumaier M, Kalthoff H, Lin SH, Najjar SM. Ceacam1 modulates epidermal growth factor receptor–mediated cell proliferation. J Clin Invest 2004;114:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989;57:327–334. [DOI] [PubMed] [Google Scholar]
- 24.Kuespert K, Pils S, Hauck CR. Ceacams: their role in physiology and pathophysiology. Curr Opin Cell Biol 2006;18:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsden R. National prospective tonsillectomy audit: final report of an audit carried out in England and Northern Ireland between July 2003. and September 2004. Available from: http://www.rcseng.ac.uk/rcseng/content/publications/docs/national_prospective.html.
- 26.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem 1989;58:453–508. [DOI] [PubMed] [Google Scholar]
- 27.Jaeken J, Detheux M, Fryns JP, Collet JF, Alliet P, Van Schaftingen E. Phosphoserine phosphatase deficiency in a patient with Williams syndrome. J Med Genet 1997;34:594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geschwind DH, Ou J, Easterday MC, Dougherty JD, Jackson RL, Chen Z, Antoine H, Terskikh A, Weissman IL, Nelson SF, et al. A genetic analysis of neural progenitor differentiation. Neuron 2001;29:325–339. [DOI] [PubMed] [Google Scholar]
- 29.Nakano I, Dougherty JD, Kim K, Klement I, Geschwind DH, Kornblum HI. Phosphoserine phosphatase is expressed in the neural stem cell niche and regulates neural stem and progenitor cell proliferation. Stem Cells 2007;25:1975–1984. [DOI] [PubMed] [Google Scholar]
- 30.Liu JO. Calmodulin-dependent phosphatase, kinases, and transcriptional corepressors involved in T-cell activation. Immunol Rev 2009;228:184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanzola MB, Kersh GJ. The dual specificity phosphatase transcriptome of the murine thymus. Mol Immunol 2006;43:754–762. [DOI] [PubMed] [Google Scholar]
- 32.Won J, Lee GH. T-cell-targeted signaling inhibitors. Int Rev Immunol 2008;27:19–41. [DOI] [PubMed] [Google Scholar]
- 33.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kheirandish L, Goldbart AD, Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in children. Pediatrics 2006;117:e61–e66. [DOI] [PubMed] [Google Scholar]
- 35.Dayyat E, Serpero LD, Kheirandish-Gozal L, Goldman JL, Snow A, Bhattacharjee R, Gozal D. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest 2009;135:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.