The interplay between electronic effects and steric effects underlies molecular conformation. For example, the common C=O⋯H–N hydrogen bonds within protein main chains may be viewed as favored by the delocalization of an oxygen lone pair (n) into the antibonding orbital (σ*) of the N–H bond, but disfavored by Pauli repulsion1 between n and the N–H bonding orbital (σ).2 Here, we report on a second example of this type of dichotomy within protein main chains.
In common elements of protein secondary structure, the oxygen (Oi–1) of a main-chain amide is proximal to the carbon (Ci′) of the subsequent amide.3 This short contact is promoted by n→π* electronic delocalization, wherein an oxygen lone pair (n) overlaps with the Ci′=Oi antibonding orbital (π*) of the subsequent peptide bond.3-5 We suspected that, as in a hydrogen bond, this electronic effect is antagonized by a steric effect, here arising from Pauli repulsion between n and the Ci′=Oi bonding orbital (π).
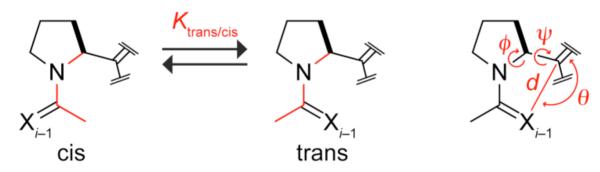
To unveil any n)(π Pauli repulsion, we sought a π system that is isosteric with a carbonyl group but provokes little n→π* interaction. We suspected that alkenyl groups, which lack the polarity of carbonyl groups, could have this attribute. To enable quantitative comparisons, we chose the AcProOMe (1) model system,6 in which n is directed towards π* in the trans conformation but not in the cis conformation (Figure 1). The value of Ktrans/cis reports on the differential stability of the trans and cis conformations and can be measured by using NMR spectroscopy. We suspected that replacing the ester of 1 with an isosteric fluoroalkene7 would attenuate the n→π* interaction. Hence, we synthesized and analyzed 1 and its fluoroalkenyl isostere, 2.
Figure 1.
Definition of equilibrium constant Ktrans/cis, distance d, planar angle θ, and dihedral angles ϕ and ψ. X = O in 1, 2, and 4–6; X = S in 3.
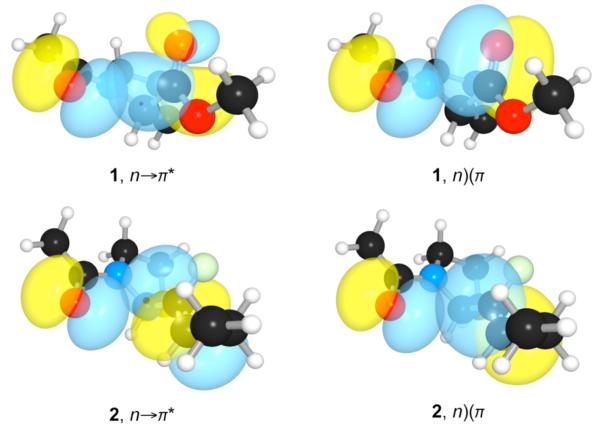
We found evidence that unfavorable Pauli repulsion can indeed antagonize a favorable n→π* interaction. Replacing the carbonyl acceptor with a fluoroalkene switches the conformational preference of the amide bond from trans to cis (Table 1). We resorted to hybrid density functional theory and Natural Bond Orbital (NBO)8 analyses to reveal the basis for this dramatic shift  in conformational preference. We performed geometry optimizations, frequency calculations, and NBO analyses at the B3LYP/6-311+G(2d,p) level of theory on eight conformations of 1 and 2 (see: Supporting Information, Tables S1 and S2). We estimated the stabilization afforded by n→π* electronic delocalization by using second-order perturbation theory, as implemented with NBO 5.0. In accord with our expectation, we found that fluoroalkene isostere 2 does not partake in an appreciable n→π* interaction (Table 1). The π* orbital of the carbonyl group in 1 is oriented properly for extensive n→π* overlap, but that of the fluoroalkenyl group in 2 is not (Figure 2). Additionally, the energy difference between the n and the π* orbitals of 2 (33.2 kcal/mol) is ~10-fold greater than that of 1 (3.5 kcal/mol). While the π* orbital of the carbonyl is located primarily on the single carbonyl carbon, the π* of the fluoroalkene isostere is distributed evenly between the two alkenyl carbons. Moreover, the distance between the donor oxygen (Oi–1) and acceptor carbon (Ci′) is short in all low energy conformations of 1 but long in 2 (Table S1). Finally, Oi–1 in the low energy conformations of 1 is along the Bürgi–Dunitz trajectory9 (θ ~100°), but Oi–1 of 2 is off of that trajectory (θ ~125°) (Table S1).
in conformational preference. We performed geometry optimizations, frequency calculations, and NBO analyses at the B3LYP/6-311+G(2d,p) level of theory on eight conformations of 1 and 2 (see: Supporting Information, Tables S1 and S2). We estimated the stabilization afforded by n→π* electronic delocalization by using second-order perturbation theory, as implemented with NBO 5.0. In accord with our expectation, we found that fluoroalkene isostere 2 does not partake in an appreciable n→π* interaction (Table 1). The π* orbital of the carbonyl group in 1 is oriented properly for extensive n→π* overlap, but that of the fluoroalkenyl group in 2 is not (Figure 2). Additionally, the energy difference between the n and the π* orbitals of 2 (33.2 kcal/mol) is ~10-fold greater than that of 1 (3.5 kcal/mol). While the π* orbital of the carbonyl is located primarily on the single carbonyl carbon, the π* of the fluoroalkene isostere is distributed evenly between the two alkenyl carbons. Moreover, the distance between the donor oxygen (Oi–1) and acceptor carbon (Ci′) is short in all low energy conformations of 1 but long in 2 (Table S1). Finally, Oi–1 in the low energy conformations of 1 is along the Bürgi–Dunitz trajectory9 (θ ~100°), but Oi–1 of 2 is off of that trajectory (θ ~125°) (Table S1).
Table 1.
Conformational properties of compounds 1–6.
| Compound | Ktrans/cisa | Chemical shift of C′i (ppm) |
d (Å)b | θ (°)b | ϕ (°)b | ψ (°)b |
n→π* (kcal/mol)b |
|---|---|---|---|---|---|---|---|
| 1 | 3.7 : 1.0 | ND | 3.08 | 99.5 | −71.12 | 152.67 | 0.40 |
| 2 | 1.0 : 1.7 | 156 | 3.28 | 124.9 | −82.81 | 117.01 | 0.01 |
| 3 | 1.0 : 2.2 | ND | 3.59 | 126.3 | −84.42 | 120.92 | 0.05 |
| 4 | 1.0 : 2.9 | 133 | 3.32 | 126.4 | −84.02 | 116.56 | 0.02 |
| 5 | 1.4 : 1.0 | ND | — | — | −78.89 | 167.16 | — |
| 6 | 1.0 : 4.0 | 105 | 3.25 | 104.1 | −80.43 | 142.03 | 0.03 |
Measured in CDCl3 at 25 °C.
Computed in the optimized conformations (trans amide bond; Cγ-endo pyrrolidine ring pucker).
Figure 2.
Overlap between n and the π* and π orbitals of 1 and 2 in their optimized geometries.
The conformational differences between 1 and 2 are evident in their computational energy landscapes (Figure 3A and 3B). As the value of d decreases, the interpenetration of the van der Waals surfaces of the donor and acceptor groups increases. That endows 1 but not 2 with conformational stability. In 1, the n)(π Pauli repulsion is offset by a strong n→π* interaction; in 2, the n→π* interaction does not overcome that repulsion. Natural Steric Analyses (NSA) supports the existence of the antagonistic Pauli repulsion in low energy conformations (Table S1).
Figure 3.
Conformational energy landscapes of (A) 1, (B) 2, and (C) 4.
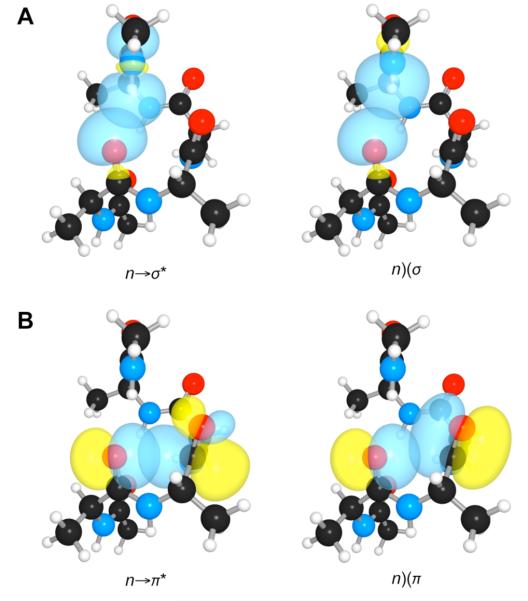
Fluoroalkene 2 lacks a favorable n→π* interaction despite restricted rotation of its Cαi–C′i bond (ψ in Figure 1). The anti rotamer is stabilized by a hyperconjugative interaction between the bonding orbital (σ) of Cα–H and the antiboding orbital (σ*) of C′i–F (Figure 4).10 This rotamer gives rise to a larger value of 3JH,F for the trans (16 Hz) than the cis (8 Hz) conformation.
Figure 4.

Overlap of the σ (Cαi–H) and σ* (C′i–F) orbitals of 2 in its optimized geometry.
If n)(π Pauli repulsion destabilizes the trans conformation of 2, then its amplification should reduce further the population of that conformation. Some of us had shown previously that the sulfur of a thioamide is a better n→π* donor than is the oxygen of an amide.4e But because sulfur is larger than oxygen and C=S bonds are longer than C=O bonds, sulfur should engender greater n)(π Pauli repulsion. To search for that manifestation, we replaced the donor oxygen (Oi–1) in amide 2 with sulfur. We found the value of Ktrans/cis for thioamide 3 to be less than that for amide 2 (Table 1). An origin in increased n)(π Pauli repulsion is supported by NSA (Table S1).
Likewise, we reasoned that attenuating any n)(π Pauli repulsion should stabilize the trans conformation. We suspected that a comparison of alkene 4 with alkane 5, which lacks the acceptor π orbital, would allow us to test our reasoning. Again, we found evidence for n)(π Pauli repulsion, as the value of Ktrans/cis for alkane 5 is greater than that for alkene 4 (Table 1).
Compound 4 offers another opportunity to probe for n)(π Pauli repulsion. The pendant fluoro group that is present in 2 but absent in 4 polarizes the π orbital, reducing the electron density on the acceptor carbon (C′i). The net effect is to diminish n)(π Pauli repulsion as evidenced by a larger value of Ktrans/cis for 2 than 4 (Table 1; Figure 3C). Accordingly, we reasoned that polarizing the π bond in the opposite direction could increase the electron density on the acceptor carbon, thereby increasing any n)(π Pauli repulsion. Indeed, the value of Ktrans/cis for 6 is less than that for both 2 and 4. The correlation between the value of Ktrans/cis for compounds 2, 4, and 6 and the 13C NMR chemical shift of each acceptor carbon (Table 1), which reports on its electron density, provides additional validation for our conclusions.
Some of us have argued4e that intimate carbonyl–carbonyl interactions, which are ubiquitous in many protein secondary structures,3 involve n→π* interactions and cannot be interpreted in terms of classical electrostatic models, such as dipole–dipole11 or charge–charge interactions.12 The results herein support this argument. First, if the interaction between adjacent carbonyl groups were manifested as a classical dipole-dipole interaction, replacing the C=O group with an C(sp2)–F group would not elicit a reversal in the conformational preference from trans to cis. Second, the value of Ktrans/cis for 3 is less than that for 2, despite the dipole moment of C=S being greater that that of C=O.13 Third, the ϕ and 𝜓 dihedral angles of 2 and 4 (which lacks a dipole) are almost identical and are distinct from those of 1 (Table 1; Figure 3C).
The Oi–1⋯C′i=Oi distance is especially small in α-helices.3 These short contacts position distal C=O and H–N groups in the main chain to form the canonical i→i+4 hydrogen bond (Figure 5). Our data indicate that n)(π Pauli repulsion deters such short contacts and would, unless counteracted by an n→π* interaction, impair α-helix formation. Indeed, others have shown that replacing a single amide bond with an alkene or a fluoroalkene isostere severely disrupts α-helical structure.14 Moreover, we put forth n)(π Pauli repulsion as the basis for the anomalous polarization of the C′i=Oi π bond towards Oi that has been observed in α-helices.15 Analogous repulsion has been observed directly by atomic force microscopy at much larger donor–acceptor distances.16
Figure 5.
Orbital overlaps that stabilize (left) and destabilize (right) the α-helical conformation of an AcAla4NHMe model system. (A) i→i+4 hydrogen bond. (B) n→π* interaction.
Finally, we note the effect of n)(π Pauli repulsion on the conformation of other molecules. The collagen triple helix has an n→π* interaction between adjacent residues.17 Each peptide bond in the triplet repeat of collagen strands has been replaced with an alkene isostere, and each substitution greatly diminishes triple-helix stability.18 Likewise, an altered conformational energy landscape could be responsible for the diminished biological activity of some small-molecule ligands containing an alkene or fluoroalkene isostere.19 These isosteres appear to be excellent mimics only for amides and esters that are not engaged in n→π* interactions. Implications for structural perturbations within more global elements of protein secondary structure remain an important avenue for further study.
Supplementary Material
Acknowledgement
We are grateful to W. L. Jorgensen and M. D. Shoulders for contributive discussions. This work was supported by NIH Grants R01 GM068649 (S.J.M.) and R01 AR044276 (R.T.R.).
Footnotes
Supporting Information Available: Procedures for syntheses and analyses reported herein, and computational data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Pauli W. Z. Phys. 1925;31:373–385. [Google Scholar]; (b) Pauli W. Z. Phys. 1925;31:765–783. [Google Scholar]; (c) Massimi M. Pauli's Exclusion Principle: The Origin and Validation of a Scientific Principle. Cambridge University Press; Cambridge, UK: 2005. [Google Scholar]
- 2.Reed AE, Curtiss LA, Weinhold F. Chem. Rev. 1988;88:899–926. Weinhold F, Landis CR. Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective. Cambridge University Press; Cambridge, UK: 2005. For another perspective, see: Khaliullin RZ, Bell AT, Head-Gordon M. Chem. Eur. J. 2009;15:851–855. doi: 10.1002/chem.200802107.
- 3.Hinderaker MP, Raines RT. Protein Sci. 2003;12:1188–1194. doi: 10.1110/ps.0241903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J. Am. Chem. Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]; (b) DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. J. Am. Chem. Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]; (c) Horng J-C, Raines RT. Protein Sci. 2006;15:74–83. doi: 10.1110/ps.051779806. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hodges JA, Raines RT. Org. Lett. 2006;8:4695–4697. doi: 10.1021/ol061569t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Choudhary A, Gandla D, Krow GR, Raines RT. J. Am. Chem. Soc. 2009;131:7244–7246. doi: 10.1021/ja901188y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Sonntag L-S, Schweizer S, Ochsenfeld C, Wennemers H. J. Am. Chem. Soc. 2006;128:14697–14703. doi: 10.1021/ja0654938. [DOI] [PubMed] [Google Scholar]; (b) Gao J, Kelly JW. Protein Sci. 2008;17:1096–1101. doi: 10.1110/ps.083439708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.As a comparitor, we chose a methyl ester rather than an amide to avoid the complications of γ-turn formation, as has been observed in AcProNHMe. See: Liang G-B, Rito CJ, Gellman SH. Biopolymers. 1992;32:293–301. doi: 10.1002/bip.360320309.
- 7.(a) Abraham RJ, Ellison SLR, Schonholzer P, Thomas WA. Tetrahedron. 1986;42:2101–2110. [Google Scholar]; (b) Boros LG, Corte BD, Gimi RH, Welch JT, Wu Y, Handschumacher RE. Tetrahedron Lett. 1994;35:6033–6036. [Google Scholar]; (c) Bartlett PA, Otake A. J. Org. Chem. 1995;60:3107–3111. [Google Scholar]; (d) Wipf P, Henninger TC, Geib SJ. J. Org. Chem. 1998;63:6088–6089. doi: 10.1021/jo981057v. [DOI] [PubMed] [Google Scholar]; (e) Jakobsche CE, Peris G, Miller SJ. Angew. Chem., Int. Ed. 2008;47:6707–6711. doi: 10.1002/anie.200802223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Weinhold F. In: Encyclopedia of Computational Chemistry. Schleyer P. v. R., Allinger NL, Clark T, Gasteiger J, Kollman PA, Shaefer HF, III, Schreiner PR., editors. Vol. 3. John Wiley & Sons; Chichester, UK: 1998. pp. 1792–1811. [Google Scholar]; (b) Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. NBO 5.0. 2001 [Google Scholar]
- 9.(a) Bürgi HB, Dunitz JD, Shefter E. J. Am. Chem. Soc. 1973;95:5065–5067. [Google Scholar]; (b) Bürgi HB, Dunitz JD, Lehn JM, Wipff G. Tetrahedron. 1974;30:1563–1572. [Google Scholar]; (c) Bürgi HB, Lehn JM, Wipff G. J. Am. Chem. Soc. 1974;96:1965–1966. doi: 10.1021/ja00819a072. [DOI] [PubMed] [Google Scholar]; (d) Bürgi HB, Dunitz JD. Acc. Chem. Res. 1983;16:153–161. [Google Scholar]; (e) Eliel EL, Wilen SH. Stereochemistry of Organic Compounds. Wiley Interscience Publication; New York: 1996. [Google Scholar]; (f) Kirby AJ. Stereoelectronic Effects. Oxford University Press; New York: 1996. [Google Scholar]; (g) Clayden J, Greeves N, Warren S, Wothers P. Organic Chemistry. Oxford University Press; New York: 2000. [Google Scholar]; (h) Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, California, USA: 2006. [Google Scholar]
- 10.Pophristic V, Goodman L. Nature. 2001;411:565–568. doi: 10.1038/35079036. [DOI] [PubMed] [Google Scholar]
- 11.(a) Paulini R, Müller K, Diederich F. Angew. Chem., Int. Ed. 2005;44:1788–1805. doi: 10.1002/anie.200462213. [DOI] [PubMed] [Google Scholar]; (b) Fischer FR, Wood PA, Allen FH, Diederich F. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17290–17294. doi: 10.1073/pnas.0806129105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Maccallum PH, Poet R, Milner-White EJ. J. Mol. Biol. 1995;248:361–373. doi: 10.1016/s0022-2836(95)80056-5. [DOI] [PubMed] [Google Scholar]; (b) Maccallum PH, Poet R, Milner-White EJ. J. Mol. Biol. 1995;248:374–384. doi: 10.1016/s0022-2836(95)80057-3. [DOI] [PubMed] [Google Scholar]; (c) Allen FH, Baalham CA, Lommerse JPA, Raithby PR. Acta Cryst. B. 1998;B54:320–329. [Google Scholar]; (d) Deane CA, Allen FH, Taylor R, Blundell TL. Protein Eng. 1999;12:1025–1028. doi: 10.1093/protein/12.12.1025. [DOI] [PubMed] [Google Scholar]
- 13.Wiberg KB, Rush DJ. J. Am. Chem. Soc. 2001;123:2038–2046. doi: 10.1021/ja003586y. [DOI] [PubMed] [Google Scholar]
- 14.Oishi S, Kamitani H, Kodera Y, Watanabe K, Kobayashi K, Narumi T, Tomita K, Ohno H, Naito T, Kodama E, Matsuoka M, Fujii N. Org. Biomol. Chem. 2009;7:2872–2877. doi: 10.1039/b907983a. [DOI] [PubMed] [Google Scholar]
- 15.Lario PI, Vrielink A. J. Am. Chem. Soc. 2003;125:12787–12794. doi: 10.1021/ja0289954. [DOI] [PubMed] [Google Scholar]
- 16.Gross L, Mohn F, Moll N, Liljeroth P, Meyer G. Science. 2009;325:1110–1114. doi: 10.1126/science.1176210. [DOI] [PubMed] [Google Scholar]
- 17.Shoulders MD, Raines RT. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Org. Lett. 2005;7:2619–2622. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]; (b) Dai N, Wang XJ, Etzkorn FA. J. Am. Chem. Soc. 2008;130:5396–5397. doi: 10.1021/ja711021m. [DOI] [PubMed] [Google Scholar]; (c) Dai N, Etzkorn FA. J. Am. Chem. Soc. 2009;131:13728–13732. doi: 10.1021/ja904177k. [DOI] [PubMed] [Google Scholar]
- 19.(a) Kaltenbronn JS, Hudspeth JP, Lunney EA, Michniewicz BM, Nicolaides ED, Repine JT, Roark WH, Stier MA, Tinney FJ, Woo PKW, Essenburg AD. J. Med. Chem. 1990;33:838–845. doi: 10.1021/jm00164a058. [DOI] [PubMed] [Google Scholar]; (b) Fincham CI, Higginbottom M, Hill DR, Horwell DC, O'Toole JC, Ratcliffe JC, Rees DC, Roberts E. J. Med. Chem. 1992;35:1472–1484. doi: 10.1021/jm00086a017. [DOI] [PubMed] [Google Scholar]; (c) Wai JS, Bamberger DL, Fisher TE, Graham SL, Smith RL, Gibbs JB, Mosser SD, Oliff AI, Pompliano DL, Rands E, Kohl NE. Bioorg. Med. Chem. 1994;2:939–947. doi: 10.1016/s0968-0896(00)82043-x. [DOI] [PubMed] [Google Scholar]; (d) Venkatesan N, Kim BH. Curr. Med. Chem. 2002;9:2243–2270. doi: 10.2174/0929867023368692. [DOI] [PubMed] [Google Scholar]; (e) Welch JT. Fluorine and Health. Elsevier B. V.; Amsterdam: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.