Abstract
Sirt1, a deacetylase involved in regulating energy metabolism in response to calorie restriction, is up regulated after chronic ethanol feeding using the intragastric feeding model of alcohol liver disease. PGC1α is also up regulated in response to ethanol. These changes are consistent with activation of the Sirt1/PGC1α pathway of metabolism and aging, involved in alcohol liver disease including steatosis, necrosis and fibrosis of the liver. To test this hypothesis, male rats fed ethanol intragastrically for 1 month were compared with rats fed ethanol plus resveratrol or naringin. Liver histology showed macrovesicular steatosis caused by ethanol and this change was unchanged by resveratrol or naringin treatment. Necrosis occurred with ethanol alone but was accentuated by resveratrol treatment, as was fibrosis. The expression of Sirt1 and PGC1α was increased by ethanol but not when naringin or resveratrol was fed with ethanol. Sirt3 was also up regulated by ethanol but not when resveratrol was fed with ethanol. These results support the concept that ethanol induces the Sirt1/PGC1α pathway of gene regulation and both naringin and resveratrol prevent the activation of this pathway by ethanol. However, resveratrol did not reduce the liver pathology caused by chronic ethanol feeding.
INTRODUCTION
In the intragastric fed rat model of alcoholic liver disease (ALD), it was shown that the gene expression of Sirt1 and 3 deacetylases (belonging to the HDAC Class III) and PGC1α (PPARγ coactivator 1α) were up regulated and PPARγ was down regulated when the blood alcohol levels were high during the urinary alcohol cycle. This raised the question as to whether these changes in this functional pathway play a role in the pathogenesis of the increased liver pathology observed at the peak alcohol levels (Bardag-Gorce et al., 2002). To attempt to answer this question, rats were fed either resveratrol or naringin, which prevent the Sirt1 induction by ethanol. Since resveratrol is present in red wine the results may be relevant to the effect of this beverage on the liver (Das et al., 1999; Ray et al., 1999). The flavonoid naringin, a potent cholesterol-lowering agent, was discovered in citrus fruit (Freedman and Merritt, 1963). Sirt1 belongs to the HDAC class III. 7 Sirt proteins were identified in humans, and contain a conserved catalytic core domain. Sirt1, a NAD+-dependant protein deacetylase, extends lifespan in diverse species and is involved in energy metabolism (Frye, 1999; Frye, 2000; Inoue et al., 2007). Sirt1 activity affects many functional gene pathways in either a positive or negative direction (Guarente and Picard, 2005). In the differentiation pathway, MEF2, MyoD, p300 are up regulated (Bouras et al., 2005; Fulco et al., 2003; Zhao et al., 2005). In the metabolic pathway, AOS, PGC1α, are up regulated and PPARγ is down regulated. In the cell survival pathway, FOXO3, KU-70 and GADD45 are up regulated and p53 and NFκB are down regulated (Engel and Mahlknecht, 2008; Hasegawa et al., 2008; Kobayashi et al., 2005; Li et al., 2007b; Luo et al., 2001; Vaziri et al., 2001; Yeung et al., 2004). In the epigenetic gene silencing pathway, Sirt1 deacetylates histones, transcriptional factors and tubulin (Inoue et al., 2007).
In the PGC1α pathway studied here, Sirt1 deacetylates PGC1α (Nemoto et al., 2005). This leads to its phosphorylation by p38, AMPK and AKT, which activates the pathways where gluconeogenesis is activated (Jager et al., 2007; Li et al., 2007a; Puigserver et al., 2001; Rodgers et al., 2008). Sirt1 affects hepatic lipid and mitochondrial metabolism through PPARγ, ERRα and HNF4α (Rodgers et al., 2008). The focus of interest here, however, is on PGC1α regulation of PPARγ. PGC1α was first identified as a cofactor for the nuclear hormone receptor PPARγ, which is required for the adaptive thermogenic response to lower temperatures (Puigserver et al., 1998).
Resveratrol’s pleiotropic properties include a marked antioxidant effect on the liver in vivo (Plin et al., 2005) and in vitro (Notas et al., 2006). Resveratrol given to rats in vivo may have protective effects on the liver. Resveratrol has a protective effect in ischemic-reperfusion experiments (Hassan-Khabbar et al., 2008). A low dose (0.02 mg) (kg) given IV decreased aminotransferase in the blood and prevented glutathione depletion caused by ischemic-reperfusion. An intermediate dose (0.2 mg/kg) increased glutathione reductase, and Cu/Zn-superoxide dismutase and catalase activities. However, at a high dose (20 mg/kg), resveratrol was pro-oxidant with increased liver injury, depletion of glutathione and antioxidant enzyme activity. Because of the adverse effects of a high dose of resveratrol, a very low intraperitoneal dose (100 µg/day) was used in the present study. Rats given 50 mg/kg rapidly conjugate resveratrol and 73% is eliminated in the urine with a terminal half life of 1–5 h (Marier et al., 2002). Red wines contain from 1.98 to 7.13 mg/L of resveratrol or 0.3 to 1.07 mg/5 ounce glass (Roy and S, 2005). Muscadine wines are richer in resveratrol (14.1–40 mg/L). Resveratrol is also present in peanuts, grapes, cranberries, and to a lesser degree in blueberries (Yu et al., 2002). Resveratrol has life extension effects and mimics several biochemical effects of caloric restriction (Baxter, 2008). Resveratrol activates Sirt1 and PGC1γ and improves mitochondrial function (Lagouge et al., 2006). Hence, this is the rationale for the present study, in which ethanol feeding induces Sirt1 expression and liver pathology.
METHODS
Animal model of alcoholic liver disease
Male Wistar rats weighing between 220 to 280 g from Harleco (Hollister, CA) were used. The rats were fed a liquid diet containing ethanol (~ 14 g/kg body weight/day) intragastrically at a constant rate for 1 month (Li et al., 2000). Five rats were fed ethanol alone, 4 were fed ethanol and resveratrol (100 µg/day by intraperitoneal injection) (Sigma, St. Louis, MO) and 4 were fed ethanol and naringin orally (100 mg/day) (Sigma, St. Louis, MO). Four were fed the diet with isocaloric glucose instead of ethanol. The ethanol dose was adjusted in order to maintain high blood ethanol levels based on the 24 hour measured in the urine. The urine was collected under toluene using metabolic cages, one rat/cage and measured using the Saliva alcohol test kit (QED) A150 (STC Technologies Bethlehem, PA). Blood alcohol levels were measured by enzymatic reaction. The rats were maintained according to the Guidelines of Animal Care, as described by the National Academy of Sciences and published by the National Institutes of Health (1996).
Liver Histology
Livers were weighed. A portion was fixed in zinc-formalin and the remaining liver was quick frozen and stored at −80°C until use. Formalin-fixed tissue was embedded in paraffin, cut and stained with hemotoxylin and eosin, reticulin and Sirus red. The pathology score was done blind and scored as follows: macrovesicular and microvesicular fat 0–4+, inflammation and necrosis 0–2+, and fibrosis number of scarred areas.
Quantitative Real-time RT-PCR Assay
Total liver RNAs were extracted with Trizol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA) as described previously (Li et al., 2008). Sequence of PCR primers for:
| Sirt1 | NM_001107627 | Forward | TGACTTCAGATCAAGAGATGGTATTTATG |
| Reverse | TGGCTTGAGGATCTGGGAGAT | ||
| Sirt3 | NM_001106313 | Forward | GGCTGCTTCACGACAAGGA |
| Reverse | CTCTCAAGCCCGTCGATGTT | ||
| PGC1α | NM_031347 | Forward | GCGCCAGCCAACACTCA |
| Reverse | TGGGTGTGGTTTGCATGGT | ||
| PPARγ | NM_013124 | Forward | GACCTGAAGCTCCAAGAATACCA |
| Reverse | TAGAGTTGGGTTTTTTCAGAATAATAAGG |
Statistical analysis
P values were determined by ANOVA and Student-Newman-Keuls for multiple group comparisons (Sigma-Stat software, San Francisco, CA).
RESULTS
Liver weights varied from 10.5 to 16.8 g. The average for ethanol was 12.7 g; ethanol+naringin 13.2 g; ethanol+resveratrol 11.5 g (no significant difference). Terminal urinary alcohol levels (UAL) varied from 280–450. The average for ethanol was 344 mg%; ethanol+naringin, 375 mg%; ethanol+resveratrol, 325 mg% (no significance difference) (Data not shown).
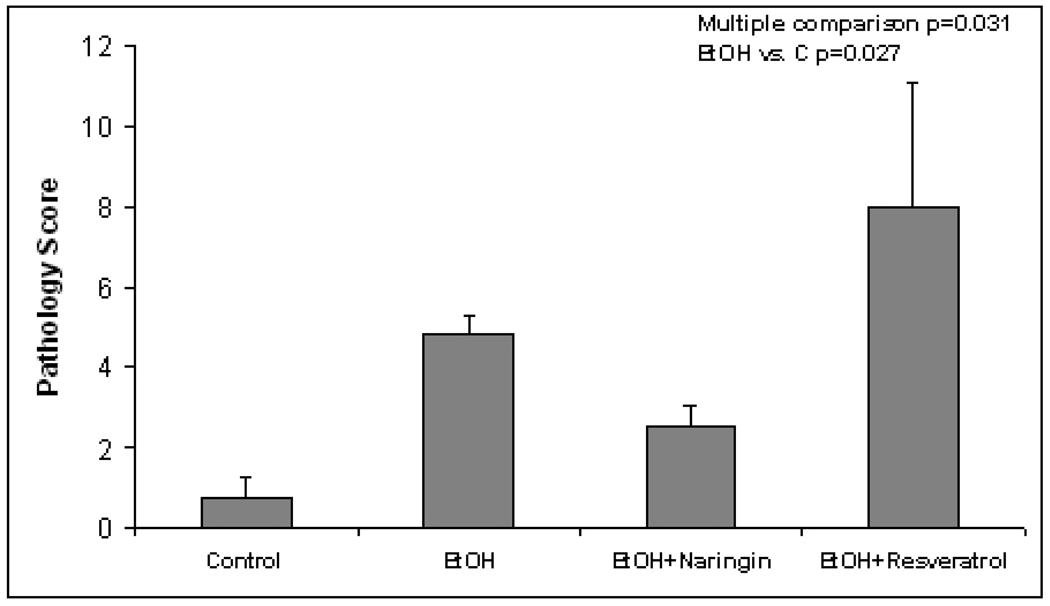
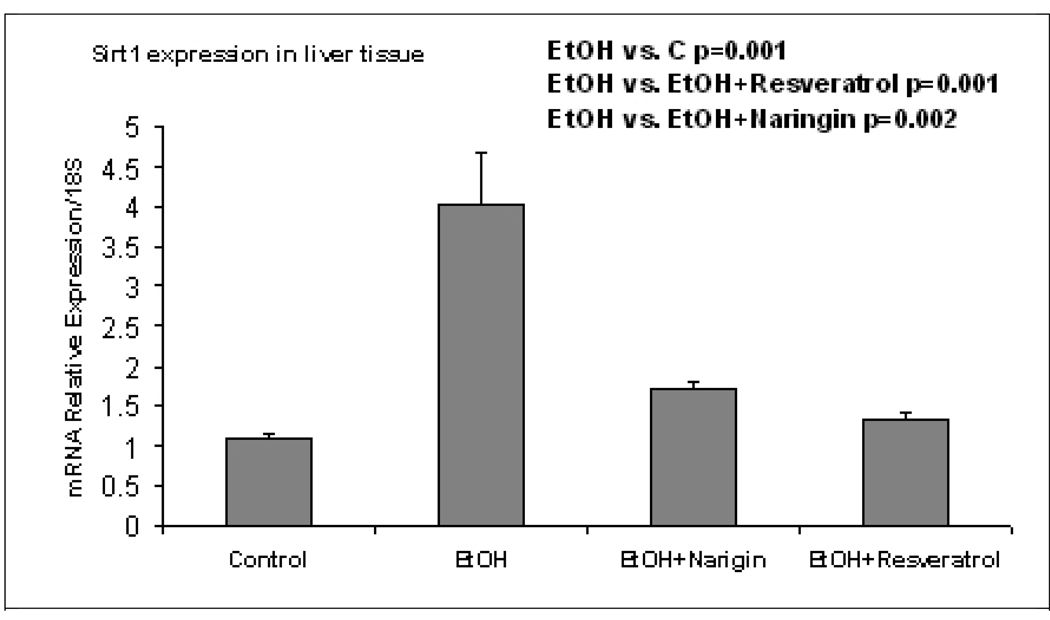
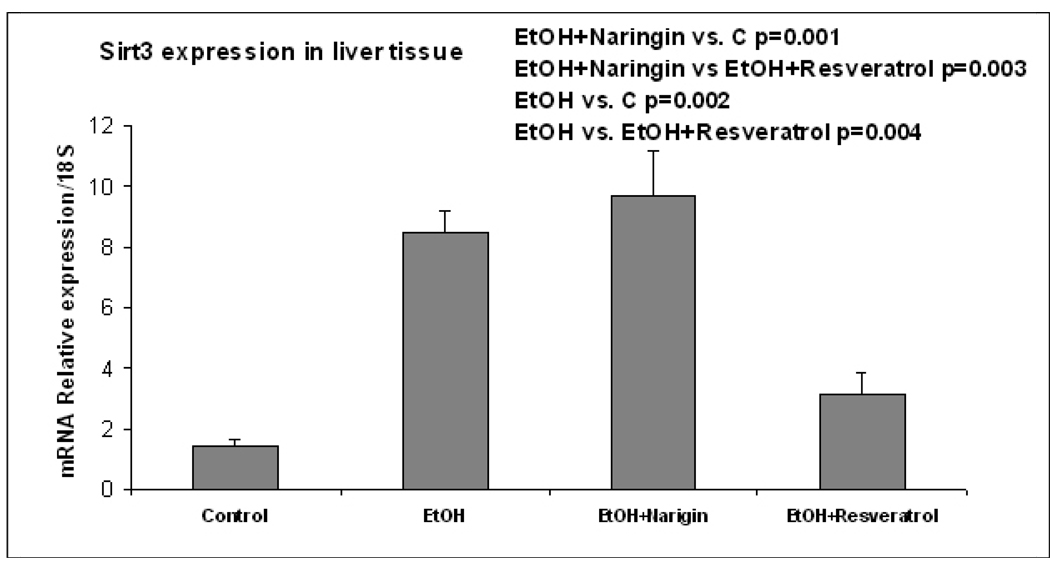
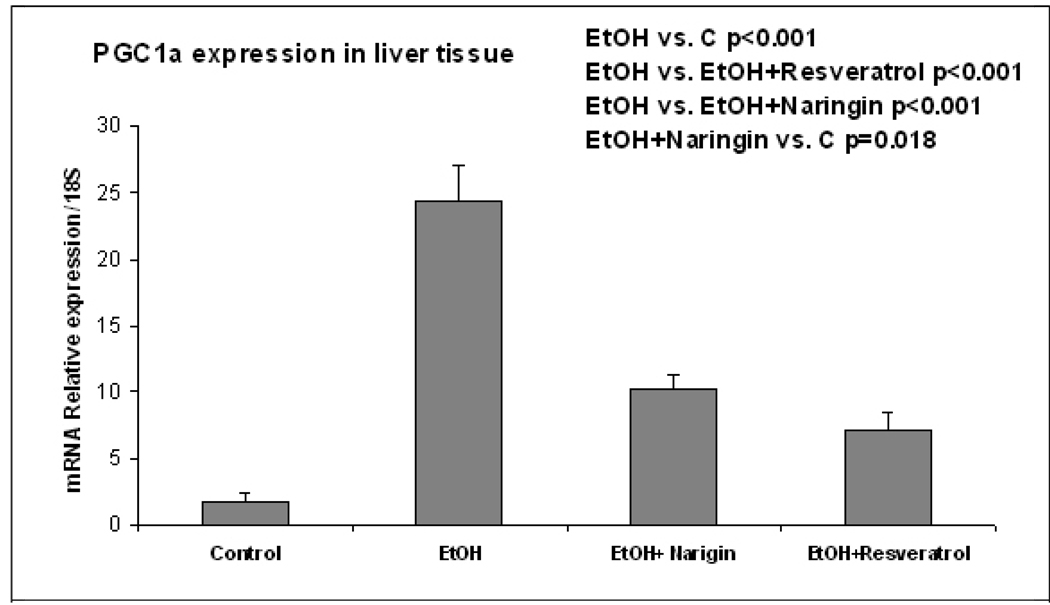
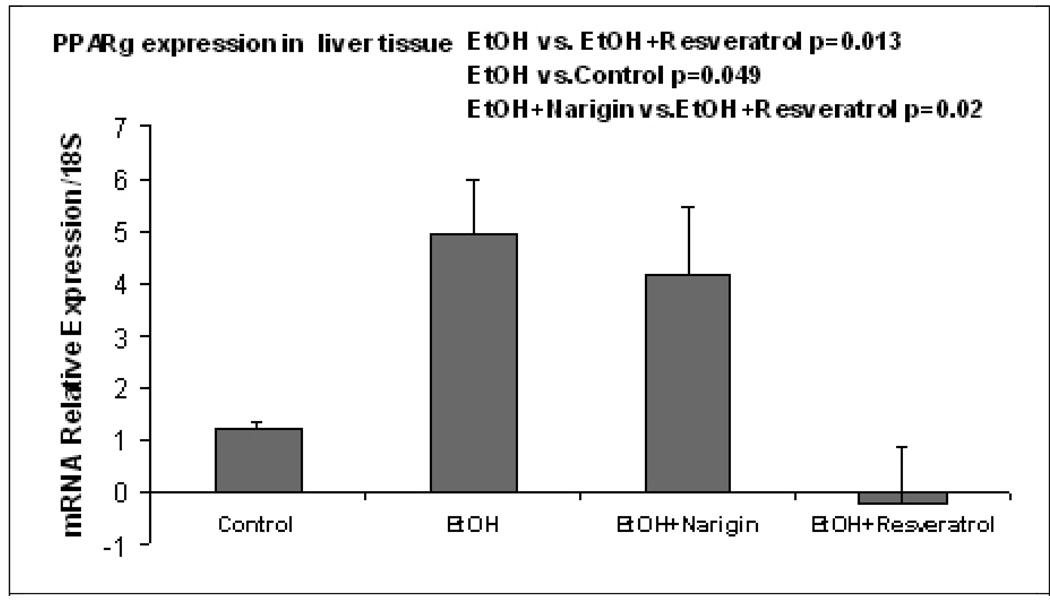
The pathology score (Table 1) showed an increase in macrovesicular fat in all three ethanol fed groups without a significant difference between the ethanol treatment groups (Fig 1). Ethanol+naringin reduced the microvesicular fat to control levels. Necrosis was increased by ethanol and by the ethanol+resveratrol group (Fig 2). Naringin partially prevented the increase of necrosis induced by ethanol (Fig 2). The number of scars was increased in the ethanol fed group and this was further increased by ethanol+resveratrol (Fig 3). As a consequence the total pathology score was increased in the ethanol+resveratrol group compared with the ethanol fed group (Fig 4). Ethanol+naringin reduced the total pathology score compared with the ethanol fed group. Using multiple group comparisons all the groups differed significantly (p=0.031). The expression of Sirt1 was increased by ethanol, but was reduced to control levels when naringin or resveratrol was given with ethanol (Fig 5). Ethanol alone caused a significant increase in expression of Sirt3 and this was not inhibited by naringin. However, resveratrol fed with ethanol did prevent the induction of Sirt3 (Fig 6). PGC1α expression was similarly induced by ethanol alone but naringin and resveratrol partially inhibited this response to ethanol (Fig 7). The expression of PPARγ was increased by ethanol. Resveratrol, not naringin, prevented this increase caused by ethanol feeding (Fig 8). In fact, resveratrol reduced the expression to below control levels.
TABLE 1.
Pathology Score of livers from 4 groups of rats fed by intragastric tube 24h/day.
| N= | Macro Fat |
Micro Fat |
Inflammation | Necrosis | Fibrosis | Blood Alcohol mg% |
|
|---|---|---|---|---|---|---|---|
| Cont | 4 | 0.5 | 0.25 | 0 | 0 | 0 | 0 |
| ROH | 5 | 2 | 1 | 0.6 | 0.4 | 1 | 505.82 ±46.3 |
| ROH+N | 4 | 1.5 | 0.25 | 0.25 | 0 | 0.5 | 372.25±41.6 |
| ROH+R | 4 | 1.5 | 1.25 | 0.25 | 1 | 4 | 437.4±85.9 |
ROH = ethanol, N=naringin, R=resveratrol, Macro= macrovesicular, Micro=microvesicular
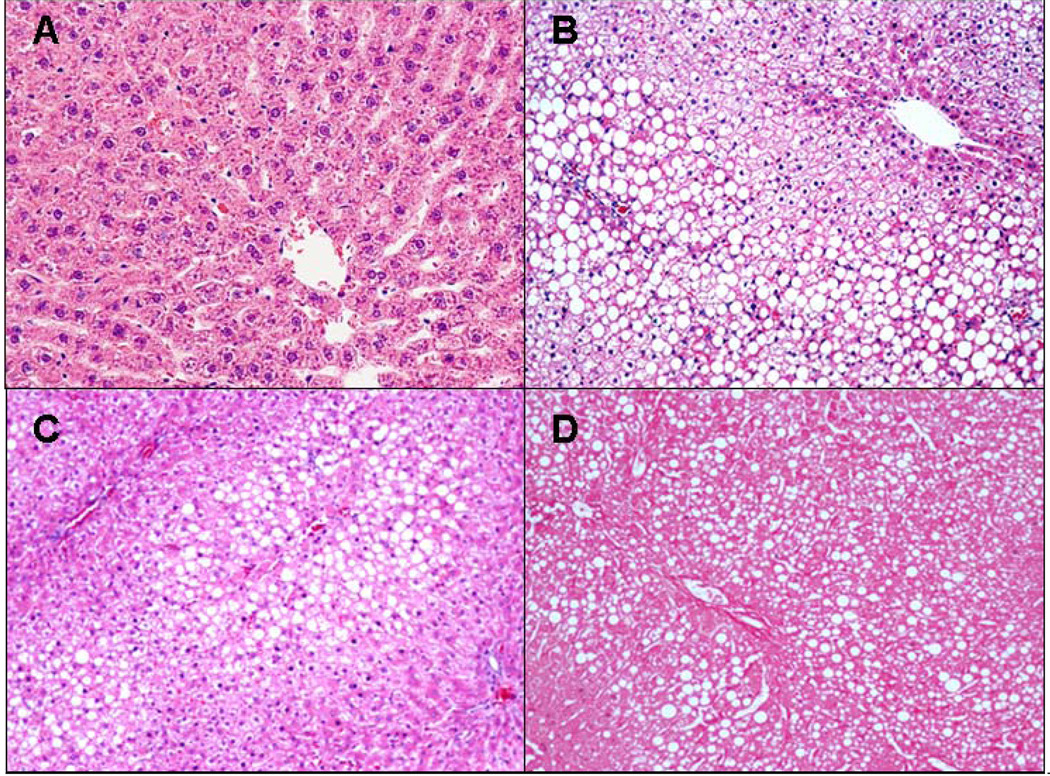
Fig. 1.
A) Control B) Ethanol C) Ethanol+Naringin D) Ethanol+Resveratrol. Macrovesicular fat was found in all rats fed ethanol (B,C,D) where fat was absent in controls (A). H&E ×10.
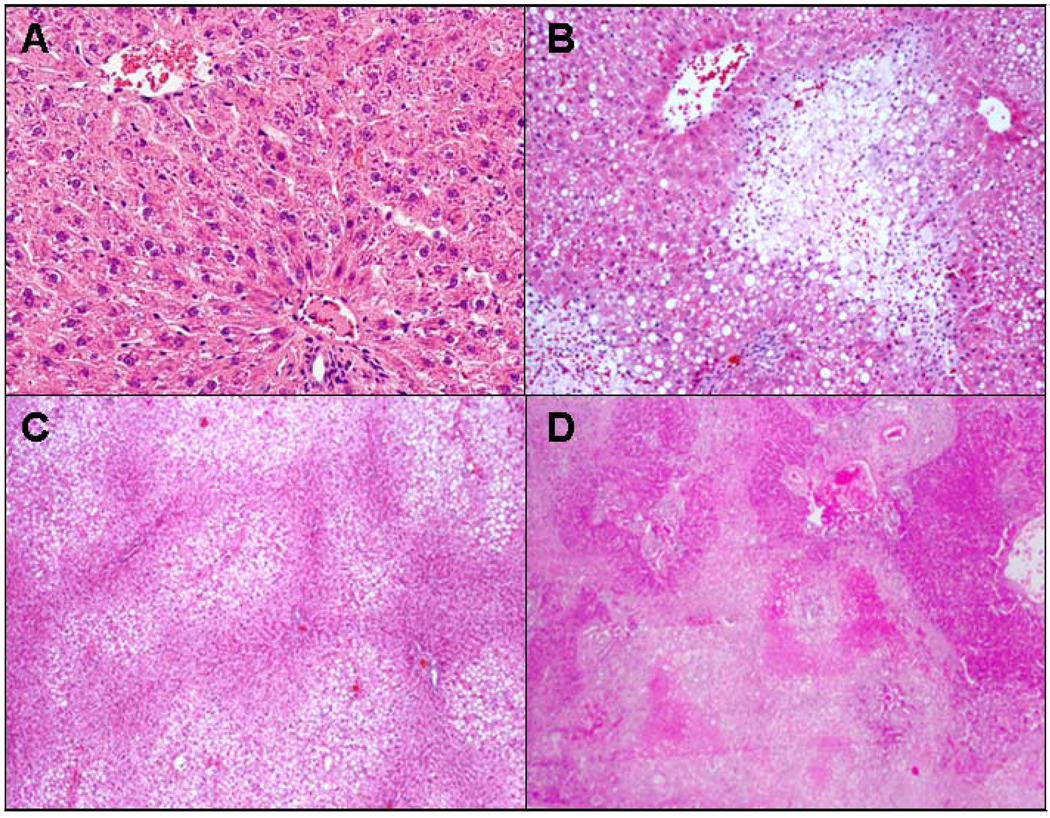
Fig. 2.
A) Control B) Ethanol C) Ethanol+Naringin D) Ethanol+Resveratrol. Necrosis was present focally in the liver of the rats fed ethanol alone (B) and in the group fed ethanol and resveratrol (D), compare to the (A). The necrosis was much more extensive when resveratrol was fed with ethanol. H&E ×10 except ethanol plus naringin ×4.
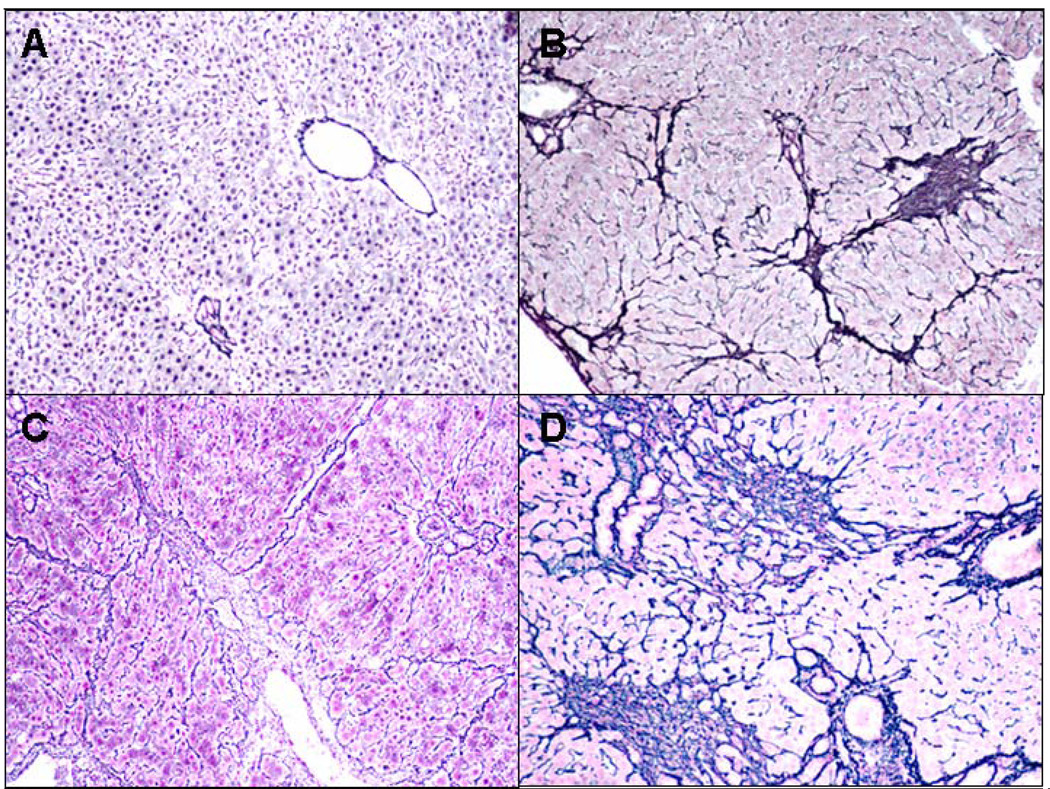
Fig. 3.
A) Control B) Ethanol C) Ethanol+Naringin D) Ethanol+Resveratrol. Fibrosis was present focally in all three groups fed ethanol (B,C,D) but it was much more extensive in the ethanol plus resveratrol group (D) and less prominent in the ethanol plus naringin fed group (C), compare to the control (A). Reticulin ×10.
Fig. 4.
Total pathology score comparing the controls with ethanol fed alone, ethanol fed with naringin and ethanol fed with resveratrol (Mean±SEM, n=4–5) (Multiple comparison p=0.031, EtOH vs. C p=0.027)
Fig. 5.
Ethanol fed alone up regulated the expression of Sirt1 (p= 0.001) and both naringin and resveratrol fed with ethanol prevented the up regulation by ethanol alone (p=0.001 and 0.002 respectively). (Mean±SEM, n=3).
Fig. 6.
Ethanol fed ethanol up regulated the expression of Sirt 3 (p=0.002). Naringin fed with ethanol did not inhibit this response to ethanol but resveratrol fed with ethanol did (p=0.004). (Mean±SEM, n=3).
Fig. 7.
Ethanol fed alone increased the expression of PGC1α (p<0.001). Both naringin and resveratrol significantly inhibited this effect by ethanol (p<0.001) (Mean±SEM, n=3).
Fig. 8.
Ethanol fed alone increased the expression of PPARγ compared with controls (p=0.049). Rats fed naringin with ethanol differed significantly with the group fed ethanol and resveratrol (p=0.02) as did the group fed ethanol alone (p=0.013) (Mean±SEM, n=4).
DISCUSSION
Naringin gave some protection from the ethanol-induced liver pathology and the up regulation of Sirt1, and PGC-1α but not the up regulation of Sirt3 and PPARγ. In contrast, resveratrol increased the necrosis and fibrosis of the liver when fed with ethanol. Resveratrol fed with ethanol prevented the up regulation of Sirt1 and 3 and PGC1α and further reduced PPARγ to below control levels. The negative effect of resveratrol on ethanol-induced pathology and PPARγ was not anticipated but may be important to wine drinkers, since red wine is rich in both ethanol and resveratrol.
The effect of down regulating PPARγ by resveratrol fed with ethanol may be important since drugs that enhance PPARγ play a significant therapeutic role in fatty liver disease seen with nonalcoholic liver disease (NASH). They play a role in improving insulin sensitivity (Issemann and Green, 1990). Drugs which enhance the PPARγ effects on peripheral fat stores and fatty acid mobilization, reduce fatty liver due to wine consumption (Yu et al., 2006). The negative effect of resveratrol in this study is surprising because an experimental NASH model fed resveratrol responded by decreasing fatty liver and increasing the phosphorylation of AMPK. Abdominal obesity, and insulin resistance were markedly improved in this study (Shang et al., 2008).
There have been contradictory results regarding the activation of sterol receptor binding protein (SREBP1c) by chronic ethanol feeding. Mice fed ethanol (You et al., 2008), where liver pathology was minimal, showed an increase in SREBP1c activation after chronic ethanol feeding. However, when rats are fed ethanol continuously intragastrically, severe liver steatoses, necrosis and fibrosis develop and SREBP expression is increased (French et al., 2005). In mouse and rat models where ethanol is fed ad lib, Sirt1 and PGC1α are down regulated (Lieber et al., 2008; You et al., 2008). This was interpreted as indicating that SREBP activation by acetylation was increased because Sirt1 was decreased due to ethanol. The steatosis observed resulted from the SREBP1c activation of enzymes involved in increasing fatty acid synthesis. The decrease in Sirt1 induced by ethanol feeding was blocked by resveratrol but the effect of resveratrol on liver steatosis caused by ethanol has not been reported (You et al., 2008).
Acknowledgments
The authors thank Adriana Flores for typing the manuscript. The study was supported by NIH/NIAAA grants 8116 and the Alcohol Center Grant on Liver and Pancreas P50-011999, including the morphology core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardag-Gorce F, et al. The importance of cycling of blood alcohol levels in the pathogenesis of experimental alcoholic liver disease in rats. Gastroenterology. 2002;123:325–335. doi: 10.1053/gast.2002.34177. [DOI] [PubMed] [Google Scholar]
- Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008;7:2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- Bouras T, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- Das DK, et al. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25:115–120. [PubMed] [Google Scholar]
- Engel N, Mahlknecht U. Aging and anti-aging: unexpected side effects of everyday medication through sirtuin1 modulation. Int J Mol Med. 2008;21:223–232. [PubMed] [Google Scholar]
- Freedman L, Merritt AJ. Citrus flavonoid complex: chemical fractionation and biological activity. Science. 1963;139:344–345. doi: 10.1126/science.139.3552.344. [DOI] [PubMed] [Google Scholar]
- French BA, et al. Microarray analysis of gene expression in the liver during the urinary ethanol cycle in rats fed ethanol intragastrically at a constant rate. Exp Mol Pathol. 2005;79:87–94. doi: 10.1016/j.yexmp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Fulco M, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372:51–56. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- Hassan-Khabbar S, et al. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14:451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]
- Inoue T, et al. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle. 2007;6:1011–1018. doi: 10.4161/cc.6.9.4219. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jager S, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li J, et al. S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting the epigenetic memory. Hepatology. 2008;47:613–624. doi: 10.1002/hep.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Mechanism of the alcohol cyclic pattern: role of the hypothalamic-pituitary-thyroid axis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G118–G125. doi: 10.1152/ajpgi.2000.279.1.G118. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007a;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ. 2007b;14:2058–2067. doi: 10.1038/sj.cdd.4402219. [DOI] [PubMed] [Google Scholar]
- Lieber CS, et al. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun. 2008;370:44–48. doi: 10.1016/j.bbrc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Luo J, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Marier JF, et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- Nemoto S, et al. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Notas G, et al. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta. 2006;1760:1657–1666. doi: 10.1016/j.bbagen.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Plin C, et al. Resveratrol protects against cold ischemia-warm reoxygenation-induced damages to mitochondria and cells in rat liver. Eur J Pharmacol. 2005;528:162–168. doi: 10.1016/j.ejphar.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Puigserver P, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Ray PS, et al. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, et al. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SK, S L. Resveratrol. Pennington Nutrition Series 7. 2005 [Google Scholar]
- Shang J, et al. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Yeung F, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- Yu C, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- Yu J, et al. Lipoprotein Lipase Activator NO-1886 Ameliorates the Severity of Dietary Steatohepatitis in Mice. Gastroenterology. 2006;131:A-825. [Google Scholar]
- Zhao X, et al. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]