Abstract
Acting through degradation of target mRNA or inhibition of translation, microRNAs (miRNAs) regulate development, differentiation, and cellular response to diverse cues. We analyzed changes in miRNA expression in human placental trophoblasts exposed to hypoxia, which may result from hypoperfusion and placental injury. Using an miRNA microarray screen, confirmed by Northern blot analysis, we defined a set of seven miRNAs (miR-93, miR-205, miR-224, miR-335, miR-424, miR-451, and miR-491) that are differentially regulated in primary trophoblasts exposed to hypoxia. We combined in silico prediction of miRNA targets with gene expression profiling data to identify a series of potential targets for the miRNAs, which were further analyzed using luciferase reporter assays. Among experimentally confirmed targets, we found that the transcriptional coactivator MED1, which plays an important role in placental development, is a target for miR-205. Using gain- and loss-of-function assays, we confirmed that miR-205 interacts with a specific target in the 3′-UTR sequence of MED1 and silences MED1 expression in human trophoblasts exposed to hypoxia, suggesting that miR-205 plays a role in trophoblast injury.—Mouillet, J.-F., Chu, T., Nelson, D. M., Mishima, T., Sadovsky, Y. MiR-205 silences MED1 in hypoxic primary human trophoblasts.
Keywords: placenta, hypoxia, microRNA, 3′-UTR
The placental trophoblasts govern placental gas exchange, nutrition, waste removal, endocrine function, and immunological support for the developing fetus. Early placental development occurs in an environment of relative hypoxia, which promotes trophoblast invasion and angiogenesis (1,2,3,4). Once placental perfusion has been fully established after the first trimester of human pregnancy, hypoxia causes trophoblast injury (5,6,7,8,9). Hypoxia regulates trophoblast gene expression and, consequently, modulates cell survival, differentiation, metabolic function, and apoptosis, which probably contribute to the pathophysiology of placental dysfunction and fetal growth restriction (10, 11).
The noncoding, 20- to 24-nucleotide microRNAs (miRNAs) are abundant in all metazoan eukaryotes, where they act post-transcriptionally to silence gene expression (12,13,14,15). These small RNAs are processed from longer transcripts carrying a characteristic hairpin secondary structure. Currently, the number of validated mature human miRNAs approaches 1000. Individual miRNAs can repress multiple genes (16,17,18), which implies a broad regulatory potential. Not surprisingly, miRNAs have emerged as important regulators of virtually every biological process associated with tissue development, differentiation, cellular proliferation, cell type-specific function, and homeostasis. Consequently, dysregulation of miRNAs has been implicated in dysfunction of genetic regulatory networks. Indeed, several pathological conditions have been linked to altered expression of miRNA (19,20,21,22,23).
Hypoxia was recently shown to have an effect on miRNA expression in diverse cellular contexts (reviewed in refs. 24, 25). Because diverse placental insults culminate in trophoblast hypoxia, we sought to analyze the effect of hypoxia on human trophoblastic miRNAs and informatically assess their molecular targets. In a previous report we showed that the miRNA biosynthetic pathway was functional in human trophoblasts exposed to hypoxic stress and that the expression of key miRNA machinery proteins was not altered by hypoxia (26). In the present study we define a set of miRNAs that exhibits altered expression in hypoxic human trophoblasts. Combining high-throughput profiles of miRNAs and mRNAs from hypoxic trophoblasts with in silico prediction of miRNA targets we identified candidate miRNA target genes in trophoblasts. Among the experimentally confirmed miRNA targets we found that miR-205 regulates the expression of the mediator complex coregulator MED1 (formerly termed PBP, TRIP220, and DRIP205), an important regulator of murine placental development (27, 28). We further showed that only one of the several miR-205 target elements within the 3′-UTR of MED1 is functional in normoxic or hypoxic trophoblasts.
MATERIALS AND METHODS
Cell culture
Primary human trophoblasts (PHTs) were prepared from normal term placentas using the trypsin-deoxyribonuclease-dispase/Percoll method as described by Kliman et al. (29), with previously published modifications (30). All placentas were obtained after term delivery using a protocol approved by the Institutional Review Board at the University of Pittsburgh. Cultures were plated at a density of 350,000 cells/cm2 and maintained in DMEM (Sigma-Aldrich Corp., St. Louis, MO, USA) containing 10% FBS (HyClone, Logan, UT, USA) and antibiotics at 37°C in a 5% carbon dioxide-air atmosphere. After 4 h, designed to allow cell attachment, the culture plates were allocated to standard or hypoxic growth conditions. Culture in hypoxic conditions was maintained in a hermetically enclosed incubator (Thermo Electron, Marietta, OH, USA) that provided a hypoxic atmosphere, defined as fraction of inspired oxygen (FiO2) = <1% O2 (5% CO2, 10% H2, and 85% N2) with continuous computerized monitoring of atmospheric oxygen using a sensor connected to a data acquisition module (Scope; Data Translation, Marlboro, MA, USA). The changes in expression of key miRNA species were also confirmed using cells cultured in FiO2 = 8 vs. <1%, as 8% is comparable to the oxygen level observed in the human placenta in vivo after the first trimester of pregnancy (4). All media were pre-equilibrated to the gas mixture before addition to the culture plate and refreshed every 24 h. Cultures in standard or hypoxic conditions continued for 48 h or as indicated in the figures. Differentiation was routinely monitored by medium hCG levels, obtained using an ELISA (DRG International, Mountainside, NJ, USA), showing a characteristic increase in medium hCG as cytotrophoblasts differentiate into syncytiotrophoblasts, with attenuation of this process in hypoxic cells (9, 30). Immortalized human first trimester extravillous trophoblast cells, provided by C. H. Graham (Queen’s University, Kingston, ON, Canada) (31), were cultured in RPMI 1640 (Cellgro, Manassas, VA, USA), supplemented with 5% bovine growth serum (HyClone) and antibiotics.
MiRNA microarray
Total trophoblastic RNA was isolated using a mirVana miRNA isolation kit (Ambion, Foster City, CA, USA). miRNA microarray expression profiling was performed by the GenoSensor Corporation (Temple, AZ, USA) using the GenoExplorer platform. Raw fluorescence data were normalized to 5S RNA as a loading control and tRNA (positive control). The results were ranked on the basis of the ratio of signal intensity between the two experimental conditions (standard and hypoxia). miRNA microarray data are available in the Supplemental Data.
Detection of miRNA by Northern blot, in situ hybridization, and RT-quantitative PCR (qPCR)
For confirmation of miRNA levels, total RNA was extracted using the guanidine thiocyanate, acid phenol:chloroform procedure of Chomczynski and Sacchi (32). Twenty micrograms of total RNA was denatured at 65°C for 10 min and subsequently resolved using 7 M urea-15% PAGE. The gel was stained using SYBR Gold (Invitrogen/Molecular Probes, Eugene, OR, USA), and RNA was electrotransferred to a nylon Hybond N+ membrane (GE Healthcare, Piscataway, NJ, USA). Hybridization was performed overnight at 37°C in 0.5 M Na2HPO4 (pH 7.4), 7% SDS, and 1 mM EDTA using DNA antisense oligonucleotide probes, which were labeled with [32P]dATP using a StarFire labeling system (IDT, Coralville, IA, USA). After washing, membranes were exposed for 16–24 h to Kodak film (Eastman Kodak, Rochester, NY, USA) for visualization and analyzed by densitometry (VisionWorks LS software, UVP BioImaging, Upland, CA, USA).
For in situ hybridization, fresh placental samples were prefixed in 4% paraformaldehyde in PBS overnight and then in 0.5 M sucrose in PBS overnight before mounting in Tissue-Tek OCT (Sakura Finetek, Torrance, CA, USA). Tissue sections (14 μM) were cut with a cryostat (Leica Microsystems, Bannockburn, IL, USA) and mounted on SuperFrost Plus glass slides (Thermo Fisher Scientific, Pittsburgh, PA, USA). Frozen sections were treated with 20 mg/ml proteinase K (Roche Diagnostics, Indianapolis, IN, USA) and then were fixed in 4% paraformaldehyde and acetylated for 10 min with a mixture of 0.25% acetic anhydride and 0.1 M triethanolamine. The postfixed tissues were subsequently incubated overnight with digoxigenin (DIG)-labeled LNA probes from Exiqon A/S (Vedbaek, Denmark) according to the manufacturer’s instructions. After stringency washes, the slides were incubated with alkaline phosphatase-conjugated anti-DIG antibody (1:500 dilution; Roche Diagnostics) and detected using BM Purple Substrate (Roche Diagnostics). Finally the slides were mounted in DPX (Fluka, Milwaukee, WI, USA). For analysis and quantification of miRNA expression levels by real-time PCR, we used the miScript PCR system (Qiagen, Valencia, CA, USA), following the manufacturer’s instructions. Total RNA input was normalized using RNU6B RNA as an endogenous control. The fold increase relative to control samples was determined by the 2−ΔΔCt method (33). Expression levels of target genes mRNA were determined by RT-qPCR using total cellular RNA purified from trophoblasts and performed in duplicate as described previously (34).
Prediction of miRNA targets
We searched for all potential target genes of the identified miRNAs using the public databases miRBase (35) and TargetScan (36), which predict putative miRNA targets mainly on the basis of the presence of miRNA binding sites in the 3′-UTR region of the genes, following the approach described by Sethupathy et al. (37). For each miRNA, we initially used TargetScan to identify genes that are predicted targets by both databases and are conserved between human and mouse. Next, we searched our previously obtained mRNA microarray data, which represent differential gene expression in trophoblasts cultured in standard or hypoxic conditions (38), for putative mRNA targets that exhibit an inverse expression pattern relative to their respective miRNAs. Because the distribution of gene expression was unknown, we used three different tests to match miRNA with putative mRNA targets: a 1-sided t test for paired samples on the original gene expression data, a 1-sided t test for paired samples on log-transformed gene expression data, and a 1-sided Wilcoxon signed-rank test for paired samples. All three tests yielded similar results. We then adjusted the P value of the tests using the method of Benjamini and Hochberg (39) to control the false discovery rate at 5%. A gene was considered up- or down-regulated if the adjusted P value for ≥2 tests was <0.1.
Plasmids, mutagenesis, transfection, and luciferase assay
miRNA expression vectors were engineered by cloning an ∼500-bp fragment of genomic DNA that harbored the miRNA precursor plus flanking sequences into pcDNA3 vector (Invitrogen, Carlsbad, CA, USA). For each miRNA, we designed a miRNA sensor construct by cloning a synthetic fragment that contained 3 perfectly matching miRNA responsive elements (MRE) into psiCHECK-2 (Promega, Madison, WI, USA). For 3′-UTR of putative miRNA target genes we PCR-amplified the relevant 3′-UTR sequences using human genomic DNA as a template and inserted them into psiCHECK-2 at the XhoI/NotI sites. Mutations in putative MREs were performed using site-directed, ligase-independent mutagenesis (40) and included deletion of 10–20 nucleotides that contained the seed sequences of the MRE within the target 3′-UTR. To reduce the risk of unintended mutations, each mutated insert was confirmed by sequencing and then subcloned back into a native psiCHECK-2 vector that was not subject to mutagenesis. Luciferase reporter constructs along with the miRNA-expression constructs were cotransfected into cells using polyethylenimine-mediated transfection (41). HTR-8/SVneo cells, plated in 12-well plates, were transfected with 10 ng of reporter construct along with increasing concentrations of CMV-based vectors (pcDNA3, Invitrogen), expressing relevant miRNAs. At 48 h after transfection, the cells were lysed with passive lysis buffer (Promega), and firefly and Renilla luciferase activities were measured consecutively with the dual luciferase reporter system (Promega). Renilla luciferase activity was normalized to the firefly luciferase control.
For expression of miRNA mimic, cells were transfected with 100 nM double-stranded miRNA mimic (miRIDIAN; Dharmacon, Lafayette, CO, USA) using HiPerfect transfection reagent (Qiagen). miR-205 knockdown was performed using a miRCURY LNA microRNA knockdown probe (100 nM; Exiqon). Transfection of the mixture of plasmid DNA and LNA oligonucleotide was performed using Attractene (Qiagen) according to the manufacturer’s instructions. For small interfering RNA (siRNA)-mediated knockdown of MED1, primary trophoblasts were transfected 24 h after plating with 100 nM of a MED1-specific siRNA (5′-GCUCUCAAAGUAACAUCUU-3′) or a control siRNA (5′-GCUAAACAAUGUACUCCUU-3′; Invitrogen) using DharmaFECT2 transfection reagent, according to the manufacturer’s instructions. Cells were harvested, and total RNA was extracted using Tri Reagent (Molecular Research Center, Cincinnati, OH, USA) 48 h after transfection.
Western blot
Cells were lysed in TBS that contained 1% Triton X-100 and protease inhibitors (5 mg/ml apoprotinin, 1 mg/ml leupeptin, and 1 mM PMSF). Lysates were separated on SDS-PAGE and transferred to PVDF membranes using standard procedures. Membranes were immunoblotted with rabbit anti-TRAP220/MED1 antibody (1 mg/ml, ab64965; Abcam, Cambridge, MA, USA) followed by anti-rabbit horseradish peroxidase secondary antibody (400 mg/ml; Sigma-Aldrich Corp.). For normalization, the same membrane was immunoblotted with antitubulin antibody (100 mg/ml, CP06; Calbiochem San Diego, CA, USA). The blots were washed and processed for chemiluminescence using SuperSignal West Dura (Thermo Fisher Scientific) and densitometrically analyzed as described above.
Statistics
All results, including all transfections, were repeated using ≥3 independent experiments. All transfection and qPCR data were obtained in duplicate. For the studies shown in Figs. 3, 4B, 5B, 6B, C, and 7, the comparison of each experimental paradigm group to the control was performed using a linear mixed-effect model, with the random effect representing the experiment-to-experiment variation, the treatment as the fixed effect and the observed log fold change as the dependent variable (42). For data consisting of one representative of three experiments, such as the studies shown in Fig. 4D, the results were analyzed by a simple linear fixed-effect model using 1-way ANOVA, with the treatment as the fixed effect and the log fold change as the dependent variable. To obtain near-normal distribution, the relative luciferase activity data were log-transformed. To compute the P values for the null hypothesis that the treatment groups do not differ from the control groups, we used a χ2 test to compare the null models and the alternative models that include the treatment as the fixed effect. When multiple hypotheses were tested simultaneously, which is the case for all studies except for those shown in Fig. 6B, C, the P values were corrected either by the method of Benjamini and Hochberg (39) for false discovery rate or by the Bonferroni method for family-wise error rate. All analyses were performed using R statistical computing software (43). The linear mixed-effect model analysis was done using the nmle package of R (44).
Figure 1.
Effect of hypoxia on miRNA expression in primary human trophoblasts. Cells were cultured for 48 h in standard conditions (FiO2=20% O2) or in hypoxia (FiO2=<1%). A) Species of miRNA that are differentially expressed using microarray profile and confirmed as described in the text. Results are expressed as fold change in hypoxia over standard conditions, as explained in Materials and Methods, and all differences were significant (P≤0.05). B) Northern blot validation of microarray data representing transcript expression in standard conditions (S) or hypoxia (H). Blots represent 3 independent experiments that were densitometrically analyzed (not shown), confirming the results in A.
Figure 2.
Expression of regulated miRNA in term human placental villi. Expression pattern of miRNA was determined by in situ hybridization, using LNA probes labeled with digoxigenin. Whereas all miRNA species were detected in the trophoblast bilayer, note weak expression of miR-205, miR-335, and miR-491 in stromal and endothelial cells. Original view: ×400. Scale bar = 50 μm.
Figure 3.
Experimental validation of predicted mRNA targets of hypoxia-regulated miRNAs. The 3′-UTR region of predicted target genes was cloned into a bicistronic psiCHECK-2 luciferase reporter vector. HTR-8/SVneo cells were transfected with the reporter construct along with increasing concentrations (0, 5, 20, and 100 ng) of pcDNA3 vectors that expressed the relevant miRNAs. Renilla luciferase activity was normalized to firefly luciferase. Relative luciferase unit (RLU) activity from each reporter construct in the absence of transfected miRNA was set at 1. Data represent the geometric mean of the normalized luciferase activity of 3 independent experiments performed in duplicate. Significant differences were determined as described in Materials and Methods: *P ≤ 0.05 [adjusted for false discovery rate (FDR) control] for 20 and 100 ng vs. no miRNA control and ≥25% reduction in gene expression for the highest concentration. **P ≤ 0.05 (adjusted for FDR control) and ≥50% reduction in gene expression.
Figure 4.
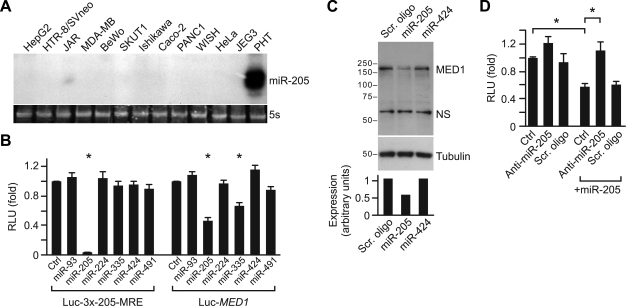
MED1 is a target for miR-205. A) Expression of mature miR-205 in cell lines and PHTs. In addition to PHTs, expression of miR-205 was assayed in cancer cell lines and several trophoblast-derived cell lines (HTR-8/SVneo, JAR, BeWo, and JEG3). 5S rRNA, stained with ethidium bromide, was used as a loading control. B) MED1 3′-UTR is selectively regulated by miR-205. A sensor construct containing 3 copies of a perfectly complementary site to miR-205 (left panel) or the MED1 3′-UTR reporter construct (10 ng each) was transfected into HTR-8/SVneo cells along with different miRNA expression plasmids or an empty control (Ctrl) plasmid (100 ng each). Relative luciferase unit (RLU) activity was determined as described in Materials and Methods. Data are geometric means of 3 independent experiments performed in duplicate. C) Expressed miR-205 silences MED1 protein in HTR-8/SVneo cells. Cells were transfected with 100 nM duplex scramble RNA (Scr. oligo), miR-205, or miR-424 RNA. Endogenous MED1 protein was analyzed 72 h later and quantified as described in Materials and Methods. Tubulin was used as a loading control (NS, nonsignificant band). Results are representative of 3 experiments. D) Anti-miR-205 inhibits the silencing effect of miR-205. HTR-8/SVneo cells were transfected with the MED1 3′-UTR reporter along with either an empty vector or a miR-205 expression plasmid. Anti-miR-205 LNA (anti-miR-205) or scrambled miRNA or no oligonucleotide (mock) was coexpressed, as noted. RLU activity was determined after 48 h as described in Materials and Methods. Data represent the geometric mean of a representative of 3 independent experiments, performed in duplicate. Significant differences were determined as described in Materials and Methods. *P ≤ 0.05 vs. control (after Bonferroni correction).
Figure 5.
A single MRE mediates the influence of miR-205 on MED1 3′-UTR. A) Schematic representation of the 3 predicted miR-205 MREs within the 3′-UTR of the MED1 mRNA. Alignments between the miR-205 binding sites and miR-205 are shown. B) Deletion analysis of MED1 3′-UTR, designed to uncover the function of miR-205 putative binding sites. HTR-8/SVneo cells were transfected with the wild-type MED1 3′-UTR reporter (wt) and with mutations in each of the three miR-205 binding sites, alone or in combination. Each reporter was transfected along with a vector expressing a scramble sequence or miR-205 expression vector. Relative luciferase unit (RLU) activity from each reporter construct, normalized to firefly luciferase activity, was determined 48 h later. Data are geometric means of 3 independent experiments, performed in duplicates. Significant differences were determined as described in Materials and Methods. *P ≤ 0.05 (after Bonferroni correction), compared with control.
Figure 6.
Hypoxia regulates the expression of miR-205 and MED1. Primary human trophoblasts from 3 different term placentas were exposed to either standard conditions (S, Std) or hypoxia (H, Hpx), as described in Materials and Methods. A) Western blot (IB) analysis of MED1 (top panel) and tubulin (bottom panel) in primary trophoblasts. B) RT-qPCR analysis of MED1 mRNA (left panel) and mature miR-205 (right panel) levels in isolated primary trophoblasts. C) Role of miR-205 binding (site 3) in hypoxia effect on the MED1 gene. Primary trophoblasts were transfected with wild-type MED1 3′-UTR reporter construct or a reporter with deleted site 3 (miR-205 binding site). Relative luciferase unit (RLU) activity was determined after 48 h as described in Materials and Methods. Data are geometric means of 3 independent experiments performed in duplicate. Significant differences were determined as described in Materials and Methods. *P ≤ 0.05 vs. control.
Figure 7.
MED1 modulates differentiation of primary human trophoblasts. Primary human trophoblasts from 4 different term placentas were transfected with a siRNA targeting MED1 or nontargeting siRNA (control); total RNAs were extracted 48 h later and evaluated by RT-qPCR, as described in Materials and Methods. Data are geometric means of 4 independent experiments, each performed in duplicate. Significant differences were determined as described in Materials and Methods. *P ≤ 0.05 vs. control (adjusted for FDR control).
RESULTS
Hypoxia alters the expression of trophoblastic miRNAs
Using miRNA microarrays we profiled miRNA expression in human primary trophoblast cells cultured for 48 h in standard culture conditions (FiO2=20%) vs. hypoxia (FiO2=1%). We selected for further analysis 7 miRNA species that showed a signal intensity higher than the mean background plus 2-fold the sd of the background, showed a consistent trend in cells from two different placentas when exposed to declining oxygen concentrations, and were confirmed using Northern blot. Of these miRNAs, 6 were up-regulated in hypoxia (miR-93, -205, -224, -335, -451, and -491) and 1 (miR-424) was down-regulated in hypoxia (Fig. 1A, B). To delineate the localization of the regulated miRNAs in placental villi and ensure that these miRNAs are expressed primarily in the trophoblast bilayer in vivo, we performed in situ hybridization using LNA-based probes. All selected miRNA species were detected in term human placental villi and expressed primarily in the trophoblast layer with a weaker signal in the villous core (Fig. 2). Notably, the expression of miR-224 and miR-451 was markedly weaker, corresponding to the weaker expression in standard conditions, detected using Northern blot analysis.
Identification of miRNA targets in trophoblasts
To gain insight into the function of miRNAs in hypoxic trophoblasts, we sought to identify putative targets for hypoxia-regulated miRNAs. The silencing function of miRNAs reflects mRNA degradation and/or inhibition of translation (16,17,18, 45,46,47,48,49,50). Focusing on the influence of miRNA on mRNA, we combined our confirmed miRNA expression profiling data with previously obtained microarray profiles of mRNA expression in normoxic and hypoxic human trophoblasts (38, 51). We first used two miRNA target prediction databases (TargetScan and miRBase) to identify putative mRNA targets to the seven hypoxia-regulated miRNAs. Assuming that there would be an inverse correlation between the expression of a given miRNA and its putative target, we screened for the predicted expression pattern of these putative targets among hypoxic trophoblastic transcripts. Using a combination of a paired t test and paired Wilcoxon rank-sum test with false discovery rate controlled at 5% and adjusted P < 0.1 from ≥2 tests, we found that 5 miRNA species that were up-regulated in hypoxia may target 15 mRNAs that were down-regulated in hypoxia. For miR-424, which was down-regulated in hypoxia, we found 4 potential target genes that were up-regulated in hypoxia. We identified no putative targets for miR-451.
To experimentally validate the regulation of these mRNA targets by the corresponding miRNAs, we coexpressed in the trophoblast line HTR-8/SVneo sets of miRNA expression vectors, which include a genomic DNA fragment containing the miRNA precursor and flanking sequences, along with a vector containing the 3′ putative target reporter downstream from luciferase. All miRNA expression vectors were functional and capable of gene silencing, as determined in parallel experiments using a reporter gene that harbors three MREs downstream from luciferase (not shown). As shown in Fig. 3, we found that MED1 was silenced ≥2-fold by miR-205. Likewise, FGFR1 and MAP2K1 were silenced ≥2-fold by miR-424. This 2-fold magnitude of miRNA effect is consistent with the extent of inhibition by miRNA reported by others (17, 18, 52).
Validation of MED1 as target of miR-205
We focused on the activity of miR-205, because miR-205 has a relatively restricted expression pattern (53, 54) compared with the ubiquitously expressed miR-424 and because miR-205 modulates MED1, which has an essential role in the mediator complex and is essential for murine placental development (28, 55). We found that miR-205 is highly expressed in primary trophoblasts, has very low expression in trophoblast-derived cells, and is undetectable in several cancer cell lines (Fig. 4A). To ensure that the interaction between MED1 3′-UTR and miR-205 is specific, we examined the activity of the MED1 reporter in the presence of coexpressed miRNAs that we identified in our study. The miR-205 MRE sensor construct served as a positive control. As shown in Fig. 4B, the activity of the control reporter was nearly abolished by miR-205, with no effect on the sensor construct by other miRNA-expressing constructs. Notably, luciferase activity from the MED1 reporter was reduced to 50% by miR-205 (Fig. 4B, right panel). The partial effect of miR-335 is probably nonspecific, as we observed a similar result using multiple reporter constructs that are devoid of miR-335 binding sites (not shown). We next transfected HTR-8/SVneo cells with miR-205 double-stranded oligonucleotide mimics and found reduced expression of endogenous MED1 protein compared with either a scramble control mimic or miR-424 (Fig. 4C). Finally, we found that although overexpression of LNA-based anti-miR-205 minimally influenced MED1 reporter in HTR-8/SVneo cells, which express very low levels of miR-205, transfection of anti-miR-205 specifically enhanced the activity of the MED1 reporter, silenced by coexpressed miR-205 (Fig. 4D). Together, these results demonstrate that MED1 is a functional cellular target for miR-205.
Discrete site in MED1 3′-UTR mediates miR-205 silencing
Using a computational search for potential miR-205 binding sites within the 3′-UTR of MED1 (using miRBase and TargetScan), we identified three potential seed matches, located at positions 387–393, 439–445, and 492–498 within MED1 3′-UTR (Fig. 5A). To accurately define the MRE for miR-205 in the MED1 3′-UTR, we deleted the nucleotides corresponding to individual seed regions within the context of the MED1 reporter plasmid and transfected the mutant reporter plasmids, along with a miR-205 expression vector, into HTR-8/SVneo cells. As expected, MED1 reporter gene activity was unchanged in the presence of miRNA scramble control vector (Fig. 5B, left panel). In contrast, whereas miR-205 silenced wild-type MED1 reporter or reporters that were mutated in site 1 or 2, mutation of site 3 alone or in combination with sites 1–2 abolished the silencing effect of miR-205 (Fig. 5B, right panel). Among the computationally predicted miR-205 seed elements in MED1 gene, our data establish only site 3 as functionally relevant.
Hypoxia in primary trophoblasts regulates MED1, a modulator of trophoblast differentiation
Having demonstrated that miR-205 silences MED1 in a trophoblast-derived cell line, we sought to confirm these results in primary human trophoblasts, and assessed the influence of hypoxia on MED1 expression. As predicted by the increased expression of miR-205 in hypoxic trophoblasts, hypoxia reduced MED1 protein and MED1 RNA expression in primary human trophoblasts (Fig. 6A), while increasing miR-205 expression (Fig. 6B). Notably, whereas the activity of the MED1 3′-UTR reporter was predictably attenuated by hypoxia, this effect was abolished in the MED1 reporter that harbored a mutant site 3 miR-205 binding element (Fig. 6C), supporting the role of miR-205 in hypoxia-mediated down-regulation of MED1 expression.
Because MED1 expression is essential for murine placental development and embryonic survival (27, 28, 55, 56), we sought to assess the effect of reduced MED1 on the expression of several genes that are associated with differentiation of primary human trophoblasts (9, 38, 57). As shown in Fig. 7, reduced expression of MED1 attenuated the expression of hCG, human placental lactogen (hPL), and placental-specific protein 1 (Plac1). In contrast, there was no effect on corticotropin-releasing hormone (CRH), sirtuin 1 (Sirt1), or syncytin. These data corroborate the role of MED1 in human trophoblasts and suggest that even partial depletion of MED1 perturbs the differentiation of human trophoblasts.
DISCUSSION
Although the causes and pathophysiology of placental injury associated with substandard fetal growth remain largely unclear, histopathological and expression analyses implicate reduced placental perfusion and cellular hypoxia in the final common pathways of diverse insults (7, 30, 38, 58,59,60,61,62). Using expression profiling, we defined a set of miRNAs that exhibit altered expression in hypoxic placental trophoblasts and provided experimental support to the computationally derived miRNA targets. After validation by Northern blot and qPCR, we restricted our interest to a small subset of trophoblastic miRNA species, including the up-regulated miR-93, miR-205, miR-224, miR-335, miR-451, and miR-491 and the down-regulated miR-424. Our set of hypoxia-regulated miRNA in trophoblasts expands prior information on miRNAs that were identified in screens for hypoxia-specific miRNA in other cell types (24, 63,64,65,66,67,68). Interestingly, with our array screen and a targeted analysis using qPCR of trophoblasts we did not detect a significant change in the expression of miR-210, a species that has been regularly described as hypoxia-responsive in several cellular systems (63, 65,66,67, 69).
Our data are the first to demonstrate the regulation of miR-205 in hypoxia. In normal tissues miR-205 is implicated in the epithelial to mesenchymal transition and the maintenance of the epithelial phenotype (53, 54, 70, 71). There is growing evidence that abnormal expression of miR-205 is associated with carcinogenesis (70, 72,73,74,75,76,77), with up-regulation in ovarian and bladder tumors (78, 79), but down-regulation in breast and esophageal tumors (80, 81). Because placental trophoblasts are also epithelial cells, our data suggest that miR-205 plays a role in the adaptation of this unique placental epithelium to injury.
Our findings that miR-205 selectively down-regulates the activity of a luciferase reporter under the control of MED1 3′-UTR, an effect reversible by anti-miR-205; reduces MED1 protein expression; regulates discrete elements in MED1 3′-UTR; and exhibits reciprocal regulation relative to MED1 in response to hypoxia provide compelling support for the role of miR-205 in regulation of MED1. Cloned originally as a cofactor of several nuclear receptors (reviewed in ref. 82), MED1 interacts with other gene-specific transcriptional activators, including GATA proteins (27, 83, 84), BRCA1 (85), p53 (86), GABPα (87), and CCAAT/enhancer-binding protein-β (88). Relevant to our studies, targeted disruption of Med1 in mice results in embryonic lethality, reflecting defects in the placenta and heart (27, 28, 89). Furthermore, attenuated MED1 mRNA levels in primary human trophoblasts decreased the expression of several genes that characterize differentiation of human trophoblasts. In light of the role of MED1 in gene regulatory networks, attenuation of MED1 expression by miR-205 probably plays a role in placental development and adaptation to pregnancy-related injuries. In this context, the crosstalk between MED1 and other placental members of the mediator complex remain to be established. Notably, other miRNA targets may be relevant to placental adaptation to injury. For example, the miR-424 target MAP2K1 plays an essential role in murine placental development (90). Similarly, FGFR1 is a member of the fibroblast growth factor receptor family that is expressed in the placenta (91), but its function in this tissue remains unclear. In addition to these defined targets we found several other potential targets that were consistently but only mildly silenced by miRNAs. These include ERBB3, which was slightly inhibited by miR-205, as shown by others (72, 74, 75), and AP2M1 and DMN1, which were repressed by miR-224.
Our target predictions were based on miRBase and TargetScan. Notably, predicted MREs are not consistent among the software packages. For example, miRBase predicted only one MRE in MED1 3′-UTR, whereas four sites were predicted by TargetScan (as well as by miRDB and PITA, not shown). The first two sites relative to the stop codon are conserved across vertebrates, with the first MRE also found in MED1 3′-UTR sequences of primates. We found that site 3, which is conserved across many species but not in mice and rats, was the active MRE in trophoblasts. Interestingly, site 3 is also the only MRE identified by miRBase. In addition, a fourth MRE, computationally positioned downstream of our cloned 3′-UTR sequences, was recently identified using TargetScan. A mutation in this element did not affect the response of the reporter to miR-205 (data not shown). Although we did not study this mutation in the context of the other mutated target sites, our results suggest that it is unlikely to influence the effect of miR-205 on MED1 3′-UTR. Whether miR-205 regulates MED1 through alternate MREs in other species remains to be determined. Similarly, our data do not rule out combinatorial interaction among MREs in other cell types (92).
Supplementary Material
Acknowledgments
The authors thank Dr. C. H. Graham (Queen’s University, Kingston, ON, Canada) for the HTR-8/SVneo cells. The authors also thank Magdalena Jennings and Elena Sadovsky for technical assistance and Lori Rideout for assistance during preparation of the manuscript. The project was supported by grants from the National Institutes of Health (R01-HD29190 to D.M.N; R21-HD053878 and R01-HD45675 to Y.S).
References
- Genbacev O, Zhou Y, Ludlow J W, Fisher S J. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky C H, Polliotti B M, Fisher S J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- Fox H, Path M C. Effect of hypoxia on trophoblast in organ culture. Am J Obstet Gynecol. 1970;107:1058–1064. doi: 10.1016/0002-9378(70)90629-0. [DOI] [PubMed] [Google Scholar]
- Arnholdt H, Meisel F, Fandrey K, Lohrs U. Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:365–372. doi: 10.1007/BF02899568. [DOI] [PubMed] [Google Scholar]
- Benyo D F, Miles T M, Conrad K P. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- Alsat E, Wyplosz P, Malassine A, Guibourdenche J, Porquet D, Nessmann C, Evain-Brion D. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168:346–353. doi: 10.1002/(SICI)1097-4652(199608)168:2<346::AID-JCP13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Chen B, Nelson D M, Sadovsky Y. N-Myc downregulated gene 1 (Ndrg1) modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–2772. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- Cartwright J E, Keogh R J, Tissot van Patot M C. Hypoxia and placental remodelling. Adv Exp Med Biol. 2007;618:113–126. doi: 10.1007/978-0-387-75434-5_9. [DOI] [PubMed] [Google Scholar]
- Simon M C, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- He L, Hannon G J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Lai E C. miRNAs: whys and wherefores of miRNA-mediated regulation. Curr Biol. 2005;15:R458–460. doi: 10.1016/j.cub.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Lim L P, Lau N C, Garrett-Engele P, Grimson A, Schelter J M, Castle J, Bartel D P, Linsley P S, Johnson J M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo F D, Gygi S P, Bartel D P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Croce C M, Calin G A. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack F J. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Latronico M V, Condorelli G. RNA silencing: small RNA-mediated posttranscriptional regulation of mRNA and the implications for heart electropathophysiology. J Cardiovasc Electrophysiol. 2009;20:230–237. doi: 10.1111/j.1540-8167.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- Pandey A K, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem. 2009;23:221–232. doi: 10.1159/000218169. [DOI] [PubMed] [Google Scholar]
- Chang T C, Mendell J T. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Negrini M, Calin G A, Davuluri R V, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle. 2007;6:1426–1431. [PubMed] [Google Scholar]
- Ivan M, Harris A L, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker R B, Mouillet J F, Nelson D M, Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod. 2007;13:273–279. doi: 10.1093/molehr/gam006. [DOI] [PubMed] [Google Scholar]
- Crawford S E, Qi C, Misra P, Stellmach V, Rao M S, Engel J D, Zhu Y, Reddy J K. Defects of the heart, eye, and megakaryocytes in peroxisome proliferator activator receptor-binding protein (PBP) null embryos implicate GATA family of transcription factors. J Biol Chem. 2002;277:3585–3592. doi: 10.1074/jbc.M107995200. [DOI] [PubMed] [Google Scholar]
- Landles C, Chalk S, Steel J H, Rosewell I, Spencer-Dene B, Lalani el N, Parker M G. The thyroid hormone receptor-associated protein TRAP220 is required at distinct embryonic stages in placental, cardiac, and hepatic development. Mol Endocrinol. 2003;17:2418–2435. doi: 10.1210/me.2003-0097. [DOI] [PubMed] [Google Scholar]
- Kliman H J, Nestler J E, Sermasi E, Sanger J M, Strauss J F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Nelson D M, Johnson R D, Smith S D, Anteby E Y, Sadovsky Y. Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol. 1999;180:896–902. doi: 10.1016/s0002-9378(99)70661-7. [DOI] [PubMed] [Google Scholar]
- Graham C H, Hawley T S, Hawley R G, MacDougall J R, Kerbel R S, Khoo N, Lala P K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Schaiff W T, Bildirici I, Cheong M, Chern P L, Nelson D M, Sadovsky Y. Peroxisome proliferator-activated receptor-γ and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab. 2005;90:4267–4275. doi: 10.1210/jc.2004-2265. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock R J, van Dongen S, Bateman A, Enright A J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh K K, Johnston W K, Garrett-Engele P, Lim L P, Bartel D P. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, Megraw M, Hatzigeorgiou A G. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods. 2006;3:881–886. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- Roh C R, Budhraja V, Kim H S, Nelson D M, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- Chiu J, March P E, Lee R, Tillett D. Site-directed, ligase-independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 2004;32:e174. doi: 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S E, Staley E M, Mayginnes J P, Pintel D J, Tullis G E. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Pinheiro J C, Bats D M. New York: Springer; Mixed-Effect Models in S and S-PLUS. 2000 [Google Scholar]
- R Development Core Team Vienna, Austria: Foundation for Statistical Computing; RA Language and Environment for Statistical Computing. 2009 http://www.R-project.org. [Google Scholar]
- Pinheiro J C, Bates D, Debroy S, Sarker D, the R Development Core Team Vienna, Austria: R Project for Statistical Computing; nlmeLinear and Nonlinear Mixed Effects Models, version 3.1-96. 2009 [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli A E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb Symp Quant Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Giraldez A J, Takeda Y, Fujiwara T, Sakamoto H, Schier A F, Inoue K. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr Biol. 2006;16:2135–2142. doi: 10.1016/j.cub.2006.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco J G. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada T P, Finn K J, Ji X, Baillat D, Gregory R I, Liebhaber S A, Pasquinelli A E, Shiekhattar R. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- Eulalio A, Rehwinkel J, Stricker M, Huntzinger E, Yang S F, Doerks T, Dorner S, Bork P, Boutros M, Izaurralde E. Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 2007;21:2558–2570. doi: 10.1101/gad.443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J C, Babak T, Corson T W, Chua G, Khan S, Gallie B L, Hughes T R, Blencowe B J, Frey B J, Morris Q D. Using expression profiling data to identify human microRNA targets. Nat Methods. 2007;4:1045–1049. doi: 10.1038/nmeth1130. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey S K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D G, Oliveira-Fernandes M, Lavker R M. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- Yu J, Ryan D G, Getsios S, Oliveira-Fernandes M, Fatima A, Lavker R M. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A. 2008;105:19300–19305. doi: 10.1073/pnas.0803992105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Jia Y, Nye J S, Rao M S, Reddy J K. Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J Biol Chem. 2000;275:14779–14782. doi: 10.1074/jbc.C000121200. [DOI] [PubMed] [Google Scholar]
- Ito M, Yuan C X, Okano H J, Darnell R B, Roeder R G. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol Cell. 2000;5:683–693. doi: 10.1016/s1097-2765(00)80247-6. [DOI] [PubMed] [Google Scholar]
- Massabbal E, Parveen S, Weisoly D L, Nelson D M, Smith S D, Fant M. PLAC1 expression increases during trophoblast differentiation: evidence for regulatory interactions with the fibroblast growth factor-7 (FGF-7) axis. Mol Reprod Dev. 2005;71:299–304. doi: 10.1002/mrd.20272. [DOI] [PubMed] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller S, Buhimschi C S, Ma Y Y, Huang S T, Yang L, Kuczynski E, Zambrano E, Lockwood C J, Buhimschi I A. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88:1057–1067. doi: 10.1038/labinvest.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Smith S D, Chandler K, Sadovsky Y, Nelson D M. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol Cell Physiol. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- Kingdom J C P, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- Kingdom J C, Kaufmann P. Oxygen and placental vascular development. Adv Exp Med Biol. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- Crosby M E, Kulshreshtha R, Ivan M, Glazer P M. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimbellot J S, Erickson S W, Mehta T, Wen H, Page G P, Sorscher E J, Hong J S. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med Genomics. 2009;2:15. doi: 10.1186/1755-8794-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps C, Buffa F M, Colella S, Moore J, Sotiriou C, Sheldon H, Harris A L, Gleadle J M, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi M C, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros D, Weber B L, Simon C, Coukos G, Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha R, Davuluri R V, Calin G A, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582:2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Gregory P A, Bert A G, Paterson E L, Barry S C, Tsykin A, Farshid G, Vadas M A, Khew-Goodall Y, Goodall G J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Gregory P A, Bracken C P, Bert A G, Goodall G J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- Iorio M V, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T, Menard S, Croce C M, Tagliabue E. MicroRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69:2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, Nonaka D, Li J, Spector Y, Rosenfeld N, Chajut A, Cohen D, Aharonov R, Mansukhani M. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta P, Valdagni R, Daidone M G, Zaffaroni N. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cε. Cancer Res. 2009;69:2287–2295. doi: 10.1158/0008-5472.CAN-08-2894. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu S, Mo Y Y. Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res. 2009;19:439–448. doi: 10.1038/cr.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijckmeester W A, Wijnhoven B P, Watson D I, Leong M P, Michael M Z, Mayne G C, Bright T, Astill D, Hussey D J. MicroRNA-143 and -205 expression in neosquamous esophageal epithelium following argon plasma ablation of Barrett’s esophagus. J Gastrointest Surg. 2009;13:846–853. doi: 10.1007/s11605-009-0799-5. [DOI] [PubMed] [Google Scholar]
- Song H, Bu G. MicroRNA-205 inhibits tumor cell migration through down-regulating the expression of the LDL receptor-related protein 1. Biochem Biophys Res Commun. 2009;388:400–405. doi: 10.1016/j.bbrc.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio M V, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu C G, Alder H, Calin G A, Menard S, Croce C M. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- Gottardo F, Liu C G, Ferracin M, Calin G A, Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, Gomella L G, Croce C M, Baffa R. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Sempere L F, Christensen M, Silahtaroglu A, Bak M, Heath C V, Schwartz G, Wells W, Kauppinen S, Cole C N. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- Feber A, Xi L, Luketich J D, Pennathur A, Landreneau R J, Wu M, Swanson S J, Godfrey T E, Litle V R. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belakavadi M, Fondell J D. Role of the mediator complex in nuclear hormone receptor signaling. Rev Physiol Biochem Pharmacol. 2006;156:23–43. doi: 10.1007/s10254-005-0002-0. [DOI] [PubMed] [Google Scholar]
- Gordon D F, Tucker E A, Tundwal K, Hall H, Wood W M, Ridgway E C. MED220/thyroid receptor-associated protein 220 functions as a transcriptional coactivator with Pit-1 and GATA-2 on the thyrotropin-β promoter in thyrotropes. Mol Endocrinol. 2006;20:1073–1089. doi: 10.1210/me.2005-0115. [DOI] [PubMed] [Google Scholar]
- Stumpf M, Waskow C, Krotschel M, van Essen D, Rodriguez P, Zhang X, Guyot B, Roeder R G, Borggrefe T. The mediator complex functions as a coactivator for GATA-1 in erythropoiesis via subunit Med1/TRAP220. Proc Natl Acad Sci U S A. 2006;103:18504–18509. doi: 10.1073/pnas.0604494103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada O, Oishi H, Takada I, Yanagisawa J, Yano T, Kato S. BRCA1 function mediates a TRAP/DRIP complex through direct interaction with TRAP220. Oncogene. 2004;23:6000–6005. doi: 10.1038/sj.onc.1207786. [DOI] [PubMed] [Google Scholar]
- Frade R, Balbo M, Barel M. RB18A, whose gene is localized on chromosome 17q12–q21.1, regulates in vivo p53 transactivating activity. Cancer Res. 2000;60:6585–6589. [PubMed] [Google Scholar]
- Udayakumar T S, Belakavadi M, Choi K H, Pandey P K, Fondell J D. Regulation of Aurora-A kinase gene expression via GABP recruitment of TRAP220/MED1. J Biol Chem. 2006;281:14691–14699. doi: 10.1074/jbc.M600163200. [DOI] [PubMed] [Google Scholar]
- Li H, Gade P, Nallar S C, Raha A, Roy S K, Karra S, Reddy J K, Reddy S P, Kalvakolanu D V. The Med1 subunit of transcriptional mediator plays a central role in regulating CCAAT/enhancer-binding protein-β-driven transcription in response to interferon-γ. J Biol Chem. 2008;283:13077–13086. doi: 10.1074/jbc.M800604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Nakamura T, Kurosawa R, Miyamae T, Imagawa T, Mori M, Aihara Y, Yokota S. Glomerulonephritis in children with mixed connective tissue disease. Clin Nephrol. 2006;66:160–165. doi: 10.5414/cnp66160. [DOI] [PubMed] [Google Scholar]
- Bissonauth V, Roy S, Gravel M, Guillemette S, Charron J. Requirement for Map2k1 (Mek1) in extra-embryonic ectoderm during placentogenesis. Development. 2006;133:3429–3440. doi: 10.1242/dev.02526. [DOI] [PubMed] [Google Scholar]
- Anteby E Y, Natanson-Yaron S, Hamani Y, Sciaki Y, Goldman-Wohl D, Greenfield C, Ariel I, Yagel S. Fibroblast growth factor-10 and fibroblast growth factor receptors 1–4: expression and peptide localization in human decidua and placenta. Eur J Obstet Gynecol Reprod Biol. 2005;119:27–35. doi: 10.1016/j.ejogrb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell R B, Cohen S M. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.