Abstract
Purkinje cell degeneration (pcd) mice have a mutation within the gene encoding cytosolic carboxypeptidase 1 (CCP1/Nna1), which has homology to metallocarboxypeptidases. To assess the function of CCP1/Nna1, quantitative proteomics and peptidomics approaches were used to compare proteins and peptides in mutant and wild-type mice. Hundreds of peptides derived from cytosolic and mitochondrial proteins are greatly elevated in pcd mouse hypothalamus, amygdala, cortex, prefrontal cortex, and striatum. However, the major proteins detected on 2-D gel electrophoresis were present in mutant and wild-type mouse cortex and hypothalamus at comparable levels, and proteasome activity is normal in these brain regions of pcd mice, suggesting that the increase in cellular peptide levels in the pcd mice is due to reduced degradation of the peptides downstream of the proteasome. Both nondegenerating and degenerating regions of pcd mouse brain, but not wild-type mouse brain, show elevated autophagy, which can be triggered by a decrease in amino acid levels. Taken together with previous studies on CCP1/Nna1, these data suggest that CCP1/Nna1 plays a role in protein turnover by cleaving proteasome-generated peptides into amino acids and that decreased peptide turnover in the pcd mice leads to cell death.—Berezniuk, I., Sironi, J., Callaway, M. B., Castro, L. M., Hirata, I. Y., Ferro, E. S., Fricker, L. D. CCP1/Nna1 functions in protein turnover in mouse brain: Implications for cell death in Purkinje cell degeneration mice.
Keywords: proteasome, carboxypeptidase, aminopeptidase, protein degradation, neurodegeneration
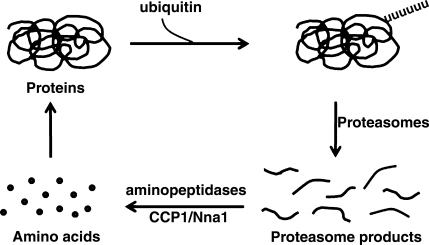
The ubiquitin proteasomal pathway is known to play a key role in protein turnover within the cell (1). However, this system produces small peptides, and additional peptidase steps are required to convert the peptides into amino acids (Fig. 1). While aminopeptidases are thought to contribute to intracellular peptide degradation, the dogma in the field is that carboxypeptidases are not involved, despite the important role that carboxypeptidases play in degradation of peptides in the intestine and other extracellular compartments (2,3,4).
Figure 1.
Model for CCP1/Nna1 function in protein turnover. Intracellular proteins are targeted for degradation by the addition of a polyubiquitin tail and undergo degradation by the 26S proteasome complex. Products of proteasome-mediated degradation are peptides, typically ranging from 10 to 20 aa. These peptides are cleaved by aminopeptidases, as well as other enzymes (oligoendopeptidases, dipeptidases) into amino acids. Hypothesized role for CCP1/Nna1 in peptide turnover is indicated.
Because of a spontaneous mutation that arose in 1976, Purkinje cell degeneration (pcd) mice lose their Purkinje cells beginning around 3 wk postnatal and also undergo loss of retinal photoreceptor cells, olfactory bulb mitral cells, and some thalamic neurons (5, 6). In addition to the original pcd mutation, there are a number of additional spontaneous mutations, chemically induced mutations, and targeted gene disruptions of the same gene loci, all of which produce ataxia due to Purkinje cell death, regardless of mutation or strain of mouse (7). In 2002, the mutation was mapped to a gene encoding a protein with homology to metallocarboxypeptidases, named Nna1 (8). Unlike other metallocarboxypeptidases that are secreted or function within the secretory pathway, Nna1 lacks a signal peptide, contains a putative ATP/GTP binding domain, and is cytosolic (9, 10). Recently, 5 other members of this metallocarboxypeptidase family were found, all lacking signal peptides, and were named cytosolic carboxypeptidase (CCP) 2-6; Nna1 was renamed CCP1/Nna1 (10, 11). CCP1/Nna1 mRNA is broadly distributed in mouse brain and other tissues (9), while levels of CCP2-6 mRNA are lower than CCP1/Nna1 in brain and undetectable in Purkinje cells (10); this led to the hypothesis that Purkinje cells die in the pcd mice because they lack sufficient levels of another CCP that can compensate for the absence of CCP1/Nna1 activity. Although a Caenorhabditis elegans orthologue of CCP6 was found to have carboxypeptidase activity (11), attempts to purify and characterize the substrate specificity of mammalian CCPs have not been successful, possibly because of the need for a cofactor or specific conditions to activate the enzymes. It is likely that CCP1/Nna1 encodes an active enzyme; expression of the wild-type (WT) form of CCP1/Nna1 in Purkinje cells of pcd mice led to survival of these cells, whereas expression of a modified CCP1/Nna1, in which 1–2 conserved substrate binding and/or catalytic residues were mutated was unable to keep the Purkinje cells alive (12, 13).
In the present study, the possibility that CCP1/Nna1 functions in cytosolic peptide degradation after proteasome function was tested using a quantitative peptidomics technique to compare peptide levels in pcd and WT mouse brain. This technique uses stable isotopic tags and mass spectrometry to detect, quantify, and identify peptides (14). While many of the peptides detected in brain are neuropeptides or other products of secretory pathway proteins, a large number of peptides derived from cytosolic and mitochondrial proteins are usually detected in peptidomics studies (15, 16). In the present study, hundreds of peptides derived from cytosolic and mitochondrial proteins were found to be greatly elevated in pcd mouse brain, suggesting a role for CCP1/Nna1 in turnover of intracellular peptides into amino acids. Because protein levels are not altered in pcd mouse brain regions such as cortex and hypothalamus, and proteasome activity is normal in these regions of pcd mouse brain, it is most likely that the increase in cellular peptide levels in the mutant mice is due to reduced degradation of the peptides into amino acids. Because low levels of amino acids can trigger autophagy (17), we examined pcd mouse brains for evidence of autophagy using microtubule-associated protein 1 light chain 3 (LC3) as a marker. In several regions of adult pcd mouse brain, but not in WT mouse brain, LC3 shows a punctate distribution consistent with enhanced autophagy in the pcd mice. On the basis of the results in nondegenerating brain regions, it was predicted that Purkinje cells would undergo similar if not more dramatic changes due to the absence of other CCPs in these cells that could partially compensate for the absence of CCP1/Nna1 in pcd mice. Purkinje cells of 2- to 3-wk-old pcd mice showed pronounced punctate distribution in many Purkinje cells, supporting this hypothesis. Taken together, these results support a role for CCP1/Nna1 in protein turnover and raise the possibility that Purkinje cell death in the pcd mouse occurs as a result of reduced peptide degradation into amino acids.
MATERIALS AND METHODS
Animals
Several breeder pairs of pcd3J heterozygous mice in the BALB/C background (BALB/cByJ-Agtpbp1pcd-3J/J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice homozygous for the pcd mutation and WT littermates were bred within the Animal Institute’s barrier facility at the Albert Einstein College of Medicine. Both males and females were used in the experiments, with no apparent differences among sexes. Mice were group-housed and maintained on a 12-hour light-dark cycle. Genotyping was done as described by Rong et al. (18).
Quantitative peptidomics
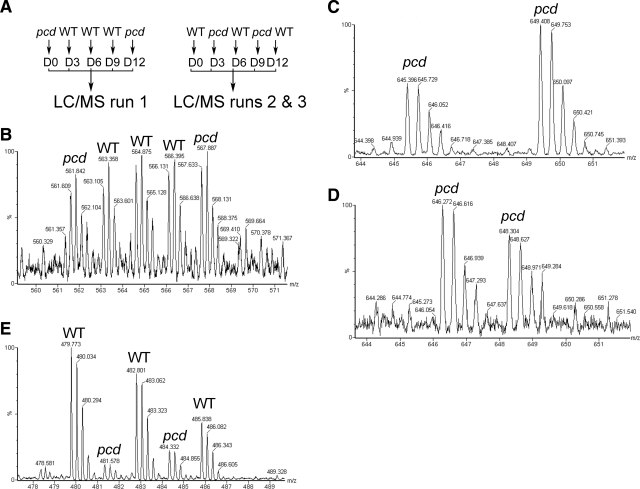
For analysis of peptides in hypothalamus and amygdala, 3 groups of mice were used. All groups consisted of 3 WT and 2 pcd mice, each analyzed separately. Group 1 consisted of 3 WT females, 1 pcd male, and 1 pcd female, all 19 to 21 wk old. Brain extracts from these mice were labeled (described below) using the scheme shown in the left panel of Fig. 2A. Groups 2 and 3 were labeled using the scheme shown in the right panel of Fig. 2A. These groups consisted of 8- to 13-wk-old mice, either all female (group 2) or all male (group 3). This strategy resulted in a total of 6 pcd mice and 9 WT mice analyzed individually for both hypothalamus and amygdala. For the other three brain regions analyzed (prefrontal cortex, cortex other than prefrontal cortex, and striatum), only group 1 was analyzed, and for these brain regions, the labeling strategy of the right panel of Fig. 2A was used.
Figure 2.
Outline of quantitative peptidomics strategy and representative MS spectra. A) Labeling scheme. For hypothalamus and amygdala, 3 groups of mice were used. Left panel: for group 1, peptide extracts from each WT mouse were labeled with D3-, D6-, or D9-TMAB, and extracts from pcd mice were labeled with D0- or D12-TMAB. Right panel: for groups 2 and 3, extracts from other pcd and WT mice were labeled as indicated (WT with D0-, D6-, or D12-TMAB and pcd with D3- or D9-TMAB). After quenching the labeling reagents, the samples within each group of mice were pooled, filtered through a 10-kDa cutoff filter, and then analyzed by LC/MS. Peptide levels were calculated from the peak intensities of each isotopic form. B) MS spectra of a peptide subsequently identified from MS/MS as the 4+ form of a C-terminal fragment of thymosin β-10 with 2 isotopic tags (monoisotopic mass 1988.043 without tags). This peptide is present in pcd mouse hypothalami at levels comparable to WT mouse hypothalami. C, D) Example of MS spectra showing a peptide that is detectable only in the pcd amygdala and not in the WT amygdala. This peptide was subsequently identified from MS/MS sequencing as the acetylated N-terminal fragment (residues 2–16) of heat shock protein 1 containing 1 isotopic tag and 2 protons (monoisotopic mass 1805.983 without isotopic tags). Panel C uses the labeling scheme for run 1; panel D uses the scheme for Run 2. E) Example of MS spectra showing a peptide present in WT mouse cortex that is greatly decreased in pcd mouse cortex. This peptide was subsequently identified from MS/MS sequencing as an N-terminal fragment of myelin basic protein with 2 isotopic tags and two protons (monoisotopic mass 1660.910 without isotopic tags). The y-axes show relative intensity (% maximum). m/z, mass to charge ratio.
Brain tissue heat treatment using microwave irradiation, peptide extraction, differential isotopic labeling, and sample preparations were performed generally, as described previously (19), except that multiple isotopic labels were used, and the labeling protocol was slightly altered (described below). The isotopic labels are active esters of trimethylammoniumbutyrate (TMAB), originally developed by Regnier and colleagues (20). In addition to the compounds containing no deuterium atoms (D0-TMAB; previously referred to as H9-TMAB) and 9 deuterium atoms (D9-TMAB), previously described by Zhang et al. (20) and Che et al. (21), compounds containing 3 deuterium atoms (D3-TMAB) and 6 deuterium atoms (D6-TMAB) were used; the synthesis and characterization of these tags was recently reported (22). An additional tag containing 9 atoms of deuterium and 3 atoms of 13C was synthesized using a similar synthetic strategy; because this tag is 12 Da more than the mass of the D0-TMAB reagent, it is referred to as D12-TMAB. Initial labeling reactions using the standard ratio of TMAB labels to brain extracts revealed that some of the peptides incorporated fewer tags than the predicted amount, suggesting that the brain extracts from the pcd mice contained a greater number of total peptides. Therefore, to achieve full labeling of the peptides the amount of TMAB reagent was increased by 50%, and 8 additions of label were performed over a reaction time of 2.5 h. Thus, a total of 7.5 mg TMAB was added per each brain region.
Extracted peptides were labeled with the various isotopic tags as indicated in Fig. 2A. Following labeling, excess reagent was quenched with Gly; the samples were pooled, filtered through YM-10 (Amicon Ultra; Millipore, Billerica, MA, USA), treated with hydroxylamine to remove TMAB groups from Tyr residues, and then desalted using C18 spin columns (Pierce, Rockford, IL, USA), as described previously (22). Samples were analyzed by liquid chromatography/mass spectrometry (LC/MS) on a Waters Q-TOF–Ultima Mass Spectrometer (Micromass, Manchester, UK) or an API Q-Star Pulsar-i quadrupole time-of-flight mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA). The peptide mixture was desalted online for 15 min using a Symmetry C18 trapping column (5-μm particles, 180 μm ID × 20 mm; Waters, Milford, MA, USA), and the trapped peptides were then separated by elution with a water/acetonitrile 0.1% formic acid gradient through a BEH 130-C18 column (1.7-μm particles, 100 μm ID × 100 mm; Waters). Data were acquired in data-dependent mode, and selected peptides dissociated by 10- to 30-eV collisions with argon; the collision energy was dynamically changed based on the m/z value and charge state of the ion. The liquid chromatography and electrospray ionization conditions included a flow rate of 600 nl/min, nanoflow capillary voltage of 3.5 kV, block temperature of 100°C, and cone voltage of 100 V.
Peptides were identified from tandem mass spectrometry (MS/MS) analysis, as described previously (23). In brief, raw data files were converted to a peaklist format (mgf) by the software Mascot Distiller 2.1.1. (Matrix Science, London, UK) and analyzed using the search engine Mascot 2.2 (Matrix Science) with variable modifications of TMAB [these are named GIST-Quat (K) and GIST-Quat (N-term) in Mascot]. Mascot searches were followed by manual interpretation to eliminate false positives. Several criteria were used to accept or decline the peptides that were identified by Mascot: 1) >80% of the major MS/MS fragment ions matched predicted a, b, or y ions, or parent ions with loss of trimethylamine; 2) a minimum of 5 fragment ions matched b or y ions; and 3) the number of tags incorporated into the peptide matched the number of free amines (N terminus and side chains of Lys). In addition, the Mascot score was either the top score of all potential sequences, or the other peptides with comparable scores could be excluded by the above criteria, leaving only one top-scoring peptide that matched all criteria. This “manual validation” approach was applied to all of the peptides reported in this study. Quantification was performed by measuring the ratio of peak intensity for the various TMAB-labeled peptides pairs in the MS spectra. For this analysis, the monoisotopic peak and the peaks containing 1 and 2 atoms of 13C were used. Multiple scans of the MS spectra were combined prior to quantitation. For those peptides detected with all 5 TMAB tags (such as the example shown in Fig. 2B), the WT value represented the intensity of each of the WT samples divided by the average of the other 2 WT samples. Because these were, by definition, either 1.0 or close to it, the important parameter is the variation representing the sd of these 3 values (Supplemental Table 1). For the pcd samples, the ratio of each pcd peak intensity vs. the average WT peak intensity was calculated, and the average of the two pcd measurements in each replicate was determined along with sd (which for n=2 corresponds to the range of the 2 values). In cases in which one or more of the peaks could not be detected for the WT samples (Fig. 2C, D), a minimum value based on the detection limit from the background signal was determined and used for calculations, with the resulting value entered as <5.0 (if the detection limit was 1/5 of the peak intensity). In some cases, the background signal allowed for higher or lower thresholds for the increase/decrease (see Supplemental Table 1).
Quantitative proteomics using differential in gel electrophoresis
Brain regions from WT and pcd mice were sonicated on ice in lysis buffer [7 M urea, 2 M thiourea, 30 mM Tris, and 4% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) containing dithiothreitol, 4-amidinophenylmethanesulfonyl fluoride, and pepstatin A]. After sonication, samples were left on ice for 2 h, then centrifuged at 50,000 rpm for 1 h at 20°C. The pH was adjusted to 8.5 with 50 mM Tris, and the protein concentration was determined using the 2D Quant Kit (GE Life Sciences, Piscataway, NJ, USA). The samples were then labeled with CyDye differential in gel electrophoresis (DIGE) minimal dye (GE Life Sciences), according to the manufacturer’s protocol. Replicates were performed in which pcd and WT samples from different mice were labeled with the Cy3 or Cy5 dyes in the reverse order to control for preferential labeling by one of the dyes. Altogether, 2 replicates were performed for the cortex and hypothalamus, and 5 replicates were performed for the cerebellum. The labeled proteins were focused on a 24-cm nonlinear immobilized pH gradient strip of pH range 3–10 for a total of 50,000 V-h and then separated by size on a denaturing 12% polyacrylamide gel. After imaging using the Typhoon 9400 (GE Life Sciences), the gels were analyzed for differentially expressed protein using Decyder 6.5 (DIA module). Another 2D gel of the unlabeled sample (WT or pcd) containing 4 times more protein was run using the same conditions for the Cy3/Cy5-labeled gels then stained with Coomassie dye. The spots of interest based on the Decyder analysis were cut from the Coomassie-stained gel and digested using modified trypsin (Promega, Madison, WI, USA) and analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a Voyager-DE STR (PerSeptive Biosystems, Foster City, CA, USA) in the reflector mode at 20-kV accelerating voltage. The differentially expressed proteins were identified by peptide fingerprinting using ProteinProspector (http://prospector.ucsf. edu/prospector/mshome.htm).
Proteasome activity assay
The measurement of proteasome activity was performed on 4 WT and 4 pcd mice (7–9-mo old) using a protocol related to those previously described (24,25,26). Hypothalamus and cortex were rapidly dissected out, and half of each region was homogenized in 300 μl (hypothalamus) or 400 μl (cortex) of proteasome assay buffer (50 mM Tris, pH 7.5; 40 mM KCl; 5 mM MgCl2; 0.5 mM ATP; and 1 mM DTT). Lysates were centrifuged at 13,000 rpm for 15 min at 4°C, and then an additional 400 μl of the assay buffer was added to the cortex supernatant. The supernatant (40 μl) or the pellet (40 μl; after suspension of the pellet in the same volume of the assay buffer as used for homogenization) was incubated with either 20 μM epoxomicin (Calbiochem, La Jolla, CA, USA) or with equal volume of the assay buffer for 1 h at 37°C. All assays were performed in duplicate. Chymotrypsin-like activity was measured using the substrate succinyl-LLVY-aminomethylcoumarin (suc-LLVY-AMC, 100 μM; Sigma-Aldrich, St. Louis, MO, USA) in a final volume of 200 μl. Incubations were performed at 37°C for 60 or 90 min, and terminated by addition of 2 ml of ice-cold assay buffer. Fluorescence was measured using a Perkin Elmer LS-3B spectrofluorometer (Perkin Elmer, Wellesley, MA, USA) at 380-nm excitation and 460-nm emission. Specific chymotrypsin-like proteasome activity was expressed as nmol AMC/min/mg of the total protein. Protein content was measured using a Bradford protein assay (Bio-Rad, Hercules, CA, USA).
Amino acid measurements
Brain regions from 4 WT and 4 pcd mice used for the proteasome activity measurement (described above) were divided into two halves; one half was used for proteasome activity, the other half was weighed and homogenized in 100 mM HCl for amino acid measurements. In addition to the cortex and hypothalamus described above, the amygdala was also dissected and used for amino acid measurements. The homogenate was centrifuged at 4°C for 15 min at 13,000 rpm, and the supernatant was removed, frozen, and lyophilized. To measure amino acid levels, the extracts were resuspended in 0.2 N sodium citrate, pH 2.2, and the amino acid contents were determined using a Shimadzu HPLC-LC10A/C-R7A system (Shimadzu Corporation, Tokyo, Japan), with o-phthalaldehyde as the derivatizing reagent. The equipment was calibrated with an amino acid standard (Sigma-Aldrich) containing 125 pmol of each amino acid, as described previously (27).
Immunohistochemistry
Young (2- to 3-wk-old) and adult (7- and 22-wk-old) WT and pcd mice were anesthetized with ether and transcardially perfused with 20 ml of cold PBS, pH 7.4, followed by 20 ml of cold 4% paraformaldehyde (PFA). Brains were removed and postfixed in 4% PFA overnight at 4°C, cryoprotected with 30% sucrose in PBS for 6 h, embedded in optimal cutting temperature (OCT) compound, and frozen in isopentane at −50°C. Brain and cerebellum coronal sections (14 μm thick) and eye horizontal and vertical sections (10 μm thick) were cut on a sliding microtome at −20°C and thaw mounted onto superfrost/Plus slides (Fisher Scientific, Hampton, NH, USA). After being washed in PBS, the sections were blocked with 5% BSA in PBS containing 0.5% Triton X-100 for 1.5–2 h at room temperature and incubated for 24 h at 4°C with either anti-LC3 (1:400 dilution; Novus Biologicals, Manchester, UK), anti-Calbidin-D28k (Sigma-Aldrich, 1:3000), or anti-phospho-p70-S6 kinase (1:50; Cell Signaling Technology, Danvers, MA, USA). After washing with PBS containing 0.2% Tween-20, secondary Cy3- and Cy2-conjugated antibodies (Jackson Immunoresearch, West Grobe, PA, USA) were applied. After overnight incubation at 4°C, the sections were washed and mounted in antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA, USA).
RESULTS
A quantitative peptidomics technique was used to examine peptides in several brain regions of pcd mice. The brain regions chosen for this analysis have higher levels of CCP1/Nna1 mRNA than other members of the CCP family (9, 10) but are not known to undergo neurodegeneration. Thus, changes in peptide levels in these brain regions between pcd and WT mice should reflect the consequences of CCP1/Nna1 absence and not secondary changes due to cell death. Altogether, the hypothalami and amygdalas from 6 pcd mice and 9 WT mice were individually analyzed using the peptidomics scheme shown in Fig. 2A, with one set of mutant and WT mice labeled using the strategy on the left and two other sets of mutant and WT mice labeled using the strategy on the right. In addition, the cortex, prefrontal cortex, and striatum from 2 mutant and 3 WT mice were analyzed using the strategy on the right. For the vast majority of peptides, results among replicates were highly reproducible for the same brain region as well as among brain regions (Supplemental Table 1). Over 2000 peptides were detected in the 9 LC/MS runs performed for this study, and ∼1/3 of these were identified by MS/MS sequencing using Mascot and manual verification of the data. The 795 identified peptides in each of the LC/MS runs represented 260 distinct peptides, with most of these found in multiple replicates from the same brain region and/or from the different brain regions analyzed. Of the 260 distinct peptides, 99 are neuropeptides and other peptides derived from secretory pathway proteins, and 161 are fragments, primarily of cytosolic and mitochondrial proteins. Because the focus of the present study was on peptides that could be substrates for CCP1, the secretory pathway peptides were not further considered. The 161 peptides derived from cytosolic, mitochondrial, and other nonsecretory pathway proteins represent 42 different protein precursors (Supplemental Table 1).
Some of the peptides showed no difference in levels between the pcd and WT mouse brain (Supplemental Table 1), with mass spectra showing 5 peaks of roughly equal peak height (Fig. 2B). Other peptides were detected as peak pairs corresponding to the isotopic tags used to label the pcd mouse brain extracts, with no detectable signals from the WT samples (Fig. 2C, D). When no signal was detected for the peptide in WT mice, the ratio of the signal intensity in the pcd vs. WT mice was usually listed in Supplemental Table 1 as >5.0 based on the detection limit due to background noise; the actual increase in the pcd mouse brain could be much larger than 5-fold. A small number of peptides showed a higher signal for the 3 peaks corresponding to the isotopic tags from the WT mice, with weaker signals from the pcd mouse brain extracts (Fig. 2E). In this example and all others showing a similar trend, there was considerable variability in the level among the WT mice, which is reflected in the large sd shown in Supplemental Table 1. The ratio of peak intensity for the two pcd peaks relative to the average WT peak intensity is indicated in Supplemental Table 1 for each of the replicates in which the peptide was detected.
The peptides were divided into one of five groups based on the ratio of the peptide in the pcd mouse relative to the level in the corresponding WT mouse brain region within each LC/MS run. One group was present in the pcd mice at levels less than 1/3 of those in the WT mice (pcd/WT ratio<0.33), and there was only one peptide in this group (Supplemental Table 1). The second group showed a small decrease in the pcd mice, with levels between 1/3 and 2/3 of the WT levels (ratio 0.33 to 0.66); there were 6 peptides in this group. The third group was generally similar between pcd and WT mice, with a ratio between 2/3 and 3/2 (0.67 to 1.49); there were 89 peptides in this group. The fourth group was slightly elevated in the pcd mice, with a ratio between 3/2 and 3 (1.5 to 3.0); there were 24 peptides in this group. The fifth group was elevated in the pcd mice to a level >3.0 times that of WT mice (ratio>3.0); most of the peptides detected in the present study fell into this group, with 455 peptides (Supplemental Table 1). These divisions are somewhat arbitrary, and some replicates of a peptide were divided between neighboring groups (big vs. small increase or decrease, or no change vs. small increase or decrease). The simplest explanation for the increase in levels in the pcd mouse brain is that these peptides represent CCP1/Nna1 substrates, which are normally rapidly degraded by the enzyme, and which are therefore greatly elevated in the absence of the enzyme. In addition, it is likely that peptides unaffected by the mutation are not CCP1/Nna1 substrates.
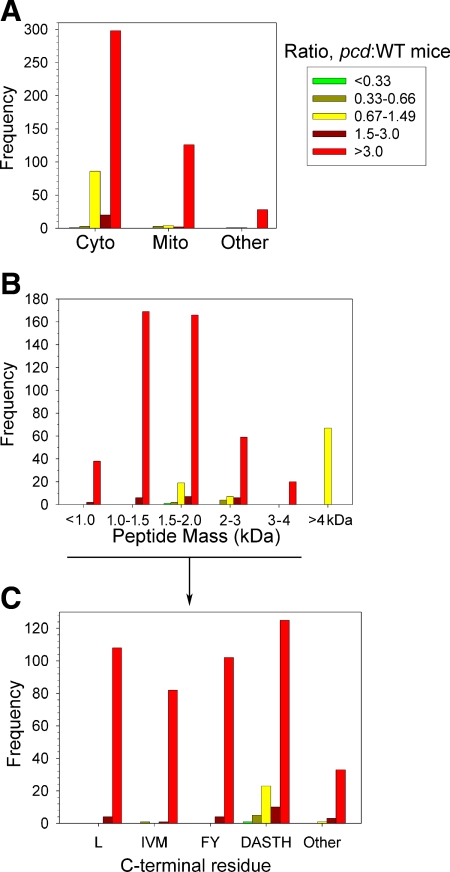
To determine whether there are physical or physiological features that distinguish the peptides affected by the CCP1/Nna1 mutation from those unaffected by the mutation, the data were further broken down into subsets based on the subcellular location of the protein. The majority of peptides derived from cytosolic proteins were greatly elevated in the pcd mice (Fig. 3A, red bars), although a sizable number of cytosolic peptides were present in the pcd and WT mice at generally equal levels (Fig. 3A, yellow bars). In contrast, virtually all of the peptides derived from mitochondrial, nuclear, lysosomal, and membrane proteins were greatly increased in the pcd mice (Fig. 3A). When the results were sorted by mass, all of the peptides that greatly increased in the pcd mice (Fig. 3B, red bars) were <4 kDa while ∼75% of the peptides unchanged in the pcd mice (Fig. 3B, yellow bars) were >4 kDa. This result suggests that CCP1/Nna1 has a preference for smaller peptides and is not able to cleave peptides >4 kDa. Further analysis of the C-terminal amino acid of the peptides under 4 kDa was undertaken to test whether there is an amino acid preference for CCP1/Nna1 substrates. Leu was the most common C-terminal residue, and peptides with this C-terminal residue were either greatly or partially increased in the pcd mice (Fig. 3C). In addition, other hydrophobic residues such as Ile, Val, Met, Phe, and Tyr were, with one exception, not found in the nonsubstrate group. Peptides with small and/or polar C-terminal residues also appeared to be substrates, with the majority of these peptides in the group that was greatly increased in the pcd mice (Fig. 3C). However, ∼15% of the peptides with a C-terminal Asp, Ala, Ser, Thr, or His were found in the unaffected group (Fig. 3C), suggesting that although CCP1/Nna1 can cleave a wide range of amino acids, the enzyme has a slight preference for hydrophobic residues. The number of peptides that were unaffected in the mutant mice was too small to permit further analysis of additional residues that may contribute to substrate preference (such as the P2 position).
Figure 3.
Summary of quantitative peptidomics results. All identified peptides were divided into 5 categories: greatly decreased in pcd mice compared to WT (pcd:WT ratio<0.33); slightly decreased (pcd:WT ratio 0.33–0.66); peptides that are present in roughly equal levels in pcd and WT brains (pcd:WT ratio 0.67–1.49); peptides that are slightly increased in pcd mice (pcd:WT ratio 1.5–3.0); and greatly increased in mutants (pcd:WT ratio>3.0). The y-axes indicate number of lines of Supplemental Table 1 that match each category (each line of Supplemental Table 1 represents a peptide in an LC/MS run). A) Subcellular location of protein precursor of peptides. Cyto, cytosol; Mito, mitochondria; other, includes nucleus, lysosomes, and plasma membrane. B) Peptides derived from cytosolic, mitochondrial, and other nonsecretory pathway proteins were divided into the indicated mass ranges. C) Analysis of the C-terminal residues of the <4-kDa peptides from cytosolic, mitochondrial, and other nonsecretory vesicle proteins. To provide a larger n for each group, several similar amino acids were pooled in this analysis. Leu constituted a large enough group to be analyzed individually. Pools of data included aliphatic (Ile, Val, Met), aromatic (Phe, Tyr), some small and/or polar side chain (Asp, Ala, Ser, Thr, His), and other residues (Gly, Asn, Gln, Cys, Glu, Lys, and Arg). Neither Trp nor Pro was detected in the C-terminal position in any of the observed peptides, so these residues could not be included in the analysis.
On the basis of the results of the peptidomics analysis of mouse brain, it is likely that CCP1/Nna1 plays a role in the degradation of peptides produced by the proteasome (Fig. 1). However, there are alternative possible explanations of the data. For example, if CCP1/Nna1 has a global effect on protein expression and/or turnover, the changes in peptide levels in pcd mutant mouse brain could arise from changes in proteins and/or their degradation. To test this, two approaches were applied; a quantitative proteomics technique using 2D-DIGE labeling was performed to compare protein levels in the WT and pcd mouse cortex (Fig. 4) and hypothalamus (data not shown), and the chymotryptic activity of the proteasome was assayed for these two brain regions of WT and pcd mice (Fig. 5). The 2D-DIGE showed no substantial difference in relative levels of proteins between WT and pcd mouse cortex (Fig. 4). As a control, we also compared cerebellum from adult WT and pcd animals, expecting to see some protein changes based on the loss of Purkinje cells in the mutant mice. While the majority of the proteins in the cerebellum were present at comparable levels between the WT and pcd mice, a handful of proteins showed decreased levels (green spots, Fig. 4) or increased levels (red spots, Fig. 4) in the pcd mice. Four of the proteins that changed consistently among 5 replicate gels, including 2 gels run with the reverse labeling scheme, were identified by tryptic peptide fingerprinting. The other proteins that appear red or green in Fig. 4 either did not change consistently and significantly among replicates, or else they were not identified. One protein that decreased in the pcd mice (synaptosomal-associated 25-kDa protein) is known to be selectively expressed in Purkinje cells and not in glia (28), while the 3 proteins that increased in the pcd mice (peroxiredoxin 6, glial fibrillary acidic protein, and glutamine synthetase) are selectively expressed in glia (29, 30). This finding is consistent with the loss of Purkinje cells and increase in glia in the mutant mice (31).
Figure 4.
Proteomics analysis of adult WT and pcd mouse brain, analyzed using 2-D-differential in-gel electrophoresis (2D-DIGE). Brain regions from WT mice were labeled with Cy3 (green); cortex and cerebellum from pcd mice were labeled with Cy5 (red). Replicate samples from additional mice were labeled with reverse orientation of dyes. Left panel: cortex. No changes in spot intensity between WT and pcd cortex were detected; all spots appear yellow. Right panel: cerebellum. Several spots were higher in the WT samples than the pcd samples (green); other spots were higher in the pcd samples (red). Spots confirmed to change in a total of 5 replicate gels from distinct animals are numbered. Bottom: cerebellar proteins that changed consistently were identified by mass spectrometry. MW Obs, observed molecular weight; pI Obs, observed isoelectric point; pcd/WT, spot volume ratio for the pcd sample vs. the WT sample (n=5). Difference in spot volume between the two samples was found to be statistically significant using Student’s t test; P values are indicated.
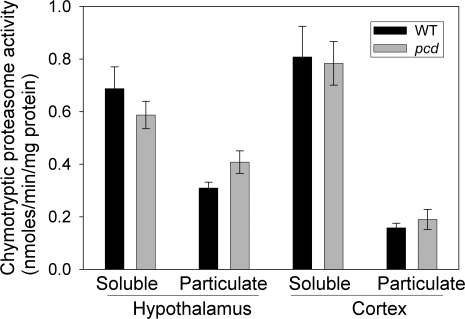
Figure 5.
Chymotrypsin-like proteasome activity in the hypothalamus and cortex of 7- to 9-mo-old WT and pcd mice. Activities of soluble and particulate fractions from the hypothalamus (left) and cortex (right) of WT and mutant animals were measured by detecting the fluorescence of free AMC released after the hydrolysis of fluorogenic substrate Suc-LLVY-AMC (excitation 380 nm, emission 460 nm). There was no statistical difference between the level of proteasome activity in WT vs. pcd mice for either the soluble or particulate fractions of the hypothalamus or cortex.
The proteasome assay was conducted using a substrate selective for the chymotrypsin-like active site, which represents the major activity of the proteasome. Because the majority of peptides that increased in the pcd mice resulted from chymotryptic-like cleavages (Supplemental Table 1), this was the most important subunit to test for up-regulation in the mutant mice. There was no significant difference in proteasome activity between WT and pcd mice for either of the brain regions examined (Fig. 5). Although there was a slight trend toward lower levels of proteasome activity in the soluble fraction of pcd mouse hypothalamus, this difference was not statistically significant and was offset by a small trend toward higher proteasome activity in the particulate fraction of mutant mouse hypothalamus (Fig. 5). The total levels of proteasome activity in the hypothalamus and cortex were unchanged between the WT and mutant mice.
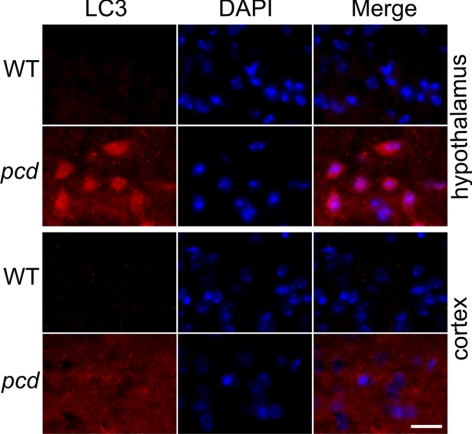
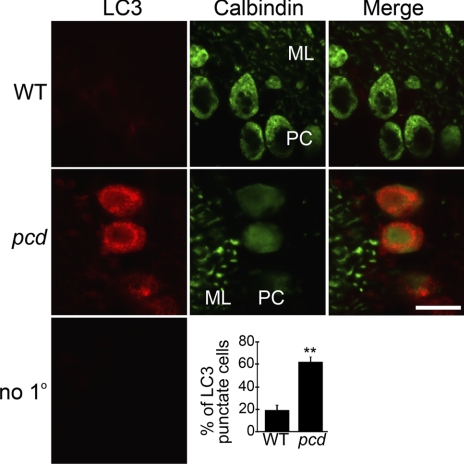
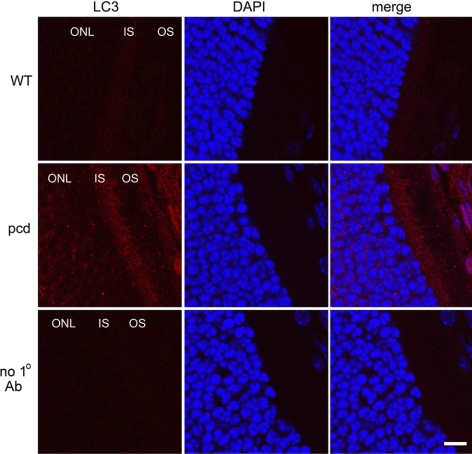
The above results on peptide, protein, and proteasome activity levels in the WT vs. pcd mice support the hypothesis that CCP1/Nna1 is involved in the conversion of proteasomally produced peptides into amino acids. In the absence of CCP1/Nna1 activity, the dramatic build-up in levels of cytosolic peptides predicts that cellular levels of amino acids will be lower in pcd mouse brain. To test this, we examined levels of amino acids in several brain regions (Supplemental Table 2). Although there was a trend toward lower levels of amino acids in the amygdala and hypothalamus of pcd mice, with levels ∼88–89% of WT levels, the differences were not statistically significant, except for the level of Asp, which decreased in the pcd amygdala (85% of WT levels), and Gly, which increased in the pcd cortex (158% of WT levels). Because amino acids in extracts of brain regions reflect levels in many different cell types, whereas the absence of Nna1/CCP1 in the pcd mice is likely to have an effect on a more limited number of cell types (based on the selective loss of Purkinje and several other cell types), we also examined pcd mouse brain for signs of autophagy, which is known to be triggered by decreased cellular levels of amino acids (17, 32, 33). Adult pcd mice showed punctate LC3 staining in hypothalamus and cortex, whereas WT mice showed less intense staining and very few cells with puncta (Fig. 6). Similar differences between WT and pcd mice were also observed in amygdala of the adult mice, with comparable results observed in multiple sections from different WT mice and pcd mice at either 7 or 22 wk of age (data not shown). Similarly, Purkinje cells of 2- to 3-wk-old pcd mice showed a punctate distribution for LC3, whereas few WT Purkinje cells from age-matched mice showed LC3 puncta (Fig. 7). Quantitation by a researcher masked to the genotype revealed significantly more Purkinje cells with punctate LC3 distribution in the pcd mice compared to WT mice (Fig. 7, bottom panel). Other cell types in the cerebellum of 3-wk-old pcd mice did not show punctate LC3 distribution (Fig. 7). Retinal sections from 3-wk-old pcd mice showed more LC3 staining in the outer nuclear layer and the inner segment than age-matched WT mice, consistent with elevated autophagy in retinal photoreceptors (Fig. 8).
Figure 6.
Distribution of LC3 in 22-wk-old WT and pcd hypothalamus and cortex. Two top panels show WT and pcd hypothalamic sections; two bottom panels show sections of WT and pcd cortex. Tissues were probed with an antibody against LC3 (marker for autophagosome) and with DAPI (marker for cell nuclei). LC3 shows more intense and punctate staining in the hypothalamus and cortex of pcd mice. Comparable results were seen with 7-wk-old WT and pcd mice (data not shown). Scale bar = 10 μm.
Figure 7.
Distribution of LC3 in Purkinje cells of 3-wk-old WT and pcd cerebellum. Top and middle panels show WT and pcd cerebellar sections, respectively, both subjected to immunostaining with antibodies against LC3 (a marker for autophagosome) and calbindin (a marker for Purkinje cells). Molecular layer (ML) shows Purkinje cell dendrites (green); Purkinje cell layer (PC) shows Purkinje cell bodies (green). LC3 (red) shows a diffuse distribution in Purkinje cells of WT mice. In pcd Purkinje cells, LC3 shows strong staining with a punctate distribution in the cell bodies. Bottom panel shows cerebellar section probed with secondary antibodies only (negative control). Percentage of LC3 punctate cells in cerebellum of WT and pcd mice was determined by an observer who was masked to the genotype. At least 40 calbindin-positive cells were counted in each cerebellar section; several sections were analyzed for each mouse; 3 mice/group. **P < 0.01; Student’s t test. Scale bar = 10 μm.
Figure 8.
Distribution of LC3 in 3-week-old WT and pcd retina. Upper and middle panels show WT and pcd retinal sections, respectively, subjected to immunostaining with an antibody against LC3 (marker for autophagosome) and stained with DAPI (marker for cell nuclei). LC3 shows more intense and punctate staining in the retina of pcd mice. Bottom panel shows retinal section probed with secondary antibodies only (negative control). ONL, outer nuclear layer; IS, inner segments; OS, outer segments. Scale bar = 10 μm.
DISCUSSION
A major finding of the present study is that pcd mice show greatly elevated levels of many peptides derived from cytosolic and mitochondrial proteins. The vast majority of the peptides in cortex, hypothalamus, amygdala, and other brain regions examined are affected by the mutation, with >90% of the peptides smaller than 4 kDa showing a dramatic increase in the pcd mouse brain. This result is not limited to the 161 peptides identified in the present study; the vast majority of the ∼1000 unidentified peptides also showed similar increases in the pcd mice (data not shown). Thus, the effect of the mutation of CCP1/Nna1 is broad, implying a role in a general cellular process. Although other CCPs are also present in many of the same brain regions as CCP1, their levels are likely to be much lower based on their mRNA levels (10), and therefore, they are not likely to be able to fully compensate for the defect in CCP1/Nna1 in the pcd mice. The simplest interpretation of these data is that CCP1/Nna1 functions in protein turnover following the action of endoproteases, such as the proteasome, which does not directly convert proteins into amino acids but instead generates small peptides. Because peptides cannot be directly recycled into new proteins, they must be degraded by additional cellular peptidases (34). While it intuitively makes sense that efficient peptide degradation proceeds from both ends using amino- and carboxypeptidases, only cytosolic aminopeptidases are thought to contribute to protein turnover, and cytosolic carboxypeptidases have not previously been implicated (2, 3, 34). This conclusion was largely based on a study incubating cytosolic extracts that had been centrifuged for 7 h at 100,000 g with an 11-mer peptide; this study detected mainly N-terminal trimming and only small amounts of C-terminal trimming of the peptide (2). It is likely that this previous study did not detect CCP1/Nna1 activity for at least two reasons; first, on the basis of the peptidomics results, CCP1/Nna1 appears to convert peptides mainly into amino acids or small peptides not readily detected by mass spectrometry. Second, although CCP1/Nna1 is cytosolic, the vast majority of the protein is particulate when extracts are centrifuged (unpublished results), and therefore little CCP1/Nna1 would have been present in the extracts tested by Beninga et al. (2).
While the simplest explanation of the peptidomics results is that CCP1/Nna1 cleaves many cytosolic peptides into amino acids (Fig. 1), alternative explanations of the peptidomics data are possible. For example, if CCP1/Nna1 inhibited proteasome activity, the absence of CCP1/Nna1 activity in the pcd mice could cause an up-regulation of endoproteolytic activity but not the subsequent degradative steps, leading to an increase in levels of peptides derived from cytosolic and mitochondrial proteins. Another possibility could be that the CCP1/Nna1 mutation caused an increase in protein levels, and this, in turn, caused the increase in peptide levels. However, the proteasome activity, as measured with a substrate for the chymotrypsin-like subunit, was no different in the pcd and WT mouse brain. The 2-dimensional gel analysis showed comparable levels of all major proteins in cortex and hypothalamus, and nearly all proteins in cerebellum, suggesting that there were no substantial changes in protein levels in the pcd mice. Therefore, it is most likely that the simplest explanation is correct, and that CCP1/Nna1 functions in the cleavage of peptides formed after the proteasome degrades proteins. A role for CCP1/Nna1 in protein turnover would be consistent with the finding that Nna1 mRNA levels are greatly increased on sciatic nerve transection or crush injury (9), treatments that are likely to cause increased protein turnover.
On the basis of the analysis of the C-terminal residue of the peptides that accumulate in pcd mice, CCP1/Nna1 appears to have a broad substrate specificity and cleave aromatic, aliphatic, basic, acidic, and other polar and nonpolar residues. A broad specificity for CCP1/Nna1 was predicted from modeling of this enzyme’s substrate-binding pocket, based on the known cleavage sites of distantly related metallocarboxypeptidases such as carboxypeptidases A, B, and D (11). CCP1/Nna1 appears to be distinct from the other metallocarboxypeptidases that have been previously studied in that peptides >4 kDa are not substrates for the enzyme (Fig. 3B), whereas other carboxypeptidases can cleave C-terminal residues from proteins (35). The size preference of CCP1/Nna1 can explain the difference between cytosolic and mitochondrial peptides observed in Fig. 3A; all of the peptides derived from mitochondrial proteins were <2.5 kDa, whereas in the cytosol, some small proteins of 5–16 kDa were detected, and these were not elevated in the pcd mice. The inability of CCP1/Nna1 to cleave proteins would prevent nonspecific degradation of cellular proteins. A potential mechanism to account for this size preference, and also explain other observations, is a process by which energy is required (and possibly another cofactor) to allow a peptide <4 kDa to enter the active site and become trapped until it is completely degraded into amino acids or small peptides. This model would explain the lack of detection of peptides from cytosolic and/or mitochondrial proteins which decreased in the pcd mice (i.e., CCP1/Nna1 products), unlike related peptidomics analysis of mice lacking carboxypeptidase E activity, which show both a large increase in substrates and a large decrease in products (21, 36, 37). This model for CCP1/Nna1 is compatible with a previous study that found a C. elegans CCP6 ortholog had carboxypeptidase activity that was enhanced by ATP and GTP (11) and by the presence of a putative ATP/GTP binding domain located within the N-terminal domain predicted to function as a regulatory domain (9,10,11). CCP1/Nna1 and all other CCPs contain a conserved ∼100 residue region to the N-terminal side of the conserved carboxypeptidase-like domain; this N-terminal domain is in a position homologous to the activation domain of procarboxypeptidase A and B. However, unlike procarboxypeptidase A and B, which are cleaved to generate the enzymatically active forms, there is no evidence that the N-terminal domain of the CCPs is cleaved (10, 11). Thus, it is possible that this N-terminal domain serves a function similar to the regulatory subunits of the proteasome (1) or the cytosolic prolyl endopeptidase (38); regulatory domains on these proteases restrict entry and exit of substrates to the enzyme’s active site. Taken together, these various findings are consistent with the model in which CCP1/Nna1 functions in an analogous way as the proteasome; substrates require energy to be loaded into the enzyme and then once in, the substrate cannot leave until degraded.
The observed difference between LC3 distribution in the WT and pcd mouse brain is consistent with amino acid starvation due to a deficiency in the recycling pathway from peptide to amino acid. Supporting this, Valero et al. (39) found evidence for inhibition of global transcription, modification in the cytoplasmic translation machinery, and the replacement of rough endoplasmic reticulum cisternae by a larger amount of free ribosomes. These changes could be due to the decrease in free amino acid levels in the pcd mice. Alternatively, it is possible that the increased levels of intracellular peptides in the pcd mouse brain interfere with cellular processes and cause some of the changes noted by many previous studies (39,40,41,42,43,44). The introduction of peptides into cells can influence protein-protein interactions (45), and it is possible that the build-up of one or more peptides in the pcd mice causes changes in cellular processes.
Previous studies on genes differentially expressed in WT and pcd mouse brain identified hundreds of genes that were up- or down-regulated in the pcd mice, although there was little overlap among the three different studies (18, 44, 46). Many of the genes found to be altered in the pcd mouse brain function in metabolism, signaling, and other physiological processes, consistent with the changes observed using peptidomics analysis in the present study. However, there was no overlap between the genes identified from microarray studies (18, 44, 46) and the genes encoding the 42 proteins that were detected in the present study (Supplemental Table 1), suggesting that the dramatic increases in many peptides detected in the present study is not simply due to increased transcription. This interpretation is also supported by the results of the 2D gel analysis showing similar levels of proteins between WT and pcd mouse cortex (Fig. 4).
Although the peptidomics analysis was performed using brain regions that do not show neural degeneration in the pcd mice, it is likely that the peptide changes seen in the regions studied are similar to those that occur in cell types destined to die in the pcd mice. Since Purkinje cells have undetectable levels of other CCPs, which may partially compensate for the absence of CCP1/Nna1, the changes in peptide and amino acid levels in this cell type should be more pronounced than in cells that contain other CCPs in addition to CCP1/Nna1. The peptidomics results, and likely role for CCP1/Nna1 in protein turnover, provides two possible theories that could contribute to cell death of Purkinje and selected other cells. First, the large accumulation of intracellular peptides could interfere with protein-protein interactions in the pcd mice, as described above, and this could cause neurodegeneration in some cell types. Second, the absence of CCP1/Nna1 would be expected to lead to reduced amino acid levels. Although levels of most amino acids in extracts of pcd mouse amygdala, cortex, or hypothalamus were not significantly different from levels in WT brain, two amino acids were significantly altered. Asp, which was found to be 15% lower in pcd amygdala in the present study, was previously found to decrease 45–60% in the cerebellum of adult pcd mice (47). Similarly, Gly, which was found to be 58% higher in the adult pcd mouse cortex in the present study, was previously found to be 43–100% elevated in adult pcd mouse cortex (47). Two other amino acids, Glu and Ala, were also examined in the previous study and found to decrease in the pcd mouse cortex (47). However, because of the Purkinje cell death that occurs, it is hard to interpret changes in amino acid levels in the cerebellum. In the present study, the lack of major changes for most amino acids in extracts of amygdala, cortex, and hypothalamus suggests that the majority of cells in these regions have mechanisms to compensate for the absence of Nna1/CCP1 in the pcd mice. Evidence for this possibility was provided by the punctate distribution of LC3 in the cerebellar Purkinje cells of 2- to 3-wk-old pcd mice and in many brain regions of adult pcd mice; the increase in punctate LC3 distribution is a marker for autophagy, which is induced by amino acid starvation. Previous analysis of Purkinje cells and retinal photoreceptors in the pcd mouse also suggested autophagy was elevated in the mutant mice (13, 40). In support of this, elevated LC3 staining was observed in the 3-wk-old pcd mouse retina in the present study (Fig. 8). In addition, punctate LC3 staining in Purkinje cells of young pcd mice was recently reported (48), consistent with our results for these cells. While it is possible that the increase in autophagy is the first step eventually leading to Purkinje cell death through apoptosis (49), it is also possible that autophagy is a protective response that reduces the impact of amino acid starvation due to loss of CCP1/Nna1 by recycling nonessential cellular components.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants DK-51271 and DA-04494 (to L.D.F.), and by Fundação de Amparo a Pesquisa do Estado de São Paulo 04/04933-2, 04/14846-0 and Financiadora de Estudos e Projetos A-03/134 (to E.S.F.). Thanks are also due to fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (E.S.F. and L.M.C.). Mass spectrometry was performed through the Rede de Proteoma do Estado de São Paulo in the Laboratório Nacional de Luz Sincrotron (Campinas, SP, Brazil). We are thankful to Prof. Fabio Gozzo (Universidade de Campinas, Campinas, SP, Brazil) for his immeasurable support with mass spectrometry. Thanks are due to Julia Grushko and Jonathan Wardman for assistance with the handling of the pcd mice. Fluorescence microscopy was performed in the laboratory of Dr. Jonathan M. Backer (Department of Molecular Pharmacology), and confocal microscopy was performed in the Analytical Imaging Facility of the Albert Einstein College of Medicine.
References
- Goldberg A L. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Beninga J, Rock K L, Goldberg A L. Interferon-gamma can stimulate post-proteasomal trimming of the N terminus of an antigenic peptide by inducing leucine aminopeptidase. J Biol Chem. 1998;273:18734–18742. doi: 10.1074/jbc.273.30.18734. [DOI] [PubMed] [Google Scholar]
- Goldberg A L, Cascio P, Saric T, Rock K L. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Arolas J L, Vendrell J, Aviles F X, Fricker L D. Metallocarboxypeptidases: emerging drug targets in biomedicine. Curr Pharm Des. 2007;13:349–366. doi: 10.2174/138161207780162980. [DOI] [PubMed] [Google Scholar]
- Mullen R J, Eicher E M, Sidman R L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976;73:208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman S, Sidman R L. Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. I. Distribution of neuron loss. J Comp Neurol. 1985;234:277–297. doi: 10.1002/cne.902340302. [DOI] [PubMed] [Google Scholar]
- Wang T, Morgan J I. The Purkinje cell degeneration (pcd) mouse: an unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 2007;1140:26–40. doi: 10.1016/j.brainres.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez A, La Spada A R, Treadaway J, Higdon J C, Harris B S, Sidman R L, Morgan J I, Zuo J. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295:1904–1906. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- Harris A, Morgan J I, Pecot M, Soumare A, Osborne A, Soares H D. Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol Cell Neurosci. 2000;16:578–596. doi: 10.1006/mcne.2000.0900. [DOI] [PubMed] [Google Scholar]
- Kalinina E, Biswas R, Berezniuk I, Hermoso A, Aviles F X, Fricker L D. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 2007;21:836–850. doi: 10.1096/fj.06-7329com. [DOI] [PubMed] [Google Scholar]
- Rodriguez de la Vega M, Sevilla R G, Hermoso A, Lorenzo J, Tanco S, Diez A, Fricker L D, Bautista J M, Aviles F X. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007;21:851–865. doi: 10.1096/fj.06-7330com. [DOI] [PubMed] [Google Scholar]
- Wang T, Parris J, Li L, Morgan J I. The carboxypeptidase-like substrate-binding site in Nna1 is essential for the rescue of the Purkinje cell degeneration (pcd) phenotype. Mol Cell Neurosci. 2006;33:200–213. doi: 10.1016/j.mcn.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Eng J, Martinez R A, Jackson S, Huang J, Possin D E, Sopher B L, La Spada A R. The zinc-binding domain of Nna1 is required to prevent retinal photoreceptor loss and cerebellar ataxia in Purkinje cell degeneration (pcd) mice. Vision Res. 2008;48:1999–2005. doi: 10.1016/j.visres.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L D, Lim J, Pan H, Che F-Y. Peptidomics: Identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom Rev. 2006;25:327–344. doi: 10.1002/mas.20079. [DOI] [PubMed] [Google Scholar]
- Che F-Y, Lim J, Biswas R, Pan H, Fricker L D. Quantitative neuropeptidomics of microwave-irradiated mouse brain and pituitary. Mol Cell Proteomics. 2005;4:1391–1405. doi: 10.1074/mcp.T500010-MCP200. [DOI] [PubMed] [Google Scholar]
- Svensson M, Skold K, Nilsson A, Falth M, Svenningsson P, Andren P E. Neuropeptidomics: expanding proteomics downwards. Biochem Soc Trans. 2007;35:588–593. doi: 10.1042/BST0350588. [DOI] [PubMed] [Google Scholar]
- Chang Y Y, Juhasz G, Goraksha-Hicks P, Arsham A M, Mallin D R, Muller L K, Neufeld T P. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37:232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- Rong Y, Wang T, Morgan J I. Identification of candidate Purkinje cell-specific markers by gene expression profiling in wild-type and pcd(3J) mice. Brain Res Mol Brain Res. 2004;132:128–145. doi: 10.1016/j.molbrainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Che F Y, Zhang X, Berezniuk I, Callaway M, Lim J, Fricker L D. Optimization of neuropeptide extraction from the mouse hypothalamus. J Proteome Res. 2007;6:4667–4676. doi: 10.1021/pr060690r. [DOI] [PubMed] [Google Scholar]
- Zhang R, Sioma C S, Thompson R A, Xiong L, Regnier F E. Controlling deuterium isotope effects in comparative proteomics. Anal Chem. 2002;74:3662–3669. doi: 10.1021/ac025614w. [DOI] [PubMed] [Google Scholar]
- Che F-Y, Biswas R, Fricker L D. Relative quantitation of peptides in wild type and Cpefat/fat mouse pituitary using stable isotopic tags and mass spectrometry. J Mass Spectrom. 2005;40:227–237. doi: 10.1002/jms.742. [DOI] [PubMed] [Google Scholar]
- Morano C, Zhang X, Fricker L D. Multiple isotopic labels for quantitative mass spectrometry. Anal Chem. 2008;80:9298–9309. doi: 10.1021/ac801654h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti D A, Morano C, Russo L C, Castro L M, Cunha F M, Zhang X, Sironi J, Klitzke C F, Ferro E S, Fricker L D. Analysis of intracellular substrates and products of thimet oligopeptidase (EC 3.4.24.15) in human embryonic kidney 293 cells. J Biol Chem. 2009;284:14105–14116. doi: 10.1074/jbc.M807916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev A F, Goldberg A L. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 2005;398:364–378. doi: 10.1016/S0076-6879(05)98030-0. [DOI] [PubMed] [Google Scholar]
- Bett J S, Goellner G M, Woodman B, Pratt G, Rechsteiner M, Bates G P. Proteasome impairment does not contribute to pathogenesis in R6/2 Huntington’s disease mice: exclusion of proteasome activator REGγ as a therapeutic target. Hum Mol Genet. 2006;15:33–44. doi: 10.1093/hmg/ddi423. [DOI] [PubMed] [Google Scholar]
- Kwak M K, Huang B, Chang H, Kim J A, Kensler T W. Tissue specific increase of the catalytic subunits of the 26S proteasome by indirect antioxidant dithiolethione in mice: enhanced activity for degradation of abnormal protein. Life Sci. 2007;80:2411–2420. doi: 10.1016/j.lfs.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Tonelli R R, Silber A M, Almeida-de-Faria M, Hirata I Y, Colli W, Alves M J. L-proline is essential for the intracellular differentiation of Trypanosoma cruzi. Cell Microbiol. 2004;6:733–741. doi: 10.1111/j.1462-5822.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K, Grant N J. Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia. 1999;27:181–187. doi: 10.1002/(sici)1098-1136(199908)27:2<181::aid-glia8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Condorelli D F, Dell'Albani P, Kaczmarek L, Messina L, Spampinato G, Avola R, Messina A, Giuffrida Stella A M. Glial fibrillary acidic protein messenger RNA and glutamine synthetase activity after nervous system injury. J Neurosci Res. 1990;26:251–257. doi: 10.1002/jnr.490260216. [DOI] [PubMed] [Google Scholar]
- Power J H, Asad S, Chataway T K, Chegini F, Manavis J, Temlett J A, Jensen P H, Blumbergs P C, Gai W P. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A C, Ghetti B. Embryonic cerebellar graft development during acute phase of gliosis in the cerebellum of pcd mutant mice. Chin J Physiol. 1993;36:141–149. [PubMed] [Google Scholar]
- Petiot A, Pattingre S, Arico S, Meley D, Codogno P. Diversity of signaling controls of macroautophagy in mammalian cells. Cell Struct Funct. 2002;27:431–441. doi: 10.1247/csf.27.431. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Mammucari C, Sandri M. The role of autophagy in neonatal tissues: just a response to amino acid starvation? Autophagy. 2008;4:727–730. doi: 10.4161/auto.6143. [DOI] [PubMed] [Google Scholar]
- Saric T, Graef C I, Goldberg A L. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–46732. doi: 10.1074/jbc.M406537200. [DOI] [PubMed] [Google Scholar]
- Lyons P J, Callaway M B, Fricker L D. Characterization of carboxypeptidase A6, an extracellular-matrix peptidase. J Biol Chem. 2008;283:7054–7063. doi: 10.1074/jbc.M707680200. [DOI] [PubMed] [Google Scholar]
- Fricker L D. Neuropeptidomics to study peptide processing in animal models of obesity. Endocrinology. 2007;148:4185–4190. doi: 10.1210/en.2007-0123. [DOI] [PubMed] [Google Scholar]
- Zhang X, Che F Y, Berezniuk I, Sonmez K, Toll L, Fricker L D. Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. J Neurochem. 2008;107:1596–1613. doi: 10.1111/j.1471-4159.2008.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Khosla C. Prolyl endopeptidases. Cell Mol Life Sci. 2007;64:345–355. doi: 10.1007/s00018-006-6317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero J, Berciano M T, Weruaga E, Lafarga M, Alonso J R. Pre-neurodegeneration of mitral cells in the pcd mutant mouse is associated with DNA damage, transcriptional repression, and reorganization of nuclear speckles and Cajal bodies. Mol Cell Neurosci. 2006;33:283–295. doi: 10.1016/j.mcn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Blanks J C, Mullen R J, LaVail M M. Retinal degeneration in the pcd cerebellar mutant mouse. II. Electron microscopic analysis. J Comp Neurol. 1982;212:231–246. doi: 10.1002/cne.902120303. [DOI] [PubMed] [Google Scholar]
- Blanks J C, Spee C. Retinal degeneration in the pcd/pcd mutant mouse: accumulation of spherules in the interphotoreceptor space. Exp Eye Res. 1992;54:637–644. doi: 10.1016/0014-4835(92)90019-o. [DOI] [PubMed] [Google Scholar]
- Landis S C, Mullen R J. The development and degeneration of Purkinje cells in pcd mutant mice. J Comp Neurol. 1978;177:125–143. doi: 10.1002/cne.901770109. [DOI] [PubMed] [Google Scholar]
- Okubo A, Sameshima M, Unoki K, Uehara F. The ultrastructural study of ribosomes in photoreceptor inner segments of the pcd cerebellar mutant mouse. Jpn J Ophthalmol. 1995;39:152–161. [PubMed] [Google Scholar]
- Ford G D, Ford B D, Steele E C, Jr, Gates A, Hood D, Matthews M A, Mirza S, Macleish P R. Analysis of transcriptional profiles and functional clustering of global cerebellar gene expression in PCD3J mice. Biochem Biophys Res Commun. 2008;377:556–561. doi: 10.1016/j.bbrc.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F M, Berti D A, Ferreira Z S, Klitzke C F, Markus R P, Ferro E S. Intracellular peptides as natural regulators of cell signaling. J Biol Chem. 2008;283:24448–24459. doi: 10.1074/jbc.M801252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Park Y, Dirisala V R, Zhang Y P, Um S J, Lee H T, Park C. Identification of genes differentially expressed in wild type and Purkinje cell degeneration mice. Mol Cells. 2005;20:219–227. [PubMed] [Google Scholar]
- McBride W J, Ghetti B. Changes in the content of glutamate and GABA in the cerebellar vermis and hemispheres of the Purkinje cell degeneration (pcd) mutant. Neurochem Res. 1988;13:121–125. doi: 10.1007/BF00973323. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Eng J, Ivanov N, Garden G A, La Spada A R. Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol Brain. 2009;2:24. doi: 10.1186/1756-6606-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuhou S, Kato N, Gemba H. Emergence of endoplasmic reticulum stress and activated microglia in Purkinje cell degeneration mice. Neurosci Lett. 2006;396:91–96. doi: 10.1016/j.neulet.2005.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.