Abstract
Maternal obesity in pregnancy predisposes offspring to insulin resistance and associated cardiovascular disease. Here, we used a well-established sheep model to investigate the effects of maternal obesity on cardiac functions. Multiparous ewes were assigned to a control (CON) diet [100% of National Research Council (NRC) recommendations] or an obesogenic (OB) diet (150% of NRC recommendations) from 60 d before conception to necropsy on d 135 of pregnancy. Fetal blood glucose and insulin were increased (P<0.01, n=8) in OB (35.09±2.03 mg/dl and 3.40±1.43 μU/ml, respectively) vs. CON ewes (23.80±1.38 mg/dl and 0.769±0.256 μU/ml). Phosphorylation of AMP-activated protein kinase (AMPK), a cardioprotective signaling pathway, was reduced (P<0.05), while the stress signaling pathway, p38 MAPK, was up-regulated (P<0.05) in OB maternal and fetal hearts. Phosphorylation of c-Jun N-terminal kinase (JNK) and insulin receptor substrate-1 (IRS-1) at Ser-307 were increased (P<0.05) in OB fetal heart associated with lower downstream PI3K-Akt activity (P<0.05), indicating impaired cardiac insulin signaling. Although OB fetal hearts exhibited a normal contractile function vs. CON fetal hearts during basal perfusion, they developed an impaired heart-rate-left-ventricular-developed pressure product in response to high workload stress. Taken together, fetuses of OB mothers demonstrate alterations in cardiac PI3K-Akt, AMPK, and JNK-IRS-1 signaling pathways that would predispose them to insulin resistance and cardiac dysfunction.—Wang, J., Ma, H., Tong, C., Zhang, H., Lawlis, G. B., Li, Y., Zang, M., Ren, J., Nijland, M. J., Ford, S. P., Nathanielsz, P. W., Li, J. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart.
Keywords: cardiac dysfunction, signaling transduction, insulin resistance
The incidence of maternal obesity has increased dramatically during the past 20 yr, by 42% in African-American women and 29% in white women (1). It is now clear that maternal obesity constitutes an important public health issue since it is associated with many obstetric complications, such as gestational diabetes mellitus, type 2 diabetes, and high blood pressure. In addition, maternal obesity leads to higher prenatal morbidity (1,2,3), in particular, macrosomia with a positive correlation of birth weight to maternal body mass index (BMI) (4). Moreover, it has been proposed that altered fetal growth in the setting of maternal obesity increases offspring disease risk for insulin resistance, diabetes, obesity, hypertension, and other cardiovascular diseases (3, 5,6,7).
Insulin resistance induced by obesity is considered to be a major contributor to cardiovascular disease (8,9,10). Insulin resistance in adipose tissue impairs fat cell uptake of fatty acids, resulting in more fatty acid flux into the liver and overproduction of very low density lipoprotein (VLDL) (10). Increased circulating VLDL levels result in accumulation in the blood vessel wall to form atherosclerotic plaques (11), predisposing the individual to coronary artery disease (CAD). In addition to the vascular changes, obesity and insulin resistance are associated with myocardial structural and functional abnormalities often termed “diabetic cardiomyopathy” (12). Kenchaiah et al. (13) reported a 5% increased risk of clinical heart failure (e.g., myocardial infarction) in men and a 7% increased risk in women with every 1 kg/m2 increase in BMI (13). Cardiac ejection fraction (EF%) increases across categories of worsening insulin sensitivity in mildly obese patients, indicating the presence of cardiac damage (14). These features of impaired cardiac function are at least in part due to many complications resulting from insulin resistance, including left ventricle (LV) remodeling, as well as alterations in cardiac metabolism and hemodynamics (15).
Recent studies indicate that activity of c-Jun N-terminal kinase (JNK), a protein activated in response to physiological stress, is elevated in obese tissue, suggesting a crucial role in obesity and insulin resistance (16). Elevation of JNK would phosphorylate its downstream target, insulin receptor substrate 1 (IRS-1) at Ser-307, which, in turn, impairs the insulin-induced IRS-1 tyrosine phosphorylation and attenuates sensitivity to insulin (17, 18). The provoked JNK signaling is attributed to various factors, such as inflammation, oxidative stress, and endoplasmic reticulum (ER) stress (19, 20), which are commonly elevated in obese individuals (21). The influence of inflammation and oxidative stress on the JNK-IRS-1 pathway interferes with insulin signaling, thereby inducing insulin resistance, a feature of type 2 diabetes.
A clear understanding of the mechanisms that affect insulin-dependent pathways in the fetal heart associated with maternal obesity and gestational diabetes is central to the need for information to develop prevention strategies for clinical management. To date, there is no report on alterations of these pathways in the hearts of fetuses of obese mothers. The pregnant sheep model has been extensively used to study fetal development due to its similarity to human pregnancy (22). Compared with rodents, sheep are monotocous (rarely carry more than twins) and are precocial, as are pregnant women. The present study was designed to test the hypothesis that maternal obesity resulted from overnutrition alters JNK-IRS-1 signaling pathways in the fetal heart in ways that would decrease insulin sensitivity.
MATERIALS AND METHODS
Animals
All animal care and treatment were performed in accordance with the guidelines of the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia ewes were fed either a highly palatable diet at 100% of National Research Council (NRC) recommendations as a control (CON) group (n=8) or 150% of the recommended energy requirements as an obesogenic (OB) group (n=8), starting 60 d before conception and continuing through d 135 of gestation (first day of mating: d 0). All ewes were weighed weekly, and body condition score (BCS) was attained at monthly intervals to evaluate changes in fatness. A BCS of 1 (emaciated) to 9 (obese) was assigned independently by 2 trained individuals. BCS is highly related to carcass lipids and can be used to estimate energy reserves available to ewes (23). Details of the management and maternal and fetal outcomes have been reported elsewhere (24).
On d 135 of gestation, sheep bearing singleton fetuses were sedated with ketamine (10 mg/kg) and maintained under isoflurane inhalation general anesthesia (2.5%). Immediately following laparotomy, maternal blood was sampled via jugular venipuncture, and fetal blood was collected from the umbilical vein. A sample of blood was collected in a prechilled tube (heparin plus sodium fluoride; Sigma, St. Louis, MO, USA) for plasma glucose measurement. For insulin assay, the blood sample was collected in prechilled nonheparinized Vacutainer tube (Sigma). Umbilical vein serum and plasma were collected and stored at −80°C. Ewes and fetuses were euthanized by exsanguination while still under general anesthesia. The fetal and maternal hearts were quickly removed from 5 CON and 5 OB animals, and LV heart samples were collected and frozen at −80°C until assayed for protein concentration.
Isolated perfusion fetal heart and cardiac function measurement
After the fetus was removed from the maternal body, the heart was quickly excised and retroperfused in the perfusion heart system (Radnoti Glass Technology Inc., Monrovia, CA, USA) with 95% O2 and 5% CO2 equilibrated Krebs-Henseleit buffer (KHB) containing 118 mM NaCl, 4.75 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 1.4 mM CaCl2, 10 μU/ml insulin, 7 mM glucose, 1% BSA, and 0.4 mM oleate. After 15 min baseline perfusion, hearts were challenged with 10 μM isoprenaline for high-workload stimulation. The LabChart6 software (ADInstruments, Colorado Springs, CO, USA) was used to monitor the heart rate and left ventricle developed pressure (LVDP).
Immunoblotting
The heart tissue samples were homogenized with 700 μl of ice-cold lysis buffer (25). The protein concentration of lysate was determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA) (26). Heart homogenate proteins were then resolved by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. For reprobing, membranes were stripped with 50 mM Tris-HCl, 2% SDS, and 0.1 M-mercaptoethanol (pH 6.8). Rabbit polyclonal antibodies against JNK, phosphor-JNK at Thr183/Tyr185, insulin receptor (IR) β, IRS-1, phosphor-IRS-1 at Ser307, p38/MAPK, phosphor-p38 at Thr180/Tyr182, Akt, phosphor-Akt at Ser473, mammalian target of rapamycin (mTOR), phosphor-mTOR at Ser2448, AMP-activated protein kinase (AMPK), phosphor-AMPK at Thr172, PP2Cα, GLUT1, GLUT4, and horseradish peroxidase-linked secondary antibody were purchased from Cell Signaling (Danvers, MA, USA). Antibodies against phosphor-IR at Tyr1150/Tyr1151, fatty acid transporter proteins FATP1 and FATP4, and CD36 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Immunoprecipitation
Immunoprecipitation was conducted as described previously (27). Heart homogenates were incubated with the appropriate antibody precoupled to protein G/A-Sepharose (GE Healthcare, Piscataway, NJ, USA) at 4°C overnight. The beads were washed 3 times with lysis buffer and then twice with lysis buffer containing 0.5 M LiCl. Proteins were eluted in SDS sample buffer for immunoblotting analysis.
Glutathione/glutathione disulfide (GSH/GSSG) ratio
Sheep heart GSH/GSSG ratio was measured by total GSH detection kit (Assay Designs, Ann Arbor, MI, USA) (22). Heart samples were weighed and homogenized in ice-cold 5% (w/v) metaphophoric acid (20 ml/g). The homogenate was centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was collected for total GSH and oxidized GSSG measurement, according to the manufacturer’s instructions. The reduced GSH was calculated by subtracting the oxidized GSSG value from the total GSH value.
IRS-1-dependent PI3-kinase (PI3K) activity
The IRS-1-dependent PI3K activity was analyzed by immunoprecipitation with anti-IRS-1 antibody (22). Briefly, an 800-μg sample of total protein homogenate was immunoprecipitated with 5 μl of IRS-1 antibody (Santa Cruz Biotechnology) and 60 μl of a 50% slurry of protein A-Sepharose. The pellet was suspended in an assay buffer containing 50 mM HEPES (pH 7), 25 mM MgCl2, and 250 μM ATP. The PI(4,5)P2 substrate was incubated for 3 h, and the product PI(3,4,5)P3 was detected by PI(3,4,5)P3 detector provided in the PI3K ELISA kit (Echelon, Salt Lake City, UT, USA). The amount of PI(3,4,5)P3 produced by extracted PI3K from the sample was proportional to the PI3K activity.
Akt activity measurement
The Akt kinase activity was analyzed by immunoprecipitation Akt from 300 μg total protein of sample homogenate with 5 μl of anti-Akt antibody (Santa Cruz Biotechnology) and 60 μl of a 50% slurry of protein A-Sepharose (28). The pellet was washed with cold PBS 3 times and then suspended in kinase assay dilution buffer. Akt kinase activity was measured based on manufacturer’s instruction of the Akt kinase activity assay kit (Assay Designs).
Statistical analysis
Data were expressed as means ± se. Significance were tested by Student’s unpaired 2-tailed t tests or 2-way repeated-measures ANOVA with Bonferroni correction. A values of P < 0.05 was considered significant.
RESULTS
Induction of maternal obesity
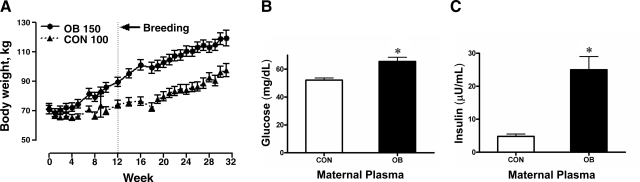
There was no difference in maternal body weight between the two groups at the beginning of the study. Body weight increased prior to and during pregnancy in OB ewes compared to CON ewes (Fig. 1A). Glucose and insulin levels were also elevated in OB maternal plasma on d 135 (Fig. 1B, C), demonstrating that OB ewes had developed obesity and hyperglycemia.
Figure 1.
Body weight (A), maternal plasma glucose (B), and maternal plasma insulin (C) in ewes fed a diet with either 100% of NRC recommendations (CON; n=8) or 150% of NRC recommendations (OB; n=8). Values are expressed as means ± se. *P < 0.05 vs. CON.
Increased JNK and p38 stress signaling and decreased insulin signaling in the maternal heart
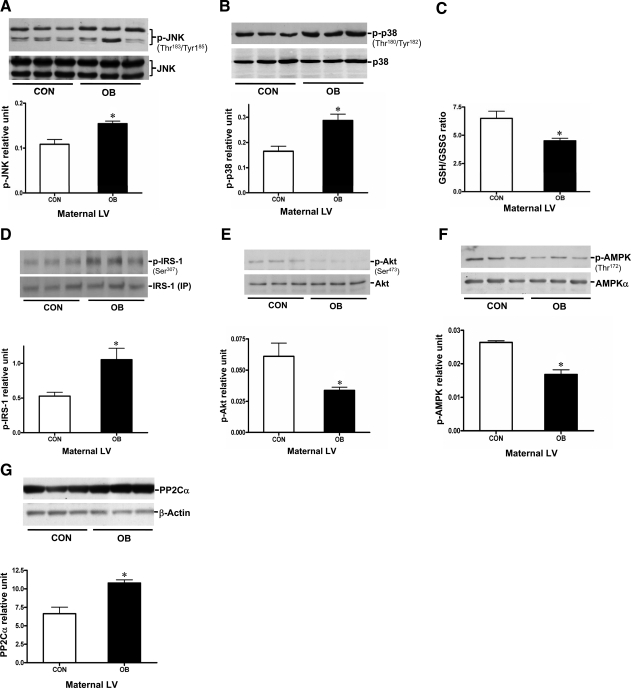
Obesity often is associated with a low-grade chronic inflammation and oxidative stress (21, 29), which lead to increased intracellular stress signaling, including JNK and p38/MAPK phosphorylation (16). To determine the effect of maternal obesity and excess consumption of a highly palatable diet on maternal inflammation during pregnancy, we examined the phosphorylation levels of both JNK and p38/MAPK in maternal hearts. Phosphor-JNK and phosphor-p38 were increased in maternal heart tissue by 51.3 and 70.6%, respectively, in the absence of change in total protein (Fig. 2A, B). Moreover, the GSH to GSSG ratio was decreased in the OB maternal heart (Fig. 2C), suggesting an increased oxidative stress associated with obesity. This elevated oxidative stress might contribute to the higher level of JNK and p38/MAPK phosphorylation in the OB maternal heart.
Figure 2.
Levels of activated JNK and p38/MAPK, GSH/GSSG ratio, and IRS-1-associated PI3K-Akt activity in heart tissue of CON and OB maternal sheep. A, B) Protein levels of JNK (A) and p38/MAPK (B), and their phosphorylation in the hearts of CON and OB pregnant sheep. Phosphorylated JNK and p38 were quantified relative to total JNK or p38 protein level, respectively. C) GSH/GSSG ratio in maternal sheep hearts. D) Phosphorylation of IRS-1 at Ser-307. E) Phosphorylation of Akt at Ser-473. F) AMPK at Thr172 in heart tissue of maternal sheep. Phosphorylated IRS-1 was quantified relative to total IRS-1 level from 500 μg total protein of sample homogenate. G) PP2Cα expression levels in CON and OB maternal sheep hearts. Values are means ± se (n=5). *P < 0.05 vs. CON.
Activated JNK can further trigger IRS-1 phosphorylation at Ser-307, resulting in IRS-1 dissociation from the IR, thereby blocking downstream PI3K/Akt pathways (17, 18). Because of the low expression level of IRS-1 in heart tissue, immunoprecipitation was performed to purify IRS-1 from maternal heart homogenates before detection of its phosphorylation at Ser-307. Immunoblotting showed that IRS-1 Ser-307 phosphorylation was increased by ∼2-fold in OB maternal heart compared to CON maternal heart (Fig. 2D). In addition, downstream Akt phosphorylation at Ser-473 and Akt kinase activity were reduced by 48.3% (Fig. 2E) and 25.9% (Supplemental Fig. 3C), respectively, despite the increased maternal plasma insulin level (Fig. 1C). These results suggest that insulin resistance develops in the obese ewes that are overfed during pregnancy. In addition, decreased AMPK phosphorylation was observed in OB maternal hearts (Fig. 2F), which was associated with increased PP2C (Fig. 2G).
Fetal plasma metabolites and heart phenotype
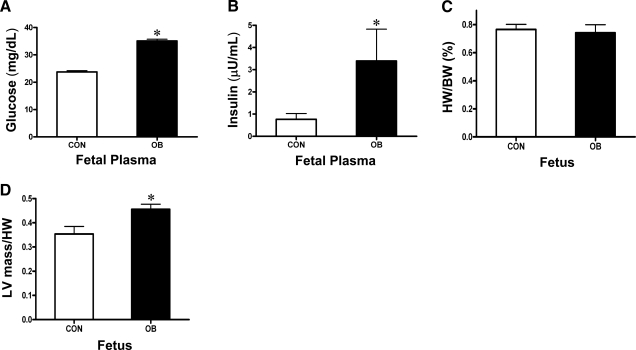
Fetal plasma glucose and insulin concentrations were increased in the OB group (Fig. 3A, B). The ratio of heart weight to body weight did not differ between the two groups (Fig. 3C), while the ratio of LV mass to heart weight was greater in OB than in CON fetal sheep (Fig. 3D). However, hematoxylin and eosin staining of the fetal sheep LV did not indicate cardiac hypertrophy or fibrogenesis (Supplemental Fig. 1). These results suggest that fetuses of OB mothers are at a high risk of developing hyperglycemia and hyperinsulinemia.
Figure 3.
Body weight (BW), heart weight (HW), LV mass, plasma metabolic parameters of LV from CON (n=8) and OB (n=8) fetal sheep at d 135 of gestation. A) Fetal glucose. B) Fetal insulin. C) Ratio of fetal HW to BW. D) Ratio of fetal LV mass to HW (D). Values are means ± se. *P < 0.01 vs. CON.
Cardiac contractile function in the fetus of obese ewes
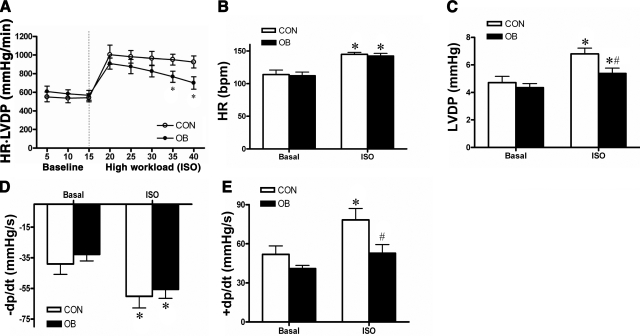
To determine whether hyperglycemia and increased LV mass induced by maternal obesity during pregnancy had any effect on fetal cardiac function, we assessed myocardial contractile function of the isolated perfused fetal heart. Under baseline conditions, both CON and OB fetal hearts maintained a heart-rate-LV pressure product of ∼600 mmHg/min (Fig. 4A). When subjected to high workload by an isoprenaline (ISO; 10 μM) challenge, the initial increase of heart-rate-LV pressure product was ∼50% in both CON and OB fetal hearts (Fig. 4A). However, after 20 min of high-workload challenge, the OB fetal hearts could not sustain the increased contractile performance (Fig. 4A), as demonstrated by the heart-rate-LV pressure product of OB fetal hearts, decreasing by 24% at the end of high-workload challenge (P<0.05 vs. CON fetal hearts).
Figure 4.
Fetal cardiac contractile function at d 135 of gestation, assessed by the Langendorff perfusion system. A) Cardiac contractile function estimated as heart rate (HR)-LVDP product. Fetal hearts were perfused for 15 min at baseline and then challenged with isoprenaline (ISO; 10 μM) at the high workload. *P < 0.05 vs. CON. B–D) Heart rate (B), LVDP (C), maximum derivative of diastolic pressure (D), and maximum derivative of systolic pressure (E). *P < 0.05 vs. basal; #P < 0.05 vs. CON ISO. Values are means ± se (n=3/group).
The impairment of cardiac contractile function in OB fetal hearts was further investigated by analyzing the heart rate and LVDP, respectively. During basal perfusion conditions, the heart rate of the 2 groups was ∼105 bpm (Fig. 4B). After treatment with ISO (10 μM), both CON and OB fetal hearts were able to achieve a heart rate of ∼150 bpm, and this value was maintained throughout the high-workload period (Fig. 4B). However, the increase of LVDP in response to ISO treatment was modestly but significantly less in OB compared to CON fetal hearts (Fig. 4C). Moreover, the maximum positive value of the first derivative of the pressure (+dp/dt) showed the same pattern as LVDP, indicating the velocity of cardiac systolic contraction in OB fetal hearts did not increase to the same degree as that in CON fetal hearts when subjected to high workload (Fig. 4E). There was no difference in the velocity of cardiac diastolic contraction (-dp/dt) between the two groups (Fig. 4D). Taken together, these findings suggest that maternal obesity might not influence normal cardiac functions in late fetal life, but rather result in less adaptive ability to high workload conditions, such as environmental stress or exercise.
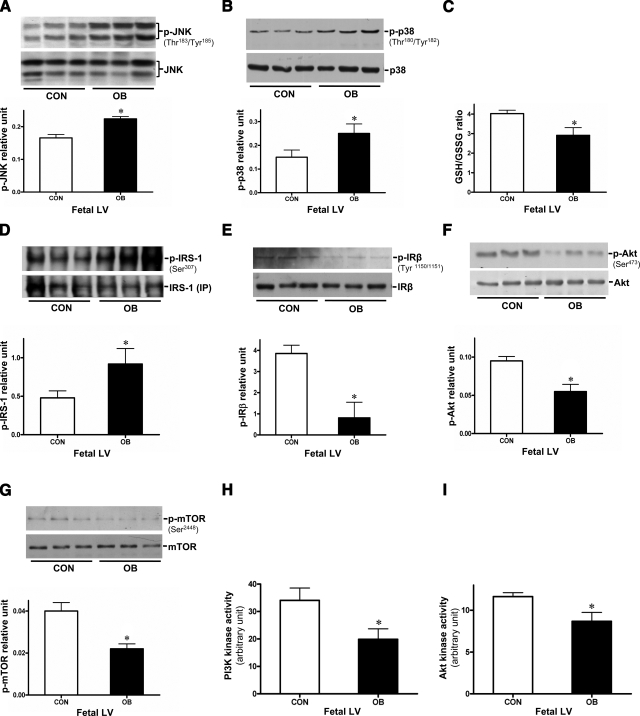
Impaired JNK-IRS-1 and insulin signaling in the heart of OB fetuses
Previous studies have revealed that insulin resistance syndromes can drive abnormal cardiac contractile function and cardiovascular diseases (10). To investigate whether the weakened adaptive ability to high-workload conditions in the OB fetal hearts was due to decreased insulin signaling, we examined JNK, p38, and insulin signaling pathways. The phosphorylation of both JNK and p38 was increased in the OB fetal hearts (Fig. 5A, B), which might be caused by an elevated oxidative stress, shown as the reduced GSH/GSSG ratio of OB fetal heart in Fig. 5C (22, 30). Since increased JNK phosphorylation might further influence insulin signaling via altering IRS-1 phosphorylation (17, 18), we further measured the activity of insulin-dependent factors in fetal hearts. Phosphor-IRS-1 at Ser-307 was increased in OB vs. CON fetal hearts (Fig. 5D), and phosphor-IRβ at Tyr1150/1151 was decreased (Fig. 5E), indicating a potential for development of insulin insensitivity in the myocardium of the OB fetus. Consistent with the altered phosphorylation of IRS-1 (Ser-307), downstream PI3K activity associated with IRS-1 was significantly lower in OB fetal hearts (Fig. 5H). Further confirmation of potential insulin resistance in the OB fetal hearts was provided by decreased Akt phosphorylation, PI3K activity, and Akt kinase activity, and reduced phosphorylation of mTOR, the direct target of Akt (31) (Fig. 5F–I). Collectively, these changes would also lead to the development of insulin resistance in the fetal heart.
Figure 5.
Altered JNK-IRS-1 signaling cascades, increased oxidative stress, and impaired insulin signaling pathways in the OB fetal hearts. A, B) Protein levels of JNK (A) and p38/MAPK (B), and their phosphorylation in the hearts of CON and OB fetal hearts. Phosphorylated JNK and p38 were quantified relative to total JNK or p38 protein level, respectively. C) GSH/GSSG ratio in fetal hearts of CON and OB sheep. D) Immunoblots of phosphor-IRS1 at Ser-307. E) Phosphor-IRβ at Tyr1150/1151 of fetal hearts. F, G) Phosphorylation of Akt at Ser-473 (F) and mTOR at Ser-2448 (G) in heart tissue of CON or OB fetus. Phosphorylated Akt and mTOR were quantified relative to total Akt or mTOR protein level, respectively. H) IRS-1-dependent PI3K activity. I) Akt kinase activity in fetal hearts, measured by immunoprecipitation kinase assays. Values are means ± se (n=5). *P < 0.05 vs. CON.
Remodeling of regulatory pathways in fatty acid uptake in the fetal heart exposed to maternal obesity
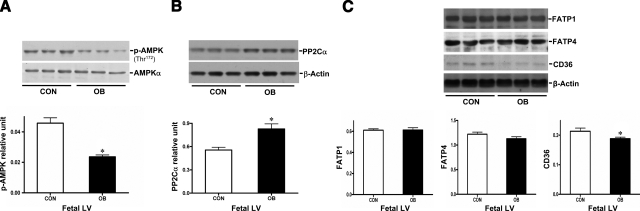
Decreased Akt phosphorylation in the presence of elevated plasma insulin and reduced phosphor-AMPK in the OB fetal heart (Fig. 6A) are associated with higher expression of PP2C in OB fetal heart (Fig. 6B) and raise the suspicion that fetal heart substrate metabolism is redirected in the setting of maternal obesity during pregnancy, since Akt and AMPK are important kinases involved in metabolic regulation (32,33,34). Intriguingly, results from this study showed no difference in the protein content of glucose transporters GLUT1 and GLUT4 (Supplemental Fig. 2) and of the fatty acid transporter proteins FATP1 and FATP4 (Fig. 6C). However, the fatty acid transporter CD36 was significantly decreased in the OB vs. CON fetal hearts (Fig. 6), whereas CD36 was increased in maternal hearts in response to high-fat diet treatment (Supplemental Fig. 4). One explanation might be that during the fetal and neonatal stages, the main energy supply is glucose, but in the adult heart, the substrate metabolism shifts from glucose to fatty acids (35,36,37). Therefore, the different pattern of fatty acid transporter CD36 in maternal and fetal heart may be due to the immaturity of the developing fetal heart. On the other hand, CD36 deficiency is associated with hypertrophic cardiomyopathy (38, 39). For this reason, the decreased CD36 expression level might also be associated with the weakened adaptive ability in OB fetal heart (Fig. 4).
Figure 6.
Alteration in AMPK signaling pathways and the expression levels of fatty acid transporters in the fetal heart. A) Phosphorylation of AMPK at Thr172 in heart tissue of CON and OB fetus. Phosphor-AMPK was quantified relative to AMPKα protein level. B) Expression levels of PP2Cα in CON and OB fetal hearts. C) Immunoblots of FATP1, FATP4, and CD36 in fetal hearts. All immunoblots were quantified relative to β-actin protein level. Values are means ± se (n=5). *P < 0.05 vs. CON.
DISCUSSION
The aim of this study was to establish, for the first time, the combined effect of preconceptional maternal obesity and high-calorie intake on the developing fetal heart in one of the most extensively studied models of fetal development, the fetal lamb. We focused on potential changes in metabolic and inflammatory mechanisms. Despite extensive studies on altricial species, such as rats and mice during the fetal period, there are no data on fetuses of precocial species, in which many of the major stages in organ development occur at a stage when key metabolic variables (PO2, glucose, glucocorticoids) are very different from those to which cells are exposed in postnatal life. Body weight, blood glucose, and serum insulin level, as well as oxidative stress level, were elevated in overfed ewes, and there was an increase in stress signaling pathways, such as JNK and p38. We also detected impaired IRS-1 activity and associated PI3K-Akt signaling in the OB maternal heart in the presence of elevated insulin levels. These data indicated that the overfeeding regimen used resulted in obesity, hyperglycemia, and insulin resistance during pregnancy. Blood glucose and insulin levels were also increased in OB fetuses. In addition, OB fetal hearts were structurally different, having a greater LV mass than CON fetal hearts, suggesting impaired cardiac function functionally related to the observation that OB fetal hearts failed to sustain high-workload contraction, with a weakened LVDP. The observed OB fetal cardiac dysfunction may be also due, in part, to cardiac insulin resistance indicated by the increased level of phosphor-JNK and phosphor-IRS-1 (Ser-307), and a reduced IRS-1-dependent PI3K-Akt signaling activity. Moreover, the higher levels of PP2C in OB heart may be the causative mechanism underlying the lower AMPK phosphorylation level in the OB heart. Although there was no difference at the protein expression level of glucose transporters (GLUT1 and GLUT4) and fatty acid transporter proteins (FATP1 and FATP4), the fatty acid transporter CD36 was decreased in the OB fetal heart, a change that could further influence fatty acid metabolism during fetal and neonatal development.
We chose to study sheep in distinction to rodents since many aspects of fetal development are similar in sheep and humans. Moreover, sheep develop diet-induced obesity easily, whereas rodents are less willing to maintain increased consumption when overfed (22). In addition, the sheep model has been extensively used to study how alterations in nutrition during pregnancy establish permanent responses as a result of fetal programming (40, 41). Our data clearly show that body weight of OB maternal sheep increased dramatically on the increased diet, with higher blood insulin and glucose levels in both OB mothers and fetuses. Similar observations have been reported in both animal and human studies.
Recently, JNK has been identified as a new target for diabetes and insulin resistance (16). db/db mice with leptin receptor deficiency exhibit hyperglycemia and hypoinsulinemia at the age of 6 wk. After being fed with the JNK inhibitor (CC105), blood glucose was lower and insulin higher, indicating improved glucose control (16). Moreover, the ob/ob mice (leptin deficient) with accompanying JNK gene knockout are more able to maintain a normal body weight and stable-state glucose and insulin level than lean wild-type littermates (17). JNK links to insulin signaling through regulation of IRS-1, a key regulatory protein involved in the insulin signaling cascade (17). When insulin binds to its receptor, IRS-1 is activated via tyrosine residue phosphorylation to initiate downstream signaling pathways such as Akt (42). On the other hand, IRS-1 can be inactivated via Ser-307 phosphorylation by JNK (18), resulting in an insensitivity to insulin signaling, a key feature of insulin resistance. In our obese pregnant sheep model, we found higher phosphor-JNK and phosphor-IRS-1 (Ser-307) levels in OB fetal hearts (vs. CON fetal hearts), with a lower Akt activity, indicating insulin resistance mediated by the JNK-IRS-1 signaling cascade. It has been proposed that augmented activation of JNK is regulated by various stress conditions, including oxidative stress and inflammation (43). The elevated oxidative stress that we have observed, represented by the decreased GSH/GSSG ratio in both OB maternal and fetal heart, could be a candidate of JNK upstream stimuli. The chronic oxidative stress and inflammation during pregnancy would alter systemic immunity and metabolism not only in mothers but also in fetuses, resulting in a long-term effect on postnatal lifetime (44).
The impaired PI3K-Akt activity and lower phosphor-mTOR level observed in the OB fetal heart may also affect postnatal development. Akt phosphorylation varies at different developmental stages, with a higher level observed during more rapid growth phases. Overexpressing Akt in cardiomyocyte-selective insulin receptor-knockout (CIRKO) mouse rescues the small heart size phenotype (31), suggesting that Akt contributes to insulin’s effect on growth and development of the heart. In addition, mTOR signaling regulates protein synthesis and inhibition of mTOR would tend to suppress cell proliferation and growth. In all, the decreased PI3K-Akt activity, as well as reduced mTOR phosphorylation observed in the OB fetal hearts during late gestation, may affect cardiac remodeling after birth.
The observed impairment of cardiac contractility occurs in the setting of blunted adaptive response, including Akt and AMPK signaling pathways. AMPK is an important energy sensor protein involved in substrate metabolism. In the presence of extracellular stress, such as hypoxia and exercise, the intracellular ATP/AMP ratio drops, leading to activation of AMPK. Activated AMPK can enhance glucose uptake and accelerate fatty acid oxidation to provide energy supply for the stressed cells (45). On the other hand, AMPK is inactivated via dephosphorylation by protein phosphatase 2C (PP2C) (46). PP2C is up-regulated by nutrient stress and insulin resistance in various tissues, including heart and skeletal muscle (47, 48). Consistent with these previous findings, PP2C protein level is also higher in OB maternal and fetal sheep hearts, and it may contribute to lower AMPK phosphorylation. The reduced AMPK activity in the OB fetal heart would make the myocardium more susceptible to stress conditions.
Altered substrate metabolism has also been observed in both animal models and patients with obesity and insulin resistance. Blunted Akt and AMPK signaling, as well as the ability of GLUT4 to translocate to the membrane to increase glucose uptake under stress conditions, might be attenuated and thus explain impaired cardiac function during high workload. Regarding fatty acid metabolism, obese women with insulin resistance show a greater fatty acid uptake and oxidation (49). Similarly, in this study, a higher expression level of fatty acid transporter CD36 was detected in the obese maternal model, suggesting that fatty acid uptake might be enhanced in maternal heart in the presence of increased dietary intake and maternal obesity. An increased utilization of fatty acid in this model could be due to two events. First, impaired insulin signaling may not be able to maintain normal glucose regulation, leading to decreased glucose uptake and utilization. As a result, more fatty acid is oxidized for energy to compensate for the decreased glucose metabolism (10). Second, up-regulating CD36 expression level may be an adaptive strategy in response to increased availability of fatty acids. Surprisingly, CD36 protein was decreased in the OB fetal heart. The reason for the suppressed CD36 expression in fetuses exposed to overnourished intrauterine environment is not clear.
Diet-induced maternal obesity in rat pregnancy and the altered in utero environment in human diabetic pregnancy both result in persistent postnatal impaired glucose tolerance, hyperinsulinemia, and defective insulin secretion (50), (51, 52). In rodent models, maternal obesity increases offspring susceptibility to myocardial ischemia/reperfusion injury (53). Maternal obesity in human pregnancy is accompanied by complex metabolic changes, including hyperglycemia and hypercholesterolemia, and is associated with disordered fetal cardiovascular development, including hypoplastic left heart syndrome, hypertrophic cardiomyopathy (54), and a greater incidence of aortic fatty streaks (55). Remodeling of the developing cardiovascular system in the presence of increased maternal nutrient availability is accompanied by greater atherogenesis and endothelial dysfunction in childhood (56, 57). Not surprisingly, therefore, maternal obesity increases the risk of neonatal mortality (58), especially in infants born after preterm premature rupture of membranes (3).
Under a basal workload, there were no functional differences between CON and OB fetal hearts in heart rate, cardiac output, and LV ±dp/dt. However, we observed a higher LV mass in OB fetal hearts, a maladaptive response resulting in impaired cardiac contractile function shown by a smaller increase in LVDP and rate of LV pressure increase under high workload stress. These observations indicate that OB fetal hearts, apparently functionally normal under basal conditions, respond poorly to increased demand, which may contribute to the increased neonatal morbidity observed in offspring of obese mothers (59).
The clinical relevance of our findings is shown by a near-50% increased rate of suboptimal sonographic visualization of fetal cardiac structure in obese pregnant women, again indicating functional deficits in OB fetal hearts. In one study using data from the Swedish Medical Health Registries, researchers found that maternal obesity was associated with an increased risk of overall and specific infant cardiovascular defects (60). The risk of late fetal death increased with prepregnancy BMI in nulliparous women, and risk of early neonatal death was nearly double among nulliparous women of high BMI. In parous women, obesity was associated with late fetal but not early neonatal death (60). The researchers conclude “Maternal overweight may be one of the most important preventable risk factors for perinatal mortality” (60). The causes of the perinatal mortality remain to be clearly established, but the impaired cardiovascular function seen clinically on ultrasound and the deficient cardiac function that we have demonstrated experimentally would impair the offspring’s ability to make the pronounced, dynamic vascular changes required to adapt to independent postnatal life at delivery.
In summary, the data provided clearly establish that fundamental fetal cardiac inflammatory and metabolic changes, associated with functional impairment, occur by the end of gestation in the setting of maternal obesity. Evaluation of changes in fetal life is an indispensible first step in understanding the effects of maternal obesity on postnatal offspring cardiovascular health. The extent to which the cardiovascular changes in fetal life that we have demonstrated persist into neonatal and later postnatal life and alter offspring cardiovascular function remains to be established. Further studies focusing on the endpoints shown to be affected will help determine the relative contributions and complex interaction of prenatal exposures and changes secondary to the development of postnatal metabolic disease and pave the way to better diagnosis, prevention, and treatment of cardiovascular dysfunction in infants exposed to the effects of maternal obesity and overnutrition.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institute on Aging (NIA)/National Institutes of Health (NIH) grant R03AG028163 (J.L.), American Federation for Aging Research grant 08007 (J.L.), NIH University of Wyoming Northern Rockies Regional Idea Network of Biomedical Research Excellence (INBRE) pilot grant 5P20RR016474 (J.L.), National Center for Research Resources pilot grant 1UL1RR025014-01 (J.L.), NIH INBRE program project P20 RR16474-04 (S.P.F.), and American Diabetes Association grant 7-08-RA-130 (J.R.).
References
- Ehrenberg H M, Dierker L, Milluzzi C, Mercer B M. Prevalence of maternal obesity in an urban center. Am J Obstet Gynecol. 2002;187:1189–1193. doi: 10.1067/mob.2002.127125. [DOI] [PubMed] [Google Scholar]
- Reece E A. Perspectives on obesity, pregnancy and birth outcomes in the United States: the scope of the problem. Am J Obstet Gynecol. 2008;198:23–27. doi: 10.1016/j.ajog.2007.06.076. [DOI] [PubMed] [Google Scholar]
- Nohr E A, Vaeth M, Bech B H, Henriksen T B, Cnattingius S, Olsen J. Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol. 2007;110:1083–1090. doi: 10.1097/01.AOG.0000286760.46679.f8. [DOI] [PubMed] [Google Scholar]
- Hull H R, Dinger M K, Knehans A W, Thompson D M, Fields D A. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416 e411–416. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- Barker D J. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Mighty H E, Fahey A J. Obesity and pregnancy complications. Curr Diabetes Reports. 2007;7:289–294. doi: 10.1007/s11892-007-0046-y. [DOI] [PubMed] [Google Scholar]
- Woods L L. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9:419–425. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Hintz K K, Aberle N S, Ren J. Insulin resistance induces hyperleptinemia, cardiac contractile dysfunction but not cardiac leptin resistance in ventricular myocytes. Int J Obes Relat Metab Disord. 2003;27:1196–1203. doi: 10.1038/sj.ijo.0802389. [DOI] [PubMed] [Google Scholar]
- Yu Y R, Li H L, Yu H L, Wang C, Pu S. The relationship between insulin resistance and endothelium-dependent vasodilatation in obese subjects. Zhonghua Yi Xue Za Zhi. 2003;83:1467–1470. [PubMed] [Google Scholar]
- Ginsberg H N. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp J H, Lespine A, Hamilton R L, Colyvas N, Chaumeton A H, Tweedie-Hardman J, Kotite L, Kunitake S T, Havel R J, Kane J P. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14:1767–1774. doi: 10.1161/01.atv.14.11.1767. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, McNeill J H. The diabetic heart: metabolic causes for the development of a cardiomyopathy. Cardiovasc Res. 1992;26:913–922. doi: 10.1093/cvr/26.10.913. [DOI] [PubMed] [Google Scholar]
- Kenchaiah S, Evans J C, Levy D, Wilson P W, Benjamin E J, Larson M G, Kannel W B, Vasan R S. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- Trovato G M, Catalano D, Caruso G, Squatrito R, Venturino M, Degano C, Fazzio S D. Relationship between cardiac function and insulin resistance in obese patients. Diabetes Nutr Metab. 2001;14:325–328. [PubMed] [Google Scholar]
- Peterson L R. Obesity and insulin resistance: effects on cardiac structure, function, and substrate metabolism. Curr Hypertens Rep. 2006;8:451–456. doi: 10.1007/s11906-006-0022-y. [DOI] [PubMed] [Google Scholar]
- Bennett B L, Satoh Y, Lewis A J. JNK: a new therapeutic target for diabetes. Curr Opin Pharm. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun C Z, Uysal K T, Maeda K, Karin M, Hotamisligil G S. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Uchida T, Yenush L, Davis R, White M F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka T A, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med. 2005;83:429–439. doi: 10.1007/s00109-005-0640-x. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir G C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. 2008;46:499–505. doi: 10.1515/CCLM.2008.096. [DOI] [PubMed] [Google Scholar]
- Zhu M J, Han B, Tong J, Ma C, Kimzey J M, Underwood K R, Xiao Y, Hess B W, Ford S P, Nathanielsz P W, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol. 2008;586:2651–2664. doi: 10.1113/jphysiol.2007.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson D W, West T R, Tatman W R, Riley M L, Judkins M B, Moss G E. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci. 1993;71:1112–1116. doi: 10.2527/1993.7151112x. [DOI] [PubMed] [Google Scholar]
- Ford S P, Zhang L, Zhu M, Miller M M, Smith D T, Hess B W, Moss G E, Nathanielsz P W, Nijland M J. Maternal obesity accelerates fetal pancreatic β cell but not α cell development in the sheep: prenatal and postnatal consequences. Am J Physiol. 2009;297:835–843. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E J, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young L H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- Zhao P, Wang J, Ma H, Xiao Y, He L, Tong C, Wang Z, Zheng Q, Dolence E K, Nair S, Ren J, Li J. A newly synthetic chromium complex-chromium (d-phenylalanine)(3) activates AMP-activated protein kinase and stimulates glucose transport. Biochem Pharm. 2009;77:1002–1010. doi: 10.1016/j.bcp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Li J, Miller E J, Ninomiya-Tsuji J, Russell R R, 3rd, Young L H. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- Hiromura M, Okada F, Obata T, Auguin D, Shibata T, Roumestand C, Noguchi M. Inhibition of Akt kinase activity by a peptide spanning the betaA strand of the proto-oncogene TCL1. J Biol Chem. 2004;279:53407–53418. doi: 10.1074/jbc.M403775200. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Holbrook N J. Common mechanisms for declines in oxidative stress tolerance and proliferation with aging. Free Radic Biol Med. 2003;35:292–299. doi: 10.1016/s0891-5849(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn C R, Abel E D, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- Dyck J R, Kudo N, Barr A J, Davies S P, Hardie D G, Lopaschuk G D. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. Eur J Biochem. 1999;262:184–190. doi: 10.1046/j.1432-1327.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- Li J, Hu X, Selvakumar P, Russell R R, 3rd, Cushman S W, Holman G D, Young L H. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrin. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- Lopaschuk G D, Spafford M A, Marsh D R. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol Heart Circ Physiol. 1991;261:H1698–H1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- Opie L H. Metabolism of the heart in health and disease. I. Am Heart J. 1968;76:685–698. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- Stanley W C, Recchia F A, Lopaschuk G D. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Okamoto F, Tanaka T, Sohmiya K, Kawamura K. CD36 abnormality and impaired myocardial long-chain fatty acid uptake in patients with hypertrophic cardiomyopathy. Jpn Circ J. 1998;62:499–504. doi: 10.1253/jcj.62.499. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Toba K, Ogawa Y, Kodama M, Hirono S, Ohkura Y, Hanawa H, Nakamura Y, Aoki Y, Fuse I, Aizawa Y, Miyajima S, Kusano Y, Nagatomo T, Hasegawa G, Naito M. Hypertrophic cardiomyopathy with type I CD36 deficiency. Jpn Circ J. 1998;62:541–542. doi: 10.1253/jcj.62.541. [DOI] [PubMed] [Google Scholar]
- Tong J, Zhu M J, Underwood K R, Hess B W, Ford S P, Du M. AMP-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3T3–L1 cells. J Animal Sci. 2008;86:1296–1305. doi: 10.2527/jas.2007-0794. [DOI] [PubMed] [Google Scholar]
- Taylor P D, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Ueki K, Kahn C R. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G R. Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle. 2007;6:888–894. doi: 10.4161/cc.6.8.4135. [DOI] [PubMed] [Google Scholar]
- Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. [E-pub ahead of print] J Perinatol. 2009 doi: 10.1038/jp.2009.182. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- Young L H, Li J, Baron S J, Russell R R. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54:2187–2196. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- Steinberg G R, Michell B J, van Denderen B J, Watt M J, Carey A L, Fam B C, Andrikopoulos S, Proietto J, Gorgun C Z, Carling D, Hotamisligil G S, Febbraio M A, Kay T W, Kemp B E. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wang M Y, Unger R H. Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–E221. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- Peterson L R, Herrero P, Schechtman K B, Racette S B, Waggoner A D, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler R J. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya J D, Strutt B, Hill D J, Patel M S. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab. 2006;290:E129–E134. doi: 10.1152/ajpendo.00248.2005. [DOI] [PubMed] [Google Scholar]
- Sobngwi E, Boudou P, Mauvais-Jarvis F, Leblanc H, Velho G, Vexiau P, Porcher R, Hadjadj S, Pratley R, Tataranni P A, Calvo F, Gautier J F. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361:1861–1865. doi: 10.1016/S0140-6736(03)13505-2. [DOI] [PubMed] [Google Scholar]
- Whitaker R C, Wright J A, Pepe M S, Seidel K D, Dietz W H. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Calvert J W, Lefer D J, Gundewar S, Poston L, Coetzee W A. Developmental programming resulting from maternal obesity in mice: effects on myocardial ischaemia-reperfusion injury. Exp Physiol. 2009;94:805–814. doi: 10.1113/expphysiol.2009.047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan N, Brazil D P, McAuliffe F. Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res A Clin Mol Teratol. 2009;85:523–530. doi: 10.1002/bdra.20567. [DOI] [PubMed] [Google Scholar]
- Napoli C, D'Armiento F P, Mancini F P, Postiglione A, Witztum J L, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Nicolaides E, Liguori A, Napoli C. Influence of maternal dysmetabolic conditions during pregnancy on cardiovascular disease. J Cardiovasc Transl Res. 2009;2:277–285. doi: 10.1007/s12265-009-9108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Glass C K, Witztum J L, Deutsch R, D'Armiento F P, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: fate of early lesions in children (FELIC) study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher N J. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112:403–408. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- Chen A, Feresu S A, Fernandez C, Rogan W J. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20:74–81. doi: 10.1097/EDE.0b013e3181878645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S, Bergstrom R, Lipworth L, Kramer M S. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.