Abstract
Apical release of ATP and UTP can activate P2Y2 receptors in the aldosterone-sensitive distal nephron (ASDN) and inhibit the open probability (Po) of the epithelial sodium channel (ENaC). Little is known, however, about the regulation and physiological relevance of this system. Patch-clamp studies in freshly isolated ASDN provide evidence that increased dietary Na+ intake in wild-type mice lowers ENaC Po, consistent with a contribution to Na+ homeostasis, and is associated with increased urinary concentrations of UTP and the ATP hydrolytic product, ADP. Genetic deletion of P2Y2 receptors in mice (P2Y2−/−; littermates to wild-type mice) or inhibition of apical P2Y-receptor activation in wild-type mice prevents dietary Na+-induced lowering of ENaC Po. Although they lack suppression of ENaC Po by dietary NaCl, P2Y2−/− mice do not exhibit NaCl-sensitive blood pressure, perhaps as a consequence of compensatory down-regulation of aldosterone levels. Consistent with this hypothesis, clamping mineralocorticoid activity at high levels unmasks greater ENaC activity and NaCl sensitivity of blood pressure in P2Y2−/− mice. The studies indicate a key role of the apical ATP/UTP-P2Y2-receptor system in the inhibition of ENaC Po in the ASDN in response to an increase in Na+ intake, thereby contributing to NaCl homeostasis and blood pressure regulation.—Pochynyuk, O., Rieg, T., Bugaj, V., Schroth, J., Fridman, A., Boss, G. R., Insel, P. A., Stockand, J. D., Vallon, V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone.
Keywords: ATP, aldosterone, distal nephron, blood pressure, nucleotide receptors
NaCl and fluid homeostasis is maintained by the kidneys and is important for the regulation of blood pressure (BP) (1). The mineralocorticoid aldosterone plays a central role in adapting renal reabsorption of Na+ to altered physiological states by activation of the rate-limiting event in Na+ transport, ENaC, the luminal epithelial sodium channel, in the aldosterone-sensitive distal nephron (ASDN) (2). The importance of ENaC in the regulation of BP is highlighted by several diseases associated with gain or loss of ENaC function (3). While hormonal regulation of ENaC activity is considered pivotal to NaCl homeostasis and BP regulation, ATP is a newly recognized candidate molecule that may locally mediate control of Na+ reabsorption in the ASDN (for review, see ref. 4,5,6,7).
ATP and other nucleotides are released by renal epithelial cells in response to physiological stimuli that include flow-induced shear stress and changes in cell volume (7,8,9,10). Extracellular nucleotides regulate a broad range of physiological events through the activation of ligand-gated P2X and G-protein-coupled P2Y receptors. Previous work has implicated P2 receptors in the regulation of Na+ reabsorption and K+ secretion in the mammalian ASDN (11,12,13,14,15,16). Using gene-knockout mice, we obtained the first in vivo evidence for an inhibitory influence of P2Y2 receptors on renal NaCl and water reabsorption (17). Moreover, single-channel patch-clamp analysis of ENaC revealed that the P2Y2 agonists ATP and UTP rapidly, dynamically, and reversibly decrease ENaC open probability (Po) in principal cells of isolated connecting tubules (CNTs) and cortical collecting ducts (CCDs) (both part of the ASDN) in rats and mice, a response that is blunted in mice that lack P2Y2 receptors (P2Y2−/−) (18). P2Y2 receptors activate phospholipase C and promote hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) in the apical membrane of principal cells (18), a pathway that can inhibit ENaC Po (19,20,21). Loss of this regulation may facilitate renal Na+ retention in P2Y2−/− mice and contribute to an increase in BP in these mice (17). However, the BP phenotype in P2Y2−/− mice is not sensitive to NaCl intake, which may be a consequence of reduced aldosterone levels and renal αENaC expression in these mice (17).
A low-Na+ diet enhances renal Na+ reabsorption by increasing the expression of the αENaC subunit and regulating the trafficking of functional ENaC channels into the apical membrane of the ASDN, effects that are, at least in part, mediated by aldosterone (22). In the current studies, we show that dietary Na+ also regulates ENaC Po and that the influence of dietary NaCl on ENaC Po in the ASDN is mediated by apical P2Y2-receptor activation. On the basis of evidence that the inhibitory influence of P2Y2-receptor activation on ENaC activity and NaCl sensitivity of BP can be unmasked by prevention of the decrease of mineralocorticoid tone that occurs in P2Y2−/− mice, we conclude that ATP/UTP and P2Y2 receptors have a key role in sodium homeostasis by the mammalian kidney and in the regulation of blood pressure.
MATERIALS AND METHODS
Animals
Animal use and welfare adhered to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the Veterans Affairs San Diego Healthcare System and the University of Texas Health Science Center at San Antonio. Generation of P2Y2−/− mice has been described in detail (23). Mice have been backcrossed to the C57BL/6 background for >10 generations. Wild-type (WT) and P2Y2−/− mice were generated by breeding of heterozygotes.
For patch-clamp experiments, mice were maintained for ≥1 wk on either a nominally Na+-free diet (<0.01% Na+; TD.90228; Harlan Teklad, Madison, WI, USA), a regular diet containing 0.32% Na+ (TD.7912; Harlan Teklad), or a high-Na+ diet (2% Na+; TD.92034; Harlan Teklad) prior to experimentation. For some experiments, animals were injected subcutaneously with 2.4 mg DOCA dissolved in 200 μl of olive oil for 3 consecutive days prior to sacrifice.
Tissue preparation
Isolation of CNTs/CCDs suitable for electrophysiology has been described previously (18, 24–26). In brief, mice were sacrificed by cervical dislocation, and the kidneys were immediately removed and cut into slices (<1 mm) that were placed into ice-cold physiological saline solution buffered with HEPES (pH 7.4). CNTs/CCDs were identified as merging segments and isolated from these slices by microdissection, using watchmaker forceps under a stereomicroscope. CNTs/CCDs, used within 1–2 h of isolation, were allowed to settle onto a 5 × 5 mm cover glass coated with poly-l-lysine that was placed in a perfusion chamber mounted on an inverted Nikon TE2000 microscope (Nikon, Melville, NY, USA) and superfused with room temperature HEPES-buffered (pH 7.4) saline solution. CNTs/CCDs were split open with a sharpened micropipette controlled with a micromanipulator to gain access to the apical membrane.
Electrophysiology
ENaC activity was determined in cell-attached patches made under voltage-clamp conditions (−Vp=−60 mV) on the apical plasma membranes of principal cells in isolated, split-open CNTs/CCDs, as described previously (18, 24,25,26,27). Gap-free single-channel current data from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B (Axon Instruments, Sunnyvale, CA, USA) or EPC-9 (Heka Instruments, Bellmore, NY, USA) patch-clamp amplifier interfaced with a PC running the pClamp 9.2 suite of software (Axon Instruments). Currents were low-pass filtered at 100 Hz and digitized at a sampling rate of 2 kHz. Unitary current (i) was determined, as normal, from all-point amplitude histograms fitted with single- or multigaussian curves using the standard 50% threshold criterion to differentiate between events. All events were inspected visually prior to acceptance. Channel activity, defined as NPo, was calculated using the following equation: NPo = Σ (t1 + 2t2 + … ntn), where N and Po are the number of ENaCs in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. For calculating Po in paired experiments, N was fixed as the greatest number of active channels observed in control or experimental conditions. In such paired patch-clamp experiments, N cannot change in response to the experimental maneuver (e.g., ATP), so any detected effect must be an effect on Po. The error associated with calculating Po increases as this variable moves away from 0.5 and approaches 0 or unity. To assure reliable calculation of Po, we measured Po with standard and accepted tools using long recording times (>1 min) and patches containing 5 or fewer channels. This approach, which provides the most confidence other than using seals with only one channel, is routinely used to determine Po (28,29,30). The frequency of observing ENaC (f) for a given condition was equal to the number of patches containing at least one active channel divided by the total number of viable seals. ENaC activity, Po, N, and f for each condition were quantified from CNTs/CCDs isolated from ≥4 mice/group.
Nucleotides in urine
Collected urine was immediately frozen in liquid nitrogen and stored at −80°C until analyzed. For most animals, we collected 2–3 urine samples/diet and determined the mean values for each animal receiving a given diet. Nucleotides in urine were measured by HPLC (31) and related to urinary creatinine.
BP measurements and NaCl appetite
DOCA treatment was performed by implanting DOCA pellets (2.4 mg/d; Innovative Research of America, Sarasota, FL, USA) or sham operation (32). Systolic BP was determined using a tail-cuff system (Visitech Systems, Apex, NC, USA) after appropriate training (17, 32). BP was determined daily 5 d prior to implantation and during the entire experimental period. Mice had free access to standard rodent diet (0.42% Na+; TD 7001; Harlan Teklad) and to fluid from 2 drinking bottles. The 2-bottle choice test was performed as described previously (32, 33). The purpose of this approach was 2-fold: to vary NaCl intake, and to test for DOCA-induced NaCl appetite.
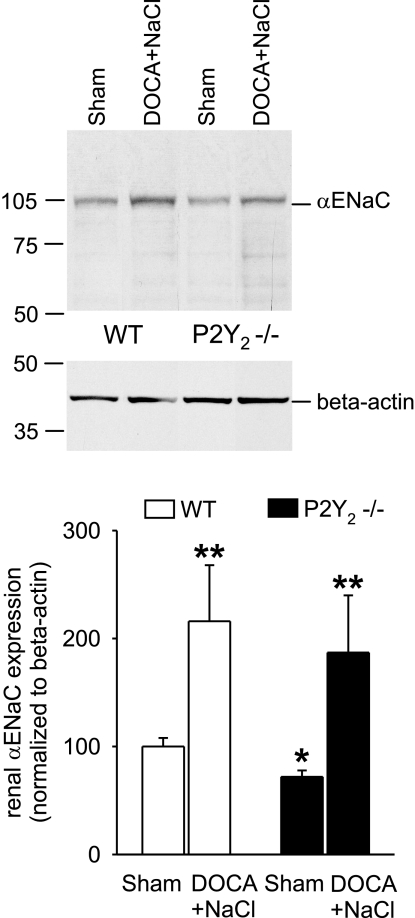
Renal αENaC expression
On d 20 after DOCA pellet implantation, mice were anesthetized, and the left kidney was excised and prepared for Western blot analysis, as described previously (26). A rabbit anti-α-ENaC antibody (1:3000; a generous gift from G. Dechenes, Assistance Publique–Hôpitaux de Paris Robert-Debré, Paris, France, and A. Doucet, Institut des Cordeliers, Paris, France; refs. 17, 26, 34) was used. Chemiluminescent detection was performed using an ECL donkey anti-rabbit IgG HRP-linked secondary antibody (1:5000; GE Healthcare, Piscataway, NJ, USA) with ECL detection reagent (GE Healthcare). Expression of αENaC was normalized to β-actin expression (monoclonal antibody A5316; Sigma-Aldrich, St. Louis, MO, USA). Densitometric analysis was performed using U.S. National Institutes of Health ImageJ Software (NIH, Bethesda, MD, USA).
Statistics and data analysis
All summarized data are reported as means ± se. Data from before and after treatment within the same experiment were compared with the paired t test. Data from different experiments were compared with a Z test, a Student’s (2-tailed) t test, or a 1-way ANOVA using the Dunnett post-test comparing treatment groups to a single control group (regular diet or high-NaCl diet). Values of P ≤ 0.05 were considered significant. For presentation, current data from some cell-attached patches were subsequently software filtered at 50 Hz, and slow baseline drifts were corrected.
RESULTS
P2Y2-receptor activation induces a tonic inhibition of ENaC Po in ASDNs of mice fed a normal-Na+ diet (0.32%)
Fig. 1A shows current traces from cell-attached patches formed on the apical membrane of principal cells in CNTs/CCDs isolated from WT (top) and P2Y2−/− (bottom) mice before and after addition of 100 μM ATP. As summarized in Fig. 1B–D, ATP, in these paired experiments, rapidly and markedly decreased ENaC Po in WT mice but not in P2Y2−/− mice. In addition, resting ENaC Po was greater in P2Y2−/− mice than in WT mice fed a normal-Na+ diet (Fig. 1E).
Figure 1.
ATP-induced inhibition of ENaC Po in ASDN is blunted in P2Y2−/− mice fed a regular-Na+ diet. A) Continuous current traces from cell-attached patches containing at ≥2 ENaCs before and after application of 100 μM ATP in CCDs isolated from WT (top) and P2Y2−/− (bottom) animals. Patches clamped to −Vp = −60 mV. Inward Li+ currents through ENaC are downward. Dashed lines indicate respective current levels, with c denoting the closed state. B, C) Summary graphs of ENaC Po changes in response to ATP for WT (B; n=8 patches, n=5 mice) and P2Y2−/− mice (C; n=9, n=5). D) Summary graph comparing the percentage decrease in ENaC Po in response to ATP in WT vs. P2Y2−/−. E) Summary graph comparing resting ENaC Po in WT vs. P2Y2−/−. Data are expressed as means ± se. *P < 0.05.
Down-regulation of ENaC Po in ASDNs by dietary NaCl intake is lacking in P2Y2−/− mice
The results in Fig. 2A, C, E and Supplemental Table 1 confirm the regulation of ENaC by dietary Na+ intake in WT mice. In CNTs/CCDs isolated from WT mice maintained on low- and high-Na+ diets, ENaC activity (which is the product of ENaC Po and the number of active ENaCs, fN), was significantly greater and smaller, respectively, compared to ENaC activity of mice fed a regular-Na+ diet (Fig. 2E), consistent with the prominent role of ENaC in Na+ homeostasis (2). This inverse relationship with dietary Na+ intake is also observed in WT mice for ENaC Po (Fig. 2C) and fN (Fig. 2B) (see the Methods for details on the measures taken to differentiate between effects on fN vs. Po).
Figure 2.
Suppression of ENaC Po but not channel number by NaCl intake is absent in ASDN of P2Y2−/− mice. To assure reliable calculation of Po, we paid particular attention to use long recording times (>1 min) and patches containing ≤5 channels. Summary graphs of resting ENaC Po, ENaC membrane levels (fN), and ENaC activity (fNPo) for WT (A, C, E) and P2Y2−/− (B, D, F) mice maintained for 1 wk on low (<0.01%)-, regular (0.32%)-, and high (2%)-Na+ diets, respectively. Data are expressed as means ± se. Numbers inside bars indicate number of experiments. *P < 0.05 vs. regular (0.32%)-Na+ diet.
Changes in dietary Na+ intake, however, failed to affect ENaC Po in CNTs/CCDs from P2Y2−/− mice, i.e., in those mice, the channel is in a hyperactive state that is independent of Na+ intake (Fig. 2B and Supplemental Table 1). ENaC Po from P2Y2−/− mice, though, was greater than that from WT mice fed normal and high-Na+ diets but was similar if the two types of mice are fed a low-Na+ diet, a condition that maximizes Po. These results suggest that dietary NaCl activates local ATP and/or UTP signaling in the ASDN and inhibits ENaC Po via P2Y2 receptors.
The apparent resistance of ENaC to NaCl intake in P2Y2−/− mice is only present for Po, since the number of active ENaC (Fig. 2D and Supplemental Table 1) was inversely related to dietary Na+ intake, an effect akin to that observed in WT mice.
Inhibition of local ATP signaling prevents down-regulation of ENaC Po in ASDNs by dietary NaCl intake in WT mice
To quantify the ambient tone of the local ATP/UTP-P2Y-receptor system under different NaCl conditions, we treated CNTs/CCDs from WT mice for 10 min with 10 U/ml hexokinase (+glucose) plus 100 μM suramin to degrade local ATP and prevent nucleotide-receptor activation, respectively (18). The number of ENaCs per patch of mice pretreated with low (<0.01%)-, regular (0.32%)-, and high (2%)-Na+ diets were 3.76 ± 0.47, 3.1 ± 0.34, and 1.87 ± 0.23, respectively. As summarized in Fig. 3C, hexokinase + suramin had little effect on ENaC Po in mice fed a low-Na+ diet. In contrast, those agents markedly increased ENaC Po in CNTs/CCDs from mice fed a high-Na+ diet. To emphasize this difference, we quantified (Fig. 3D) the fold-increase in ENaC Po and activity (fNPo) after hexokinase+suramin treatment. The results suggest that this change in ENaC activity reflects changes in Po. By comparison, application of hexokinase + suramin did not affect ENaC Po in P2Y2−/− mice fed a high-NaCl diet (from 0.52±0.05 to 0.51±0.04; n=12 patches in 4 mice; NS). These results support the conclusion that the inhibition by the ATP/UTP-P2Y2 system on ENaC Po is regulated by dietary NaCl, with greater NaCl intake inducing greater activity of the system.
Figure 3.
Inhibition of local ATP signaling prevents regulation of ENaC Po in ASDN by dietary NaCl intake in WT mice. A, B)Representative current traces from cell-attached patches containing ENaC from WT mice fed low (<0.01%)-Na+ (A) and high (2%)-Na+ diets (B) in the absence (top) and presence of hexokinase (+glucose) + suramin (bottom) to degrade local ATP and prevent nucleotide-receptor activation, respectively. Dashed lines indicate respective current levels, with c denoting the closed state. All other conditions are the same as in Fig. 1A. C) Summary of ENaC Po in the absence (control) and presence of hexokinase + suramin for mice fed <0.01, 0.32, and 2%-Na+ diets. *P < 0.05 vs. regular (0.32%)-Na+ diet; **P < 0.05 vs. respective control diet. D) Summary of fold increase in ENaC Po and ENaC activity (fNPo) in response to hexokinase + suramin in mice fed <0.01, 0.32, and 2%-Na+ diets. Data are expressed as means ± se. Numbers inside bars indicate number of experiments.
High dietary NaCl intake is associated with increased urinary UTP and the ATP hydrolytic product, ADP
To assess whether dietary NaCl may affect luminal ATP and/or UTP concentrations, we collected urine from WT mice fed control or high-NaCl diets and quantitated ATP, UTP, ADP, and UDP (Fig. 4). The nucleotide concentrations were in the low micromolar range, with ATP concentrations being the lowest. The highest ATP values were found in animals fed a high-NaCl diet, but the concentrations were variable, such that we detected no significant difference in ATP concentration for mice fed control or high-NaCl diets. Urinary concentration of the ATP hydrolytic product, ADP, however, was significantly greater in mice fed a high-NaCl diet, perhaps reflecting greater ATP release and degradation to ADP in the distal nephron. Urinary UTP was also increased in mice fed a high-NaCl vs. control diet, a result consistent with the greater activation of luminal P2Y2 receptors.
Figure 4.
High dietary NaCl intake is associated with increased urinary levels of UTP and the ATP hydrolytic product, ADP. Spontaneous collections of urine were taken in WT mice fed control or high-NaCl diets, and concentrations of ATP, UTP, ADP, and UDP were measured; n = 5 mice/group. Data are expressed as means ± se. *P < 0.05 vs. respective control diet.
Mineralocorticoid-induced increase in ENaC Po in ASDNs is lacking in P2Y2−/− mice
To test whether mineralocorticoids increase ENaC Po and whether such an effect depends on inhibition of the influence of ATP/P2Y2-receptor signaling, we studied the effects of different NaCl dietary levels on ENaC activity in CNTs/CCDs of WT and P2Y2−/− mice pretreated with the mineralocorticoid DOCA. As shown in Fig. 5A and Supplemental Table 1, DOCA increased ENaC Po in CNTs/CCDs from WT mice fed regular and high-NaCl diets, but not from mice fed a low-NaCl diet. A similar response was observed with regard to the number of active ENaCs (fN) (Fig. 5C; Supplemental Table 1). DOCA treatment of P2Y2−/− mice with regular and high NaCl intake increased the number of active ENaCs and ENaC activity, but ENaC Po, which is elevated due to loss of modulation by the P2Y2 receptors, was unresponsive (Fig. 5B, D, F and Supplemental Table 1). Thus, the results imply a mineralocorticoid-induced increase in ENaC Po in response to a low-NaCl diet by a mechanism that involves suppression of the ATP/UTP-P2Y2-receptor system.
Figure 5.
Clamping mineralocorticoids at a high level increases ENaC Po in ASDN and dampens but does not abolish the regulation of ENaC by dietary NaCl in WT but not P2Y2−/− mice. Summary of resting ENaC Po, ENaC membrane levels (fN), and ENaC activity (fNPo) for WT (A, C, E; open bars) and P2Y2−/− mice (B, D, F; solid bars) maintained for 1 wk on low (<0.01%)-, regular (0.32%)-, and high (2%)-Na+ diets, respectively, in the absence (open or solid bars) or presence of DOCA injections on the last 3 d (shaded bars). Data are expressed as means ± se. Numbers inside bars indicate number of experiments. *P < 0.05 vs. low (<0.01%)-Na+ diet; **P < 0.05 vs. respective control.
In the presence of high exogenous mineralocorticoid levels, dietary NaCl intake lowers ENaC Po and the number of active ENaC in ASDNs of WT but not P2Y2−/− mice
Despite DOCA treatment, dietary NaCl decreased the number of active ENaCs and ENaC Po in WT mice (Fig. 5A, C and Supplemental Table 1). As observed in the absence of exogenous mineralocorticoid, the dietary NaCl-induced down-regulation of ENaC Po was absent in DOCA-treated P2Y2−/− mice. Moreover, P2Y2−/− mice treated with DOCA showed a blunted decrease in active ENaC numbers in response to increased dietary NaCl. Together, these data provide strong evidence for a prominent role of P2Y2-receptor signaling in suppression of ENaC activity, when Na+ transport is activated by high levels of mineralocorticoid in combination with high NaCl intake. ATP and/or UTP release in this setting may also be due to changes in cell volume (17,35,36,37) as a consequence of DOCA-induced overactive Na+ transport.
Clamping mineralocorticoids at high levels unmasks greater NaCl sensitivity of BP in P2Y2−/− mice
Considering the blunted regulation of ENaC Po by dietary NaCl in P2Y2−/− mice, one might predict a NaCl-sensitive form of hypertension. We found, though, that the BP phenotype in these mice is NaCl resistant, perhaps, at least in part, due to a down-regulation of the renin-angiotensin-aldosterone system observed in these animals (17). To further assess this idea and the electrophysiological data above, we examined the effect of DOCA on renal αENaC expression and NaCl sensitivity of BP (17, 32). We manipulated dietary NaCl intake by providing access to 2 drinking bottles that first contained water, then switched one bottle to 1% NaCl, and finally provided access only to 1% NaCl in both bottles (Figs. 6 and 7).
Figure 6.
Clamping mineralocorticoids at a high level induces greater NaCl sensitivity of BP in P2Y2−/− than in WT mice. Systolic BP was determined in WT and P2Y2−/− mice treated with DOCA and provided access to 2 drinking bottles filled with H2O only, choice of H2O and 1% NaCl, or both filled with 1% NaCl. Changes in BP compared with basal measurements before DOCA application and with access to H2O only (A), as well as absolute BP values (B); n = 5–6 mice/group. For comparison, we adapted and included previously published data (17) in WT and P2Y2−/− mice to illustrate the BP response to variation in NaCl intake in the absence of DOCA (triangles). P2Y2−/− mice fed a high-NaCl diet (B, top solid triangle) or provided the choice of drinking H2O or 1% NaCl during treatment with DOCA (B, middle solid circle) had similar increases in NaCl intake vs. control diet, but if given DOCA had a greater BP response. Comparing similar NaCl intake levels in WT mice indicated a smaller BP response to DOCA. Data are expressed as means ± se.
Figure 7.
Mineralocorticoid-induced increase in appetite for NaCl is blunted in P2Y2−/− mice. Mice have free access to 2 bottles. Top horizontal bars indicate time of switching from H2O to 1% NaCl for bottles 1 and 2. Graphs show daily volume drunk from bottle 1 (top panel) or from bottle 2 (bottom panel) by WT and P2Y2−/− mice prior to and during DOCA treatment; n = 5–6 mice/group. Data are expressed as means ± se. *P < 0.05 vs. WT.
We found that BP was modestly greater in P2Y2−/− compared to WT mice before we administered DOCA or increased dietary NaCl (109±2 vs. 102±1 mmHg, in P2Y2−/− and WT mice, respectively; P<0.05), confirming previous results (17). Sham treatment did not significantly change BP over the 20-d observation period in P2Y2−/− or WT mice (−3±1 and −1±1 mmHg, NS). WT or P2Y2−/− mice given DOCA and only water to drink had no change in BP (Fig. 6), although P2Y2−/− mice had greater fluid intake under these conditions, which may reflect greater induction of thirst (Fig. 7). After one bottle was switched to 1% NaCl, DOCA-treated P2Y2−/− and WT mice initially (d 7 in Fig. 7) preferred 1% NaCl to water, consistent with DOCA-induced salt appetite. WT mice responded similarly on subsequent days, but P2Y2−/− mice preferred water or avoided the NaCl solution (Fig. 7). Notably, despite lower NaCl intake during this period, P2Y2−/− mice had a greater increase in BP than did WT animals (Fig. 6). If only able to drink 1% NaCl (d 15–19 in Fig. 7), WT and P2Y2−/− mice had similar NaCl intake, but P2Y2−/− mice had a greater, further increase in BP than did WT mice (Fig. 6). Consistent with the lower plasma aldosterone concentration in P2Y2−/− mice (17), sham-treated P2Y2−/− mice had lower αENaC expression than did WT mice; DOCA/high NaCl up-regulated renal αENaC expression in P2Y2−/− and WT mice (Fig. 8). This increase in αENaC expression may have helped unmask the greater ENaC Po and, as a consequence, produced greater NaCl-sensitivity of BP in P2Y2−/− mice treated with DOCA.
Figure 8.
Clamping mineralocorticoids at a high level plus high-NaCl diet increases renal αENaC expression in P2Y−/− and WT mice. Western blot analysis was performed after completing the experiments in Figs. 6 and 7; n = 5 or 6 mice/group. Data are expressed as means ± se. *P < 0.05 vs. WT; **P < 0.05 vs. sham.
DISCUSSION
Regulation of ENaC by aldosterone plays a primary role in adapting renal reabsorption of Na+ in altered physiological states. Much is known about the mechanisms that regulate ENaC trafficking, which determines the number of ENaCs in the apical membrane of the ASDN (22). The present studies suggest that dietary NaCl and mineralocorticoids also alter renal Na+ reabsorption by regulating ENaC Po. Moreover, our data indicate that this regulation involves the apical ATP/UTP-P2Y2-receptor system.
A previous study (38) compared ENaC activity in cell-attached patches of split-open CCDs in rats fed normal and low-NaCl diets and concluded that the increase in ENaC activity (NPo) in response to lowering NaCl intake was primarily due to an increased number of channels per patch. The researchers, however, also stated that the reduction in NaCl intake or increase in aldosterone may have moved the channels from a low ENaC Po state, which in rat CCD is not observable, to a high ENaC Po state with a value of ∼0.5 (38). The present studies provide strong evidence that the number of active ENaC and ENaC Po change inversely with NaCl intake in CNTs/CCDs of WT mice.
Luminal application of ATP or UTP to CNTs/CCDs of WT mice fed a low-NaCl diet acutely lowers ENaC Po from 0.5 to 0.1 (18), i.e., to levels of WT mice fed a high-NaCl diet in the present studies. Changes in luminal availability of ATP/UTP thus may contribute to the adaptation of P2Y2-receptor activity in response to changes in NaCl intake. Indeed, we found that mice fed a high-NaCl diet have greater urinary levels of UTP and ADP. The greater urinary ADP levels may reflect greater ATP release in the ASDN and hydrolysis to ADP. Further studies are necessary to test this idea.
Treatment of WT mice with DOCA increased the number of active ENaCs and ENaC Po. The observation that DOCA did not alter ENaC Po in P2Y2−/− mice is consistent with the notion that mineralocorticoids increase ENaC Po by suppressing the inhibitory influence of P2Y2-receptor activation. Previous studies have shown that mineralocorticoids enhance ENaC Po in the A6 amphibian renal cells, a model for the mammalian distal nephron (28, 39, 40). Alterations in NaCl intake and resultant changes in aldosterone concentration may regulate luminal availability of ATP and/or UTP in the ASDN. The resulting changes in apical P2Y2-receptor activation may then affect ENaC Po with respect to NaCl balance (Fig. 9). Further studies are needed to define the molecular events that link dietary NaCl intake and mineralocorticoids to the luminal ATP/UTP-P2Y2-receptor system.
Figure 9.
Model for the regulation of ENaC Po by dietary NaCl and aldosterone through altering apical P2Y2-receptor tone in the ASDN. Left: low NaCl intake and increased aldosterone levels suppress the luminal availability of ATP and/or UTP. Inner leaflet of the lipid bilayer contains high concentrations of negatively charged phosphatidylinositol 4,5-bisphosphate (PIP2), which binds to positively charged regions of the N terminus of the β-subunit of ENaC, thereby maintaining the open ENaC channel and the reuptake of Na+ (18,19,20,21). Right: high NaCl intake increases apical availability of ATP and/or UTP. Stimulation of P2Y2 receptors activates phospholipase C (PLC), which lowers the membrane concentration of PIP2. This induces a conformational change in the N terminus of β-ENaC, lowering ENaC Po and thereby Na+ reuptake. NaCl-induced changes in ENaC Po act in concert with changes in ENaC expression and trafficking (latter not shown). IP3, inositol trisphosphate.
P2Y2−/− mice have an increased BP, but amiloride-sensitive renal Na+ excretion is not significantly increased compared with WT mice fed a regular-NaCl diet (17), consistent with a similar net ENaC activity in isolated CNTs/CCDspatches that we show here. P2Y2−/− mice have lower plasma aldosterone concentrations (17), which is expected to reduce renal αENaC expression and basolateral Na+-K+-ATPase activity, the driving force for Na+ uptake. These effects may compensate for greater ENaC Po and help normalize ENaC activity in P2Y2−/− mice. Greater BP in the presence of normal net ENaC activity, however, implies that P2Y2−/− mice have additional BP-related defects; P2Y2−/− mice have increased activity of the furosemide-sensitive Na-K-2Cl cotransporter in the thick ascending limb (17), which is consistent with a role for ATP/P2Y2 receptors in that segment (8, 10).
Patch-clamp analysis of DOCA-treated mice indicated that absence of P2Y2 receptors blunts the dietary NaCl-induced suppression of the number of active ENaCs per patch. Such an influence of P2Y2 receptors on active ENaC numbers was not observed in the absence of DOCA, indicating that DOCA sensitizes active ENaC expression in the apical membrane to a P2Y2-receptor-mediated inhibitory influence; the molecular mechanisms for such interactions remain to be determined. Irrespective of the mechanism, elimination of differences in mineralocorticoid tone between genotypes unmasked deficient inhibitory tone on ENaC activity in P2Y2−/− mice, causing greater up-regulation of ENaC activity and NaCl sensitivity of BP. These effects in P2Y2−/− mice were associated with blunted DOCA-induced NaCl appetite or intake. DOCA is known to induce NaCl intake, i.e., when treated with DOCA and given the choice to drink water or NaCl solution, WT mice prefer NaCl solution. Why knockout mice prefer to drink tap water vs. 1% NaCl solution is not clear, but this decrease in ingestion of Na+ may prevent even greater increases in BP.
In summary, our results provide strong evidence for a previously unappreciated role of P2Y2 receptors in the regulation of ENaC Po by dietary NaCl and mineralocorticoids, and unmask the influence of this system on NaCl sensitivity of BP. Further studies are warranted to explore effects of P2Y2-receptor agonism on NaCl homeostasis and BP regulation and on a possible role of P2Y2-receptor polymorphisms (41) in the pathophysiology of hypertension in humans.
Supplementary Material
Acknowledgments
Research was supported by the U.S. National Institutes of Health (R01DK59594 to J.D.S.; R01GM66232 to P.A.I.; and R01DK56248, R01DK28602 and P30DK079337 to V.V.), the American Heart Association (064005N to J.D.S. and 0825062F and 09SDG2230391 to O.P.), the Department of Veterans Affairs (to V.V.), and the National Kidney Foundation of Southern California (to T.R.). The authors thank B. Koller and W. Zinzow (University of North Carolina, Chapel Hill, NC, USA) for the P2Y2−/− mouse.
References
- Guyton A C. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension. 1992;19:I2–I8. doi: 10.1161/01.hyp.19.1_suppl.i2. [DOI] [PubMed] [Google Scholar]
- Hummler E, Vallon V. Lessons from mouse mutants of epithelial sodium channel and its regulatory proteins. J Am Soc Nephrol. 2005;16:3160–3166. doi: 10.1681/ASN.2005040450. [DOI] [PubMed] [Google Scholar]
- Lifton R P, Gharavi A G, Geller D S. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Unwin R J, Bailey M A, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci. 2003;18:237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol. 2008;294:F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- Schwiebert E M, Kishore B K. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol. 2001;280:F945–F963. doi: 10.1152/ajprenal.2001.280.6.F945. [DOI] [PubMed] [Google Scholar]
- Geyti C S, Odgaard E, Overgaard M T, Jensen M E, Leipziger J, Praetorius H A. Slow spontaneous [Ca2+] oscillations reflect nucleotide release from renal epithelia. Pflügers Arch. 2008;455:1105–1117. doi: 10.1007/s00424-007-0366-4. [DOI] [PubMed] [Google Scholar]
- Hovater M B, Olteanu D, Hanson E L, Cheng N L, Siroky B, Fintha A, Komlosi P, Liu W, Satlin L M, Bell P D, Yoder B K, Schwiebert E M. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 2008;4:155–170. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M E, Odgaard E, Christensen M H, Praetorius H A, Leipziger J. Flow-induced [Ca2+]I increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18:2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- Wildman S S, Marks J, Turner C M, Yew-Booth L, Peppiatt-Wildman C M, King B F, Shirley D G, Wang W, Unwin R J. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol. 2008;19:731–742. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Deetjen P, Ko W H, Jacobi C, Leipziger J. P2Y(2) receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol. 2001;183:115–124. doi: 10.1007/s00232-001-0059-4. [DOI] [PubMed] [Google Scholar]
- Cuffe J E, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol. 2000;524:77–90. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley D G, Bailey M A, Unwin R J. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol. 2005;288:F1243–F1248. doi: 10.1152/ajprenal.00152.2004. [DOI] [PubMed] [Google Scholar]
- Lehrmann H, Thomas J, Kim S J, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002;13:10–18. doi: 10.1681/ASN.V13110. [DOI] [PubMed] [Google Scholar]
- Lu M, MacGregor G G, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol. 2000;116:299–310. doi: 10.1085/jgp.116.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T, Bundey R A, Chen Y, Deschenes G, Junger W, Insel P A, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Bugaj V, Rieg T, Insel P A, Mironova E, Vallon V, Stockand J D. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- Ma H P, Saxena S, Warnock D G. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- Ma H P, Eaton D C. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–3187. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- Butterworth M B, Edinger R S, Frizzell R A, Johnson J P. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:F10–F24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya L, Watt W C, Lazarowski E R, Koller B H, Boucher R C. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J Biol Chem. 1999;274:26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina J L, Stockand J D. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–F1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand J D. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol. 2007;18:1652–1661. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct αENaC activity. J Am Soc Nephrol. 2009;20:721–729. doi: 10.1681/ASN.2008040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochynyuk O, Tong Q, Staruschenko A, Stockand J D. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol. 2007;580:365–372. doi: 10.1113/jphysiol.2006.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemendy A E, Kleyman T R, Eaton D C. Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol Cell Physiol. 1992;263:C825–C837. doi: 10.1152/ajpcell.1992.263.4.C825. [DOI] [PubMed] [Google Scholar]
- Sackmann B, Neher E. New York: Plenum Press; Single Channel Recording. 1995 [Google Scholar]
- Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Boss G R, Erbe R W. Decreased purine synthesis during amino acid starvation of human lymphoblasts. J Biol Chem. 1982;257:4242–4247. [PubMed] [Google Scholar]
- Vallon V, Huang D Y, Grahammer F, Wyatt A W, Osswald H, Wulff P, Kuhl D, Lang F. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am J Physiol Regul Integr Comp Physiol. 2005;289:R395–R401. doi: 10.1152/ajpregu.00731.2004. [DOI] [PubMed] [Google Scholar]
- Rieg T, Schnermann J, Vallon V. Adenosine A(1) receptors determine effects of caffeine on total fluid intake but not caffeine appetite. Eur J Pharmacol. 2007;555:174–177. doi: 10.1016/j.ejphar.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Lourdel S, Loffing J, Favre G, Paulais M, Nissant A, Fakitsas P, Creminon C, Feraille E, Verrey F, Teulon J, Doucet A, Deschenes G. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol. 2005;16:3642–3650. doi: 10.1681/ASN.2005040363. [DOI] [PubMed] [Google Scholar]
- Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol. 2009;296:R419–R427. doi: 10.1152/ajpregu.90784.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A L, Kudlow B A, Marrs K L, Gruenert D C, Guggino W B, Schwiebert E M. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol Cell Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky S D, Fitz J G. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha J, Frindt G, Antonian L, Silver R B, Palmer L G. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B N, Kemendy A E, Kokko K E, Hinton C F, Marunaka Y, Eaton D C. Regulation of the amiloride-blockable sodium channel from epithelial tissue. Mol Cell Biochem. 1990;99:141–150. doi: 10.1007/BF00230344. [DOI] [PubMed] [Google Scholar]
- Duchatelle P, Ohara A, Ling B N, Kemendy A E, Kokko K E, Matsumoto P S, Eaton D C. Regulation of renal epithelial sodium channels. Mol Cell Biochem. 1992;114:27–34. doi: 10.1007/BF00240294. [DOI] [PubMed] [Google Scholar]
- Buscher R, Hoerning A, Patel H H, Zhang S, Arthur D B, Grasemann H, Ratjen F, Insel P A. P2Y2 receptor polymorphisms and haplotypes in cystic fibrosis and their impact on Ca2+ influx. Pharmacogenet Genomics. 2006;16:199–205. doi: 10.1097/01.fpc.0000189798.11468.6a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.